Diapause Induction, Color Changes, and Supercooling Point of Diapause Larvae of Tetrastichus septentrionalis Yang (Hymenoptera: Eulophidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Insect Production
2.2. Experiment 1: The Critical Photoperiod for Diapause Induction under Different Temperature Conditions
2.3. Experiment 2: Morphological Observations of the Diapause State of T. septentrionalis
2.4. Experiment 3: Sensitive Insect State Determination for Photoperiod-Induced Diapause
2.5. Experiment 4: Determination of Post-Diapause Developmental Starting Temperatures and Effective Cumulative Temperatures
2.6. Experiment 5: Measurement of the Supercooling Point and Freezing Point of T. septentrionalis
2.7. Statistical Analyses
3. Results
3.1. Experiment 1: The Effect of Photoperiod on T. septentrionalis and Its Critical Photoperiod under Different Temperature Conditions
3.2. Experiment 2: Comparison between the Diapause and Non-Diapause Insect Morphology of T. septentrionalis
3.3. Experiment 3: Photoperiod-Induced Diapause Induction of Sensitive Insect States
3.4. Experiment 4: Post-Diapause Developmental Starting Temperatures and Effective Cumulative Temperatures of T. septentrionalis
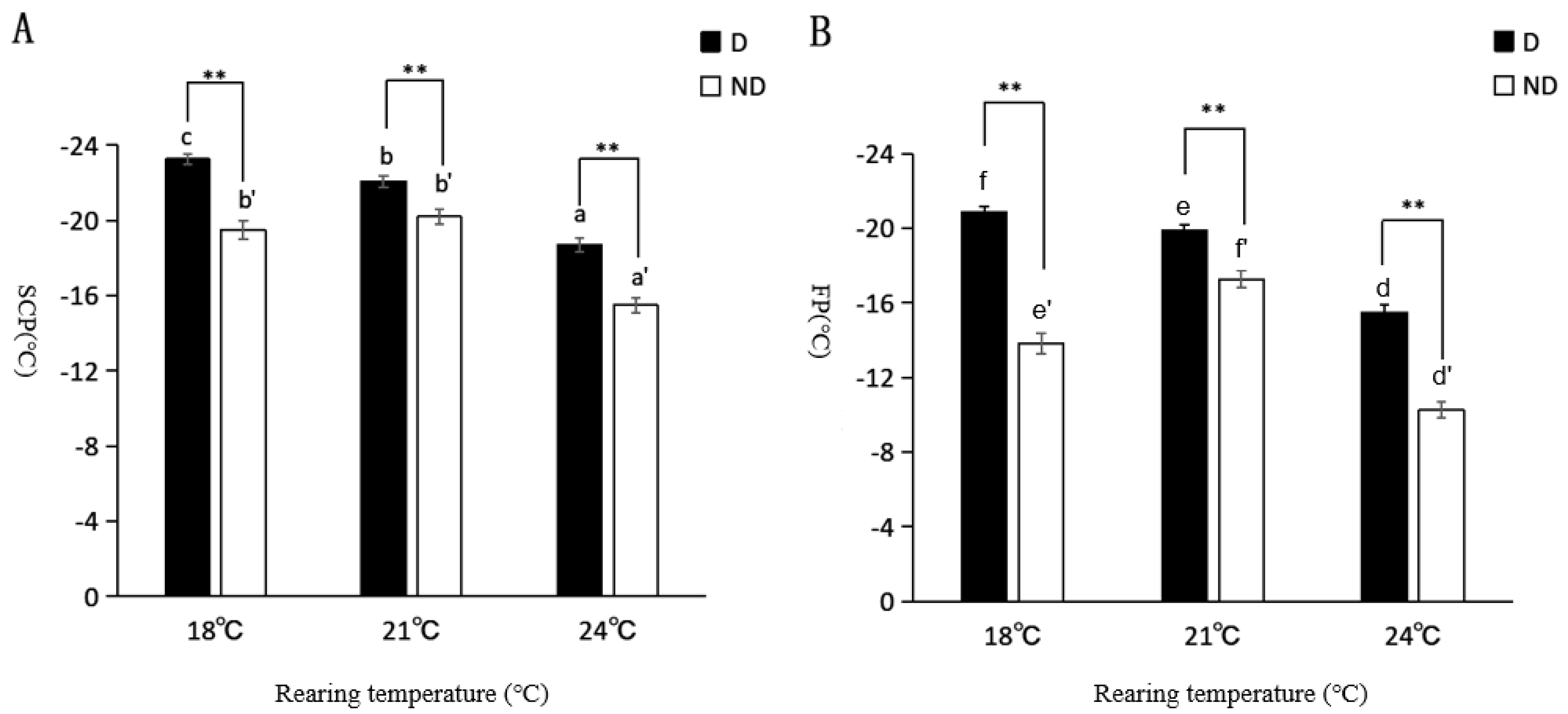
3.5. Experiment 5: Comparison of the Supercooling Point and Freezing Point between Diapause and Non-Diapause T. septentrionalis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Zhong, J.; Jiang, B.; Liang, G.; Su, J.; Ren, Y. Seasonal variation in cold tolerance of the population of pine armored scale, Hemiberlesia pitysophila Takagi (Homoptera: Diaspididae). J. Acta Ecol. Sin. 2009, 29, 5813–5822. [Google Scholar]
- Nordin, J.H.; Cui, Z.; Yin, C.M. Cold-induced glycerol accumulation by Ostrinia nubilalis larvae is developmentally regulated. J. Insect Physiol. 1984, 30, 563–566. [Google Scholar] [CrossRef]
- Denlinger, D.L. Why study diapause? J. Entomol. Res. 2008, 38, 1–9. [Google Scholar] [CrossRef]
- Tomcala, A.; Tollarova, M.; Overgaard, J.; Simek, P.; Kostal, V. Seasonal acquisition of chill tolerance and restructuring of membrane glycerophospholipids in an overwintering insect: Triggering by low temperature, desiccation and diapause progression. J. Exp. Biol. 2006, 209, 4102–4114. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L. Regulation of diapause. J. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Gill, H.K.; Goyal, G.; Chahil, G. Insect Diapause: A Review. J. Agric. Sci. Technol. 2017, 7, 20. [Google Scholar]
- Forster, J.; Hirst, A.G.; Woodward, G. Growth and Development Rates Have Different Thermal Responses. J. Am. Nat. 2011, 178, 668–678. [Google Scholar] [CrossRef]
- Fang, O.; Feng, G. Methodology of mearsuring and analyzing insect cold hardiness. J. Chin. Bull. Entomol. 2014, 51, 1646–1652. [Google Scholar]
- Jing, X.; Kang, L. Overview and evaluation of research methodology for insect cold hardiness. J. Entomol. Knowl. 2004, 41, 7–10. [Google Scholar]
- McIntyre, T.; Andaloori, L.; Hood, G.R.; Feder, J.L.; Hahn, D.A.; Ragland, G.J.; Toxopeus, J. Cold tolerance and diapause within and across trophic levels: Endoparasitic wasps and their fly host have similar phenotypes. J. Insect Physiol. 2023, 146, 104501. [Google Scholar] [CrossRef]
- Somme, L. The history of cold hardiness research in terrestrial arthropods. Cryo Lett. 2000, 21, 289–296. [Google Scholar]
- Hanson, A.A.; Venette, R.C.; Lelito, J.P. Cold tolerance of Chinese emerald ash borer parasitoids: Spathius agrili Yang (Hymenoptera: Braconidae), Tetrastichus planipennisi Yang (Hymenoptera: Eulophidae), and Oobius agrili Zhang and Huang (Hymenoptera: Encyrtidae). J. Biol. Control. 2013, 67, 516–529. [Google Scholar] [CrossRef]
- Liu, F.; Li, Q. Occurrence, Forest Cintrol Status and Prospect of Fall-webworm (Hyphantria cunea Drury) in China. J. Shenyang Agric. Univ. 2022, 53, 630–640. [Google Scholar]
- Sun, S.; Nan, J.; Yang, L.; Song, L.; Zuo, T.; Wang, Y.; Li, S. Research progress on natural enemies of the Hyphantria cunea (Drury). J. Enviro. Entomol. 2021, 43, 1331–1347. [Google Scholar]
- Zhao, X.; Geng, Y.; Hao, D.; Dai, L.; Sun, S. Research progress and prospect of the control technology of Hyphantria cunea. J. For. Pest. Dis. 2022, 41, 44–52. [Google Scholar] [CrossRef]
- Yang, Z.; Yongan, Z. Researches on techniques for biocontrol of the fall webworm, Hyphantria cunea, a severe invasive insect pest to China. Chin. J. Appl. Entomol. 2007, 44, 465–471+622. [Google Scholar]
- Guo, T.; Yan, X. Advance in parasitoids of the Tetrastichus howardi species group in Tetrastichus Haliday (Hymenoptera: Euophidae). J. Nanjing For. Univ. Nat. Sci. 2011, 35, 127–133. [Google Scholar]
- Yang, Z.; Wang, B.; Wei, J. A new species of Eulophidae (Hymenoptera: Chalcidoidea) parasitizing fall webworm in China and Korea. J. Acta Entomol. Sin. 2001, 44, 98–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, J.; Fu, X.; Yang, L.; Hu, S.; Ma, X.; Sun, S. Fecundity of Tetrastichus septentrionalis and effects of alternative hosts on the mass rearing of this Species. Chin. Bull. Entomol. 2023, 60, 872–882. [Google Scholar]
- Qu, H.; Shao, L.; Pang, H.; Nie, L.; Yang, J.; Tan, H. Threshold temperature and effective accumulated temperature of Tetrastichus septentrionalis. J. For. Pest. Dis. 2007, 4, 9–10. [Google Scholar]
- Lee, R.E.; Costanzo, J.P. Biological ice nucleation and ice distribution in cold-hardy ectothermic animals. J. Annu. Rev. Physiol. 1998, 60, 55. [Google Scholar] [CrossRef]
- Kong, W.; Wang, Y.; Guo, Y.; Gao, Y.; Zhao, F.; Li, J.; Ma, R. Supercooling points and freezing points of Grapholita molesta (Busck). J. Plant Protec. 2019, 45, 102–105. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, J. A new simple method to test insect super-cooling point. J. Entomol. Knowl. 2000, 4, 236–238. [Google Scholar]
- Zhao, L.; Xu, X.; Xu, Z.; Liu, Y.; Sun, S. Diapause induction, color change, and cold tolerance physiology of the diapausing larvae of the Chouioia cunea (Hymenoptera: Eulophidae). J. Insect Sci. 2014, 14, 2014. [Google Scholar] [CrossRef][Green Version]
- Beck, S.D. Photoperiodic Induction of Diapause in an Insect. J. Biol. Bull. 1962, 122, 1–12. [Google Scholar] [CrossRef]
- Beck, S.D. Insect Photoperiodism, 2nd ed.; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Liu, Z.; Xia, Y.; Wu, Q.; Zhao, C.; Li, D. Physiochemical changes in diapause larvae of Anastatus japonicus Ashmead. J. Plant Protec. 2021, 47, 158–165. [Google Scholar] [CrossRef]
- Macleod, E.G. Experimental induction and elimination of adult diapause and autumnal coloration in Chrysopa carnea (Neuroptera). J. Insect Physiol. 1967, 13, 1343–1349. [Google Scholar] [CrossRef]
- Numata, H.; Hidaka, T. Photoperiodic control of adult diapause in the bean bug, Riptortus clavatus Thunberg (Heteroptera: Coreidae) I. Reversible induction and termination of diapause. J. Appl. Entomol. Zool. 2008, 17, 530–538. [Google Scholar] [CrossRef]
- Fasulati, S.R. Photoperiodic reaction and coloration of Eurydema Oleracea (Heteroptera, Pentatomidae) [Pests of crucifers]. Entomol. Rev. 1979, 58, 15–23. [Google Scholar]
- Yamamoto, K.; Tsujimura, Y.; Kometani, M.; Kitazawa, C.; Islam, A.T.M.F.; Yamanaka, A. Diapause pupal color diphenism induced by temperature and humidity conditions in Byasa alcinous (Lepidoptera: Papilionidae). J. Insect Physiol. 2011, 57, 930–934. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Xu, S.; Pan, Y.; Lei, C. Morphology diapause and non-diapause pupae amd their adults of Sercinus montelus Gray (Lepidoptera: Papilionidae). J. Acta Entomol. Sin. 2008, 51, 454–458. [Google Scholar]
- Musolin, D.L.; Numata, H. Photoperiodic and temperature control of diapause induction and colour change in the southern green stink bug Nezara viridula. J. Physiol. Entomol. 2003, 28, 65–74. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y. The mechanisms of regulation and control of insect diapause. J. Shanxi Univ. Nat. Sci. Ed. 1995, 1, 105–118. [Google Scholar]
- Wang, Y.; Zhang, L.; Chen, H.; Wang, W.; Zhang, J. Temperature and photoperiodic response of diapause induction in Aphidius gifuensis. J. Appl. Entomol. 2013, 50, 718–726. [Google Scholar]
- Zhang, L.; Chen, H.; Wang, M.; Liu, C.; Zhang, Y.; Chen, C.; Wang, S. Research Prospects in Diapause of Parasitoid Wasp. J. Bio Control. 2014, 30, 149–164. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Lu, Z.; Liu, X.; Zhang, Q. Critical photoperiod and ssensitive stage of diapause induction in Microplitis mediator (Haliday) (Hymenoptera: Braconidae). J. Acta Entomol. Sin. 2008, 51, 635–639. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Vaghina, N.P.; Voinovich, N.D. Diapause induction in Trichogramma embryophagum Htg. (Hym., Trichogrammatidae): The dynamics of thermosensitivity. J. Appl. Entomol. 2008, 132, 502–509. [Google Scholar] [CrossRef]
- Sorokina, A.; Maslennikova, V. Peculiarities of photothermic reactions of some species of Trichogramma genus (Hymenoptera, Trichogrammatidae). Vestnik Len. Gos. Univ. 1986, 1, 9–14. [Google Scholar]
- Leatemia, J.A.; Laing, J.E.; Corrigan, J.E. Production of exclusively male progeny by mated, honey-fed Trichogramma minutum Riley (Hym., Trichogrammatidae). J. Appl. Entomol. 1995, 119, 561–566. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Kats, T.S. Exogenous and Endogenous Factors Inducing Diapause in Trichogramma principium Sug. et Sor. (Hymenoptera, Trichogrammatidae). J. Entomol. Rev. 2004, 83, 776–785. [Google Scholar]
- Wang, T.; Laing, J. Diapause termination and morphogenesis of Holcothorax testaceipes Ratzeburg (Hymenoptera: Encyrtidae), an introduced parasitoid of the spotted tentiform leafminer, Phyllonorycter blancardella (f.) (Lepidoptera: Gracillariidae). J. Can. Entomol. 1989, 121, 65–77. [Google Scholar] [CrossRef]
- Mehrnejad, M.R.; Copland, M.J.W. Diapause strategy in the parasitoid Psyllaephagus pistaciae. J. Entomol. Exp. Appl. 2005, 116, 109–114. [Google Scholar] [CrossRef]
- Gunie, G.; Laugé, G. Effects of high temperatures recorded during diapause completion of Trichogramma brassicae prepupae (Hym.: Trichogrammatidae), on the treated generation and its progeny. J. Entomophaga 1997, 42, 329–336. [Google Scholar]
- Pompanon, F.; Bouletreau, M. Effect of diapause and developmental host species on the circadian locomotor activity rhythm of Trichogramma brassicae females. J. Entomol. Exp. Appl. 2010, 82, 231–234. [Google Scholar] [CrossRef]
- Song, K.; Zheng, L. Study on the Diapause of Trichogramma brassicae. J. Hebei Agric. Sci. 2003, 1, 22–26. [Google Scholar] [CrossRef]
- Bale, J.S. Insect cold hardiness: A matter of life and death. J. Eur. J. Entomol. 1996, 93, 369–382. [Google Scholar]
- Eaton, M.; Kells, S.A. Freeze mortality characteristics of the mold mite Tyrophagus putrescentiae, a significant pest of stored products. J. Econ. Entomol. 2011, 4, 1423–1429. [Google Scholar] [CrossRef]
- Morey, A.; Venette, R.; Hutchison, W. Could natural selection change the geographic range limits of light brown apple moth (Lepidoptera, Tortricidae) in North America? J. NeoBiota 2013, 18, 151–156. [Google Scholar]
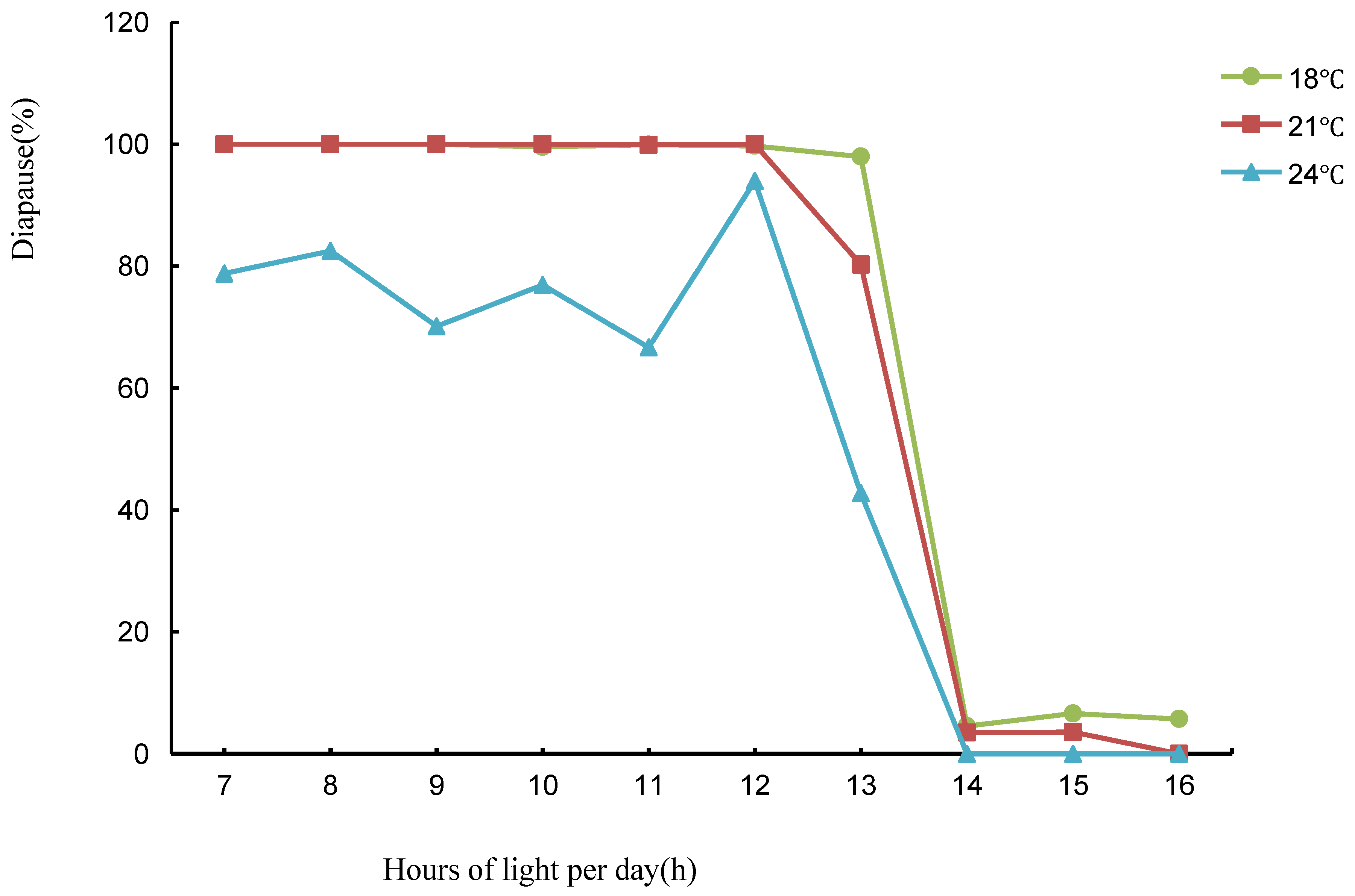

| Photoperiod (L:D) | Actual Measurement Sample No. | Diapause Rate (%) (18 °C) | Diapause Rate (%) (21 °C) | Diapause Rate (%) (24 °C) |
|---|---|---|---|---|
| 7:17 | 85 | 100.00 ± 0.00 aA | 99.97 ±0.06 aA | 97.08 ± 1.87 aA |
| 8:16 | 89 | 100.00 ± 0.00 aA | 100.00 ±0.00 aA | 82.50 ± 7.12 bB |
| 9:15 | 87 | 100.00 ± 0.00 aA | 100.00 ±0.00 aA | 70.12 ± 7.81 bB |
| 10:14 | 89 | 99.54 ± 0.46 aA | 100.00 ±0.00 aA | 76.90 ± 7.68 bB |
| 11:13 | 89 | 99.92 ± 0.08 aA | 99.89 ± 0.11 aA | 66.67 ± 8.75 bB |
| 12:12 | 89 | 99.72 ± 0.23 aA | 100.00 ± 0.00 aA | 93.96 ± 3.93 aA |
| 13:11 | 89 | 97.95 ± 1.25 aA | 80.23 ± 7.31 bB | 42.72 ± 8.95 bC |
| 14:10 | 87 | 4.55 ± 3.72 bA | 3.50 ± 3.45 cA | 0.00 ± 0.00 cB |
| 15:9 | 83 | 6.61 ± 3.98 bA | 3.57 ± 3.57 cA | 0.00 ± 0.00 cB |
| 16:8 | 88 | 0.00 ± 0.00 cA | 0.00 ± 0.00 dA | 0.00 ± 0.00 cA |
| Temperature (°C) | Fitted Equation | Coefficient of Determination (R2) | Critical Photoperiod |
|---|---|---|---|
| 18 | Y = −45.815X2 + 1143.605X − 7026.18 | 1.00 | L:D =14 h 2 min:9 h 58 min |
| 21 | Y = −28.480X2 + 692.230X − 4105.640 | 1.00 | L:D = 14 h 2 min:9 h 58 min |
| 24 | Y = 4.260X2 − 157.740X + 1373.400 | 1.00 | L:D = 14 h 0 min:10 h 00 min |
| Treatment | Approximate Stage | (Mean ± SE) Diapause Rate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg | Larval | Pupal | ||||||||||||
| 0–2 d | 2–4 d | 0–2 d | 2–4 d | 4–6 d | 6–8 d | 8–10 d | 10–12 d | 12–14 d | 0–2 d | 2–4 d | 4–6 d | 6–8 d | ||
| 1 | S | L | L | L | L | L | L | L | L | L | L | L | L | 0.00 ± 0.00% c |
| 2 | S | S | L | L | L | L | L | L | L | L | L | L | L | 0.00 ± 0.00% c |
| 3 | S | S | S | L | L | L | L | L | L | L | L | L | L | 0.11 ± 0.11% c |
| 4 | S | S | S | S | L | L | L | L | L | L | L | L | L | 1.14 ± 0.57% c |
| 5 | S | S | S | S | S | L | L | L | L | L | L | L | L | 15.08 ± 5.02% b |
| 6 | S | S | S | S | S | S | L | L | L | L | L | L | L | 16.28 ± 7.92% b |
| 7 | S | S | S | S | S | S | S | L | L | L | L | L | L | 98.66 ± 1.11% a |
| 8 | S | S | S | S | S | S | S | S | L | L | L | L | L | 99.30 ± 0.70% a |
| 9 | S | S | S | S | S | S | S | S | S | L | L | L | L | 100.00 ± 0.00% a |
| 10 | S | S | S | S | S | S | S | S | S | S | L | L | L | 100.00 ± 0.00% a |
| 11 | S | S | S | S | S | S | S | S | S | S | S | L | L | 100.00 ± 0.00% a |
| 12 | S | S | S | S | S | S | S | S | S | S | S | S | L | 100.00 ± 0.00% a |
| 13 | S | S | S | S | S | S | S | S | S | S | S | S | S | 99.66 ± 0.34% a |
| A | L | S | S | S | S | S | S | S | S | S | S | S | S | 100.00 ± 0.00% a |
| B | L | L | S | S | S | S | S | S | S | S | S | S | S | 100.00 ± 0.00% a |
| C | L | L | L | S | S | S | S | S | S | S | S | S | S | 99.26 ± 0.74% a |
| D | L | L | L | L | S | S | S | S | S | S | S | S | S | 100.00 ± 0.00% a |
| E | L | L | L | L | L | S | S | S | S | S | S | S | S | 62.19 ± 14.60% b |
| F | L | L | L | L | L | L | S | S | S | S | S | S | S | 16.31 ± 8.48% c |
| G | L | L | L | L | L | L | L | S | S | S | S | S | S | 1.26 ± 0.65% d |
| H | L | L | L | L | L | L | L | L | S | S | S | S | S | 0.72 ± 0.58% d |
| I | L | L | L | L | L | L | L | L | L | S | S | S | S | 0.00 ± 0.00% d |
| J | L | L | L | L | L | L | L | L | L | L | S | S | S | 0.00 ± 0.00% d |
| K | L | L | L | L | L | L | L | L | L | L | L | S | S | 0.00 ± 0.00% d |
| L | L | L | L | L | L | L | L | L | L | L | L | L | S | 0.00 ± 0.00% d |
| M | L | L | L | L | L | L | L | L | L | L | L | L | L | 0.00 ± 0.00% d |
| Temperature (°C) | Developmental Duration (d) | Developmental Rate (1/d) |
|---|---|---|
| 18 | 35.42 ± 0.20 a | 0.03 ± 0.02 c′ |
| 21 | 26.75 ± 0.17 b | 0.04 ± 0.02 b′ |
| 24 | 17.00 ± 0.22 c | 0.06 ± 0.08 a′ |
| Temperature (°C) | Number of Individuals | Different Treatments of Insect States | |||
|---|---|---|---|---|---|
| Diapause | Non-Diapause | ||||
| SCP (°C) | FP (°C) | SCP (°C) | FP (°C) | ||
| 18 | 60 | −23.26 ± 0.2ff5 cB | −20.87 ± 0.3ff0 cB | −19.51 ± 0.4ff8 bA | −13.84 ± 0.5ff5 bA |
| 21 | 60 | −22.09 ± 0.3ff1 bB | −19.90 ± 0.3ff1 bB | −20.20 ± 0.4ff2 bA | −17.26 ± 0.4ff7 cA |
| 24 | 60 | −18.69 ± 0.3ff7 aB | −15.49 ± 0.4ff2 aB | −15.49 ± 0.4ff2 aA | −10.25 ± 0.4ff2 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shi, J.; Yang, L.; Cheng, Y.; Liu, X.; Sun, S. Diapause Induction, Color Changes, and Supercooling Point of Diapause Larvae of Tetrastichus septentrionalis Yang (Hymenoptera: Eulophidae). Insects 2023, 14, 826. https://doi.org/10.3390/insects14100826
Li Z, Shi J, Yang L, Cheng Y, Liu X, Sun S. Diapause Induction, Color Changes, and Supercooling Point of Diapause Larvae of Tetrastichus septentrionalis Yang (Hymenoptera: Eulophidae). Insects. 2023; 14(10):826. https://doi.org/10.3390/insects14100826
Chicago/Turabian StyleLi, Zhixin, Junrui Shi, Liyuan Yang, Yiran Cheng, Xudan Liu, and Shouhui Sun. 2023. "Diapause Induction, Color Changes, and Supercooling Point of Diapause Larvae of Tetrastichus septentrionalis Yang (Hymenoptera: Eulophidae)" Insects 14, no. 10: 826. https://doi.org/10.3390/insects14100826
APA StyleLi, Z., Shi, J., Yang, L., Cheng, Y., Liu, X., & Sun, S. (2023). Diapause Induction, Color Changes, and Supercooling Point of Diapause Larvae of Tetrastichus septentrionalis Yang (Hymenoptera: Eulophidae). Insects, 14(10), 826. https://doi.org/10.3390/insects14100826





