True Parthenogenesis and Female-Biased Sex Ratios in Cicadomorpha and Fulgoromorpha (Hemiptera, Auchenorrhyncha)
Abstract
Simple Summary
Abstract
1. Introduction
2. A Brief History of Parthenogenesis in Fulgoromorpha and Cicadomorpha
3. A Brief Overview of the Patterns and Origins of True Parthenogenesis
4. Well-Documented Cases of True Parthenogenesis in Fulgoromorpha and Cicadomorpha
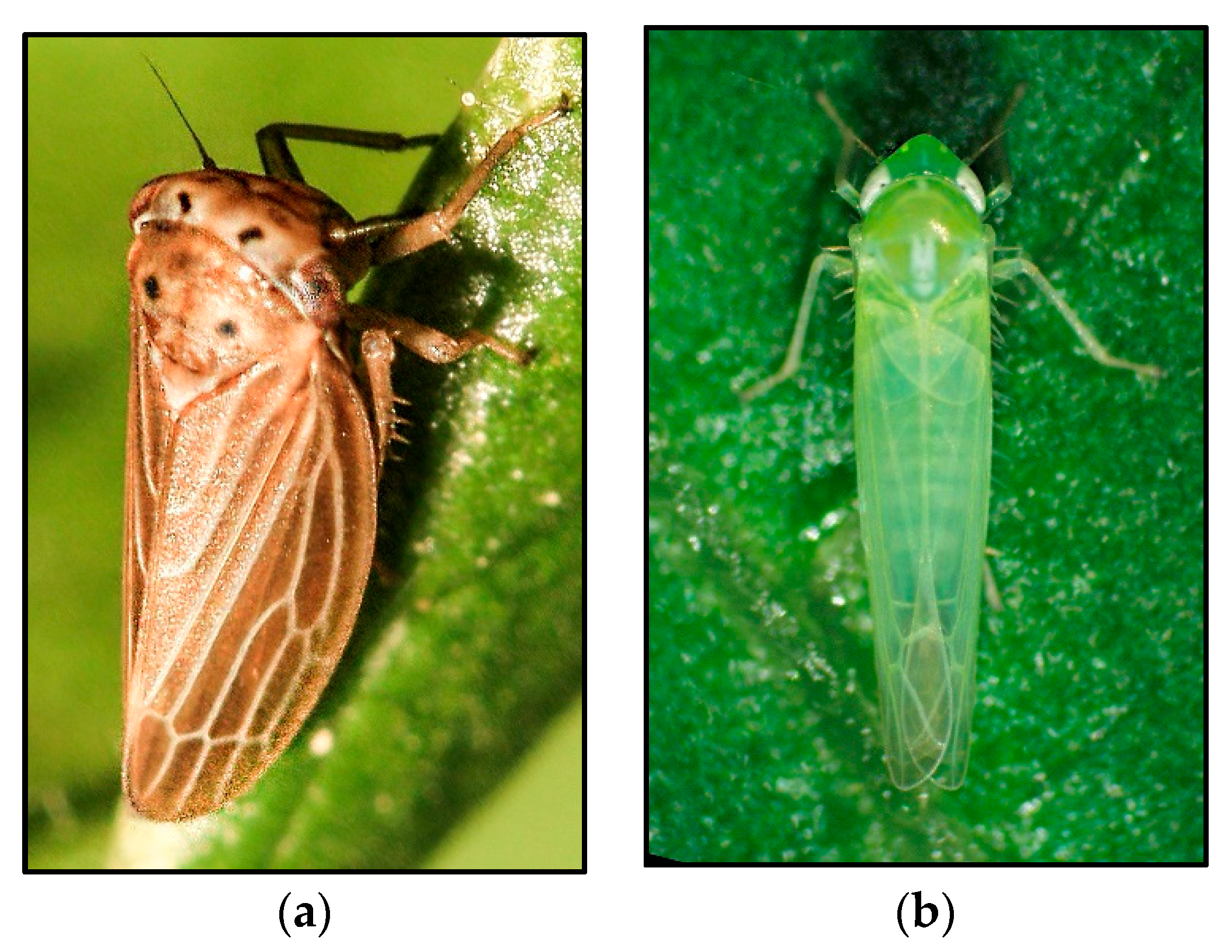
4.1. The Leafhopper Family Cicadellidae
- The speciesAgallia quadripunctata(Four-spotted Clover Leafhopper)
- Distribution and Ecology of Parthenoforms
- Origin and Genetic Diversity: Rare Males
- The genusEmpoasca
- Distribution and Ecology of Parthenoforms
- Origin and Genetic Diversity: Clones, Rare Males
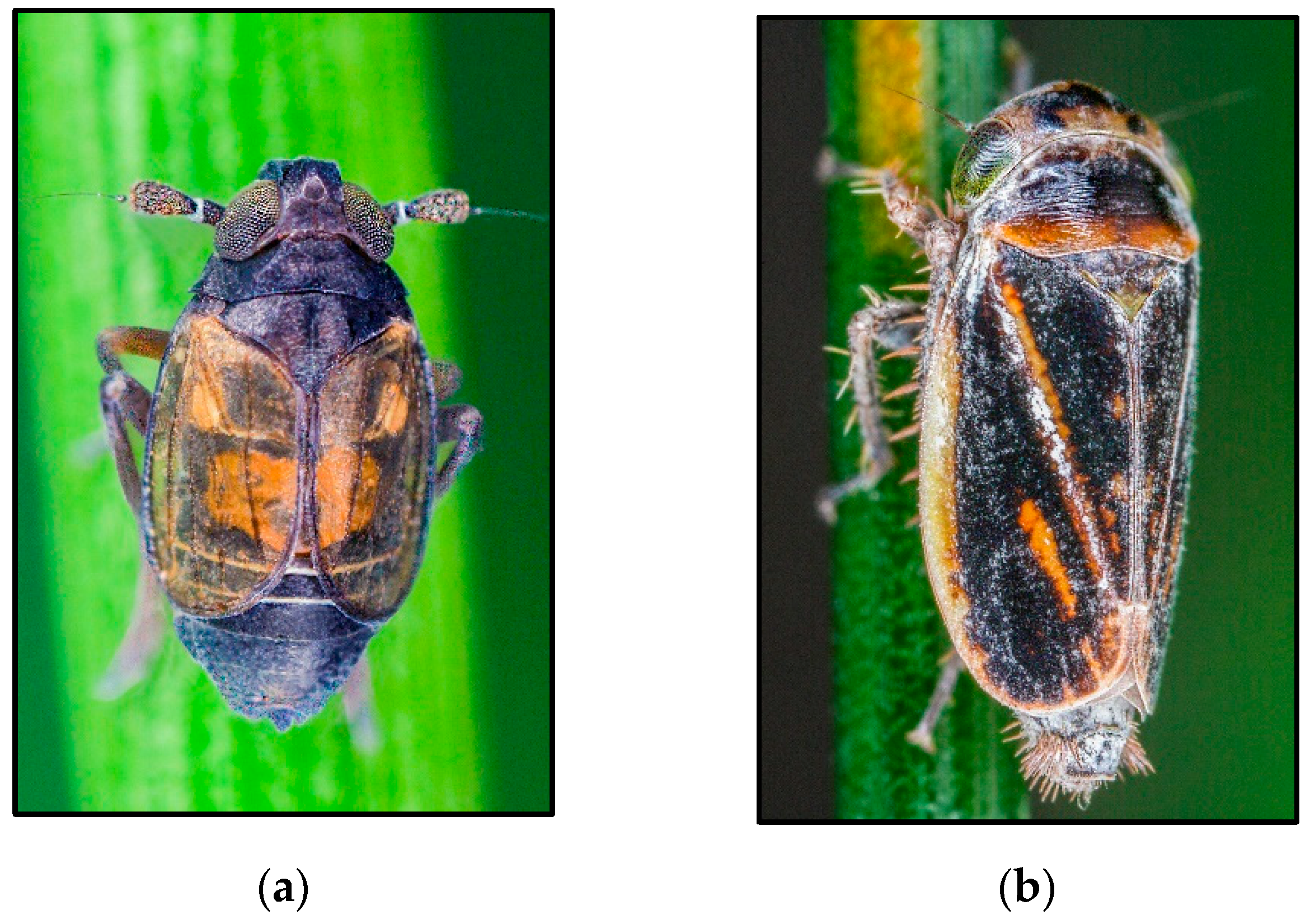
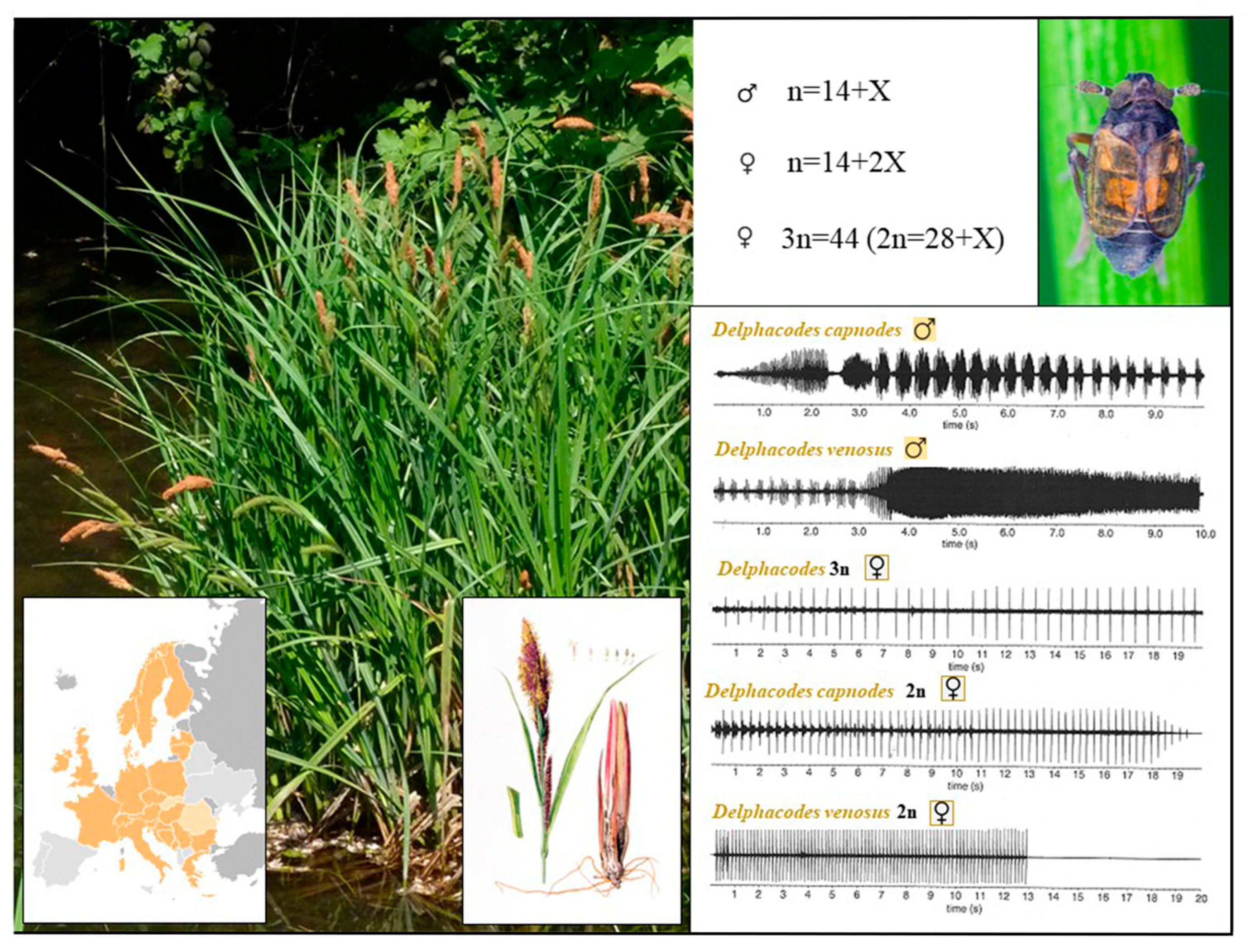
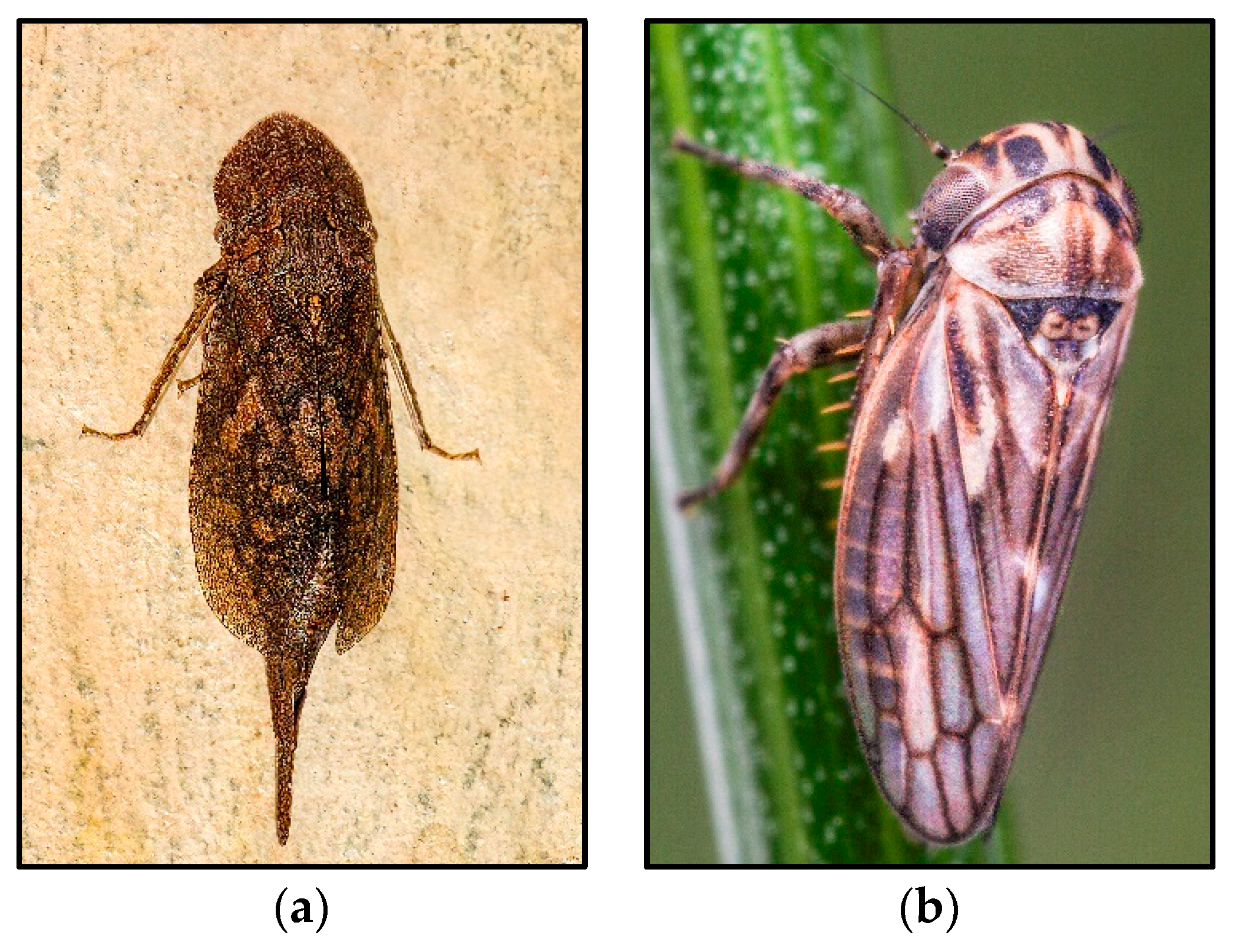
4.2. The Planthopper Family Delphacidae
- The genusDelphacodes
- Distribution and Ecology of Parthenoforms
- Origin and Genetic Diversity: Mating Behavior
5. Suspected Cases of Parthenogenesis in Fulgoromorpha and Cicadomorpha Based on Faunistic Studies
- Cicadellidae
- Issidae
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality; Croom Helm Applied Biology Series; University of California Press: Berkeley, CA, USA, 1982; pp. 1–635. [Google Scholar]
- Suomalainen, E. Parthenogenesis in animals. Adv. Genet. 1950, 3, 193–253. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, E.; Saura, A.; Lokki, J. Cytology and Evolution in Parthenogenesis; CRC Press: Boca Raton, FL, USA, 1987; pp. 1–206. [Google Scholar]
- Schön, I.; Martens, K.; van Dijk, P.J. (Eds.) Lost Sex: The Evolutionary Biology of Parthenogenesis; Springer: Dordrecht, The Netherlands, 2009; pp. 1–615. [Google Scholar] [CrossRef]
- Normark, B.B. Modes of reproduction. In The Evolution of Insect Mating Systems; Shuker, D.M., Simmons, L.W., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 1–19. [Google Scholar]
- Rodriguero, M.S. Parthenogenesis. In Reproductive Strategies in Insects, 1st ed.; Omkar, M.G., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 35–71. [Google Scholar]
- Vershinina, A.O.; Kuznetsova, V.G. Parthenogenesis in Hexapoda: Entognatha and non-holometabolous insects. J. Zool. Syst. Evol. Res. 2016, 54, 257–268. [Google Scholar] [CrossRef]
- Gokhman, V.E.; Kuznetsova, V.G. Parthenogenesis in Hexapoda: Holometabolous insects. J. Zool. Syst. Evol. Res. 2017, 56, 23–34. [Google Scholar] [CrossRef]
- Normark, B.B. The evolution of alternative genetic systems in insects. Annu. Rev. Entomol. 2003, 48, 397–423. [Google Scholar] [CrossRef]
- Simon, J.-C.; Delmotte, F.; Rispe, C.; Crease, T. Phylogenetic relationships between parthenogens and their sexual relatives: The possible routes to parthenogenesis in animals. Biol. J. Linn. Soc. 2003, 79, 151–163. [Google Scholar] [CrossRef]
- Kearney, M. Hybridization, glaciation and geographical parthenogenesis. Trends Ecol. Evol. 2005, 20, 495–502. [Google Scholar] [CrossRef]
- Lundmark, M. Polyploidization, hybridization and geographical parthenogenesis. Trends Ecol. Evol. 2006, 21, 9. [Google Scholar] [CrossRef]
- Normark, B.B.; Kirkendall, L.R. Parthenogenesis in insects and mites. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Elsevier Inc.: Berkeley, CA, USA, 2009; pp. 753–757. [Google Scholar]
- Werren, J.H.; Windsor, D. Wolbachia infection frequencies in insects: Evidence of a global equilibrium? Proc. R. Soc. Lond. B 2000, 267, 1277–1285. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Zhang, Z. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 2011, 3103, 1–82. [Google Scholar] [CrossRef]
- Weirauch, C.; Schuh, R.T. Systematics and evolution of Heteroptera: 25 years of progress. Annu. Rev. Entomol. 2011, 56, 487–510. [Google Scholar] [CrossRef]
- Bourgoin, T.; Campbell, B.C. Inferring a phylogeny for Hemiptera: Falling into the autapomorphic trap. Denisia 2002, 4, 67–82. [Google Scholar]
- Johnson, K.P.; Dietrich, C.H.; Friedrich, F.; Beutel, R.G.; Wipfler, B.; Peters, R.S.; Allen, J.M.; Petersen, M.; Donath, A.; Walden, K.K.O.; et al. Phylogenomics and the evolution of hemipteroid insects. Proc. Natl. Acad. Sci. USA 2018, 115, 12775–12780. [Google Scholar] [CrossRef]
- Kuznetsova, V.G.; Gavrilov-Zimin, I.A.; Grozeva, S.M.; Golub, N.V. Comparative analysis of chromosome numbers and sex chromosome systems in Paraneoptera (Insecta). Comp. Cytogenet. 2021, 15, 279–327. [Google Scholar] [CrossRef]
- Nur, U. Evolution of unusual chromosome systems in scale insects (Coccoidea: Homoptera). In Insect Cytogenetics; Blackman, R.L., Hewitt, G.M., Ashburner, M., Eds.; Blackwell Scientific Publications: Oxford, UK, 1980; pp. 97–117. [Google Scholar]
- Gullan, P.J.; Kosztarab, M. Adaptations in scale insects. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, I.A. A catalog of chromosome numbers and genetic systems of scale insects (Homoptera: Coccinea) of the world. Isr. J. Entomol. 2007, 37, 1–45. Available online: https://www.researchgate.net/publication/228625552 (accessed on 8 October 2023).
- Yokogawa, T.; Yahara, T. Mitochondrial phylogeny certified PGL (Paternal Genome Loss) is of single origin and haplodiploidy sensu stricto (arrhenotoky) did not evolve from PGL in the scale insects (Hemiptera: Coccoidea). Genes Genet. Syst. 2009, 84, 57–66. [Google Scholar] [CrossRef]
- Ross, L.; Pen, I.; Shuker, D.M. Genomic conflict in scale insects: The causes and consequences of bizarre genetic systems. Biol. Rev. 2010, 85, 807–828. [Google Scholar] [CrossRef]
- Ross, L.; Hardy, N.B.; Okusu, A.; Normark, B.B. Large population size predicts the distribution of asexuality in scale insects. Evolution 2013, 67, 196–206. [Google Scholar] [CrossRef]
- De la Filia, A.G.; Mongue, A.J.; Dorrens, J.; Lemon, H.; Laetsch, D.R.; Ross, L. Males that silence their father’s genes: Genomic imprinting of a complete haploid genome. Mol. Biol. Evol. 2021, 38, 2566–2581. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L. Chromosome numbers in the Aphididae and their taxonomic significance. Syst. Entomol. 1980, 5, 7–25. [Google Scholar] [CrossRef]
- Blackman, R.L.; Spence, J.M.; Normark, B.B. High diversity of structurally heterozygous karyotypes and rDNA arrays in parthenogenetic aphids of the genus Trama (Aphididae: Lachninae). Heredity 2000, 84, 254–260. [Google Scholar] [CrossRef]
- Suomalainen, E.; Saura, A. Genetic polymorphism and evolution in parthenogenetic animals. Part 9. Absence of variation within parthenogenetic aphid clones. Theor. Appl. Genet. 1980, 57, 129–132. [Google Scholar] [CrossRef]
- Wilson, A.C.C.; Sunnucks, P.; Hales, D.F. Heritable genetic variation and potential for adaptive evolution in asexual aphids (Aphidoidea). Biol. J. Linn. Soc. 2003, 79, 115–135. [Google Scholar] [CrossRef]
- Simon, J.-C.; Stoeckel, S.; Tagu, D. Evolutionary and functional insights into reproductive strategies of aphids. C. R. Biol. 2010, 333, 488–496. [Google Scholar] [CrossRef]
- Davis, G.K. Cyclical parthenogenesis and viviparity in aphids as evolutionary novelties. J. Exp. Zool. Part B 2012, 318, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Defendini, H.; Rimbault, M.; Mahéo, F.; Cloteau, R.; Denis, G.; Mieuzet, L.; Outreman, Y.; Simon, J.-C.; Jaquiéry, J. Evolutionary consequences of loss of sexual reproduction on male-related traits in parthenogenetic lineages of the pea aphid. Mol. Ecol. 2023, 32, 3672–3685. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Cahill, M. The karyotype of Bemisia tabaci (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 1997, 87, 213–215. [Google Scholar] [CrossRef]
- Taravati, S.; Mannion, C. Effect of aggregation and cage setting on some life-history parameters of Aleurodicus rugioperculatus (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2016, 109, 249–254. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): A global synthesis. J. Nat. Hist. 2009, 43, 65–179. [Google Scholar] [CrossRef]
- Hardy, N.B. The biodiversity of Sternorrhyncha: Scale insects, aphids, psyllids, and whiteflies. In Insect Biodiversity: Science and Society, II; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 591–625. [Google Scholar] [CrossRef]
- Nokkala, C.; Kuznetsova, V.G.; Nokkala, S. Rare diploid females coexist with rare males: A novel finding in triploid parthenogenetic populations in the psyllid Cacopsylla myrtilli (W. Wagner, 1947) (Hemiptera, Psylloidea) in northern Europe. Genetica 2015, 143, 589–595. [Google Scholar] [CrossRef]
- Nokkala, C.; Kuznetsova, V.G.; Shapoval, N.A.; Nokkala, S. Phylogeography and Wolbachia infections reveal postglacial recolonization routes of the parthenogenetic plant louse Cacopsylla myrtilli (W. Wagner 1947), (Hemiptera, Psylloidea). J. Zool. Syst. Evol. Res. 2022, 2022, 5458633. [Google Scholar] [CrossRef]
- Nokkala, S.; Kuznetsova, V.; Pietarinen, P.; Nokkala, C. Evolutionary potential of parthenogenesis—Bisexual lineages within triploid apomictic thelytoky in Cacopsylla ledi (Flor, 1861) (Hemiptera, Psylloidea) in Fennoscandia. Insects 2022, 13, 1140. [Google Scholar] [CrossRef]
- Shapoval, N.A.; Nokkala, S.; Nokkala, C.; Kuftina, G.N.; Kuznetsova, V.G. The incidence of Wolbachia bacterial endosymbiont in bisexual and parthenogenetic populations of the psyllid genus Cacopsylla (Hemiptera, Psylloidea). Insects 2021, 12, 853. [Google Scholar] [CrossRef] [PubMed]
- Carayon, J. Parthenogenese constante prouvee chez deux Heteropteres: Le miride Campyloneura virgula et l’anthocoride Calliodis Maculipennis. Ann. Société Entomol. Fr. 1989, 25, 387–391. [Google Scholar] [CrossRef]
- Böcher, J.; Nachman, G. Coexistence of bisexual and unisexual populations of Nysius groenlandicus in the Zackenberg Valley, Northeast Greenland. Entomol. Exp. Appl. 2011, 140, 196–206. [Google Scholar] [CrossRef]
- Drosopoulos, S. Triploid pseudogamous biotype of the leafhopper Muellerianella fairmairei. Nature 1976, 263, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Drosopoulos, S. Laboratory synthesis of a pseudogamous triploid “species” of the genus Muellerianella (Homoptera, Delphacidae). Evolution 1978, 32, 916–920. [Google Scholar]
- Den Bieman, C.F.M.; Eggers-Schumacher, H.A. Allozyme polymorphism in planthoppers of the genus Ribautodelphax (Homoptera, Delphacidae) and the origin of pseudogamous triploid forms. Neth. J. Zool. 1986, 37, 239–254. [Google Scholar] [CrossRef]
- Den Bieman, C.F.M. Karyotypic variation in bisexual species and pseudogamous forms of the planthopper genus Ribautodelphax (Homoptera, Delphacidae). Genetica 1988, 76, 101–110. [Google Scholar] [CrossRef]
- Den Bieman, C.F.M. Hybridization studies in the planthopper genus Ribautodelphax (Homoptera, Delphacidae). Genetica 1988, 76, 15–26. [Google Scholar] [CrossRef]
- Den Bieman, C.F.M. Coexistence of the pseudogamous and sexual planthoppers of the genus Ribautodelphax (Homoptera, Delphacidae). Ecol. Entomol. 1988, 13, 383–390. [Google Scholar] [CrossRef]
- Kuznetsova, V.G.; Aguin-Pombo, D. Comparative cytogenetics of Auchenorrhyncha (Hemiptera, Homoptera): A review. ZooKeys 2015, 538, 63–93. [Google Scholar] [CrossRef] [PubMed]
- Deitz, L.L. Dr Metcalf: A Resource on Cicadas, Leafhoppers, Planthoppers, Spittlebugs, and Treehoppers. 2008. Available online: http://www.lib.ncsu.edu/specialcollections/digital/metcalf/introduction.html (accessed on 6 June 2023).
- Skinner, R.K.; Dietrich, C.H.; Walden, K.K.O.; Gordon, E.; Sweet, A.D.; Podsiadlowski, L.; Petersen, M.; Simon, C.; Takiya, D.M.; Johnson, K.P. Phylogenomics of Auchenorrhyncha (Insecta: Hemiptera) using transcriptomes: Examining controversial relationships via degeneracy coding and interrogation of gene conflict. Syst. Entomol. 2020, 45, 85–113. [Google Scholar] [CrossRef]
- Hamilton, K.G.A. A new family of froghoppers from the American tropics (Hemiptera: Cercopoidea: Epipygidae). Biodiversity 2001, 2, 15–21. [Google Scholar] [CrossRef]
- Black, L.M.; Oman, P.W. Parthenogenesis in a leafhopper Agallia quadripunctacta (Provancher) (Homoptera: Cicadellidae). Proc. Entomol. Soc. Wash. 1947, 49, 19–20. [Google Scholar]
- Den Bieman, C.F.M.; de Vrijer, P.W.F. True parthenogenesis for the first time demonstrated in planthoppers (Homoptera, Delphacidae). Ann. Soc. Entomol. Fr. 1987, 23, 3–9. [Google Scholar] [CrossRef]
- Aguin-Pombo, D.; Kuznetsova, V.; Freitas, N. Multiple parthenoforms of Empoasca leafhoppers from Madeira Island. Where are these unisexual forms coming from? J. Hered. 2006, 97, 171–176. [Google Scholar] [CrossRef][Green Version]
- Aguin-Pombo, D.; Valido, L.; Sousa, F.; Arraiol, A. Differences in wing venation between parthenogenetic and bisexual species of Empoasca leafhoppers from Madeira Island. Bull. Insectol. 2014, 67, 1–12. [Google Scholar]
- Aguin-Pombo, D.; Rodrigues, M.C.P.A.; Voetdijk, B.; Breeuwer, J.A.J. Parthenogenesis and sex-ratio distorting bacteria in Empoasca (Hemiptera: Cicadellidae) leafhoppers. Ann. Entomol. Soc. Am. 2021, 114, 738–749. [Google Scholar] [CrossRef]
- Negri, I.; Pellecchia, M.; Mazzoglio, P.J.; Patetta, A.; Alma, A. Feminizing Wolbachia in Zyginidia pullula (Insecta, Hemiptera), a leafhopper with an XX/X0 sex-determination system. Proc. Biol. Sci. 2006, 273, 2409–2416. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fumiko, Y.; Masaya, M.; Noda, H. Cytoplasmic incompatibility involving Cardinium and Wolbachia in the white-backed planthopper Sogatella furcifera (Hemiptera: Delphacidae). App. Entomol. Zool. 2012, 47, 273–283. [Google Scholar] [CrossRef]
- Gnezdilov, V.M. Notes on Scorlupella montana (Becker) (Homoptera: Issidae). Zoosyst. Rossica 2001, 9, 365–366. [Google Scholar]
- White, M.J.D. Animal Cytology and Evolution, 3rd ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 1–468. [Google Scholar]
- Tvedte, E.S.; Logsdon, J.M.; Forbes, A.A. Sex loss in insects: Causes of asexuality and consequences for genomes. Curr. Opin. Insect Sci. 2019, 31, 77–83. [Google Scholar] [CrossRef]
- Ma, W.-J.; Schwander, T. Patterns and mechanisms in instances of endosymbiont-induced parthenogenesis. J. Evol. Biol. 2017, 30, 868–888. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Bouchon, D.; Boutin, S.; Bellamy, L.; Zhou, L.; Engelstädter, J.; Hurst, G.D. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 2008, 6, 27. [Google Scholar] [CrossRef]
- Jeyaprakash, A.; Hoy, M.A. Long PCR improves Wolbachia DNA amplifications: Wsp sequences found in 76% of sixty-three arthropod species. Insect Mol. Biol. 2000, 9, 393–405. [Google Scholar] [CrossRef]
- Bing, X.-L.; Zhao, D.-S.; Hong, X.-Y. Bacterial reproductive manipulators in rice planthoppers. Arch. Insect Biochem. Physiol. 2019, 101, e21548. [Google Scholar] [CrossRef]
- Hoshizaki, S.; Shimada, T. PCR-based detection of Wolbachia, cytoplasmic incompatibility microorganisms, infected in natural populations of Laodelphax striatellus (Homoptera: Delphacidae) in central Japan: Has the distribution of Wolbachia spread recently? Insect Mol. Biol. 1995, 4, 237–243. [Google Scholar] [CrossRef]
- Noda, H. Cytoplasmic incompatibility in allopatric field populations of the small brown planthopper, Laodelphax striatellus, in Japan. Entomol. Exp. Appl. 1984, 35, 263–267. [Google Scholar] [CrossRef]
- Noda, H.; Koizumi, Y.; Zhang, Q.; Deng, K. Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem. Mol. Biol. 2001, 31, 727–737. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhao, D.-X.; Hong, X.-Y. Cardinium—The leading factor of cytoplasmic incompatibility in the planthopper Sogatella furcifera doubly infected with Wolbachia and Cardinium. Environ. Entomol. 2012, 41, 833–840. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Mejdalani, G.; Coelho, L.B.N. A new species of Agallia Curtis, 1833 from Southeastern Brazil (Insecta: Hemiptera: Cicadellidae: Agalliinae) with taxonomic notes on the genus. Stud. Neotrop. Fauna Environ. 2007, 42, 221–224. [Google Scholar] [CrossRef]
- Oman, P.W. A Classification of North American Agallian Leaf Hoppers; U.S. Government Printing Office: Washington, DC, USA, 1933; Volume 372, pp. 1–94. [Google Scholar]
- DeLong, D.M.; Davidson, R.H. The genus Agallia-external characters used to distinguish the species injuring economic crops. Ohio J. Sci. 1931, 31, 377–385. [Google Scholar]
- McKamey, S.H. Taxonomic catalogue of the leafhoppers (Membracoidea). Part 1. Cicadellinae. Mem. Am. Entomol. Inst. 2007, 78, 1–394. [Google Scholar]
- Maw, H.E.L.; Foottit, R.G.; Hamilton, K.G.A.; Scudder, G.G.E. Checklist of the Hemiptera of Canada and Alaska; NRC Research Press: Ottawa, ON, Canada, 2000; pp. 1–220. [Google Scholar]
- DeLong, D.M. The leafhoppers or Cicadellidae of Illinois (Eurymelinae-Balcluthinae). Bull. Ill. Nat. Hist. Surv. 1948, 24, 97–376. [Google Scholar] [CrossRef]
- Beirne, B.P. Leafhoppers (Homoptera: Cicadellidae) of Canada and Alaska. Can. Entomol. 1956, 88 (Suppl. S2), 5–180. [Google Scholar] [CrossRef]
- Nielson, M.W. The Leafhopper Vectors of Phytopathogenic Viruses (Homoptera, Cicadellidae). Taxonomy, Biology and Virus Transmission; Technical Bulletin 1382; United States Department of Agriculture: Washington, DC, USA, 1968; 386p. [Google Scholar]
- Vandel, A. La parthenogenese geographique: Contribution L’etude biologique et cytologique de la parthenogenese naturelle. Bull. Biol. Fr. Belg. 1928, 62, 164–281. [Google Scholar]
- Lynch, M. Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Q. Rev. Biol. 1984, 59, 257–290. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Zeiders, K.; Bouseman, J.K.; Voegtlin, D.J. Inventory of Select Groups of Arthropods of Richardson Wildlife Foundation. Illinois Natural History Survey Technical Reports, No. 8. 1997, p. 24p. Available online: https://www.ideals.illinois.edu/items/10093 (accessed on 8 October 2023).
- MacLean, D.B. An annotated list of leafhoppers (Homoptera: Cicadellidae) from Watercress Marsh, Columbiana County. Ohio. Ohio J. Sci. 1984, 5, 252–254. [Google Scholar]
- Taboada, O.; Hoffman, J.R. Distribution of leafhopper vectors of plant diseases in Michigan. Trans. Am. Microsc. Soc. 1965, 84, 201–210. [Google Scholar] [CrossRef]
- Connacher, B.S. The Effects of Grazing (Bison and Elk) and Burning on Biodiversity of Hemiptera (Heteroptera; Homoptera: Auchenorrhyncha) at Prairie State Park, Missouri, Ph.D. Thesis. University of Central Missouri, Warrensburg, MO, USA, 2012.
- Hamilton, K.G.A.; Langor, D.W. Leafhopper fauna of Newfoundland and Cape Breton Islands (Rhynchota: Homoptera: Cicadellidae). Can. Entomol. 1987, 119, 663–695. [Google Scholar] [CrossRef]
- Chandler, D.S.; Hamilton, K.G.A. Biodiversity and ecology of the leafhoppers (Hemiptera: Cicadellidae) of New Hampshire. Trans. Am. Entomol. Soc. 2017, 143, 773–971. [Google Scholar] [CrossRef]
- Freytag, P.H. The Leafhoppers of Kentucky. Part 1. Agalliinae, Idiocerinae, and Macropsinae; College of Agriculture, Agricultural Experiment Station Progress Report 223; University of Kentucky: Lexington, KY, USA, 1975; pp. 1–63. [Google Scholar]
- Ball, E.D. Some Insects Injurious to Truck Crops: The Leafhoppers of the Sugar Beet and Their Relation to the “Curlyleaf” Condition; Bulletin No. 66; United States Department of Agriculture: Washington, DC, USA, 1910; pp. 33–52. [Google Scholar]
- Bentz, J.; Townsend, A.M. Spatial and temporal patterns of abundance of the potato leafhopper among red maples. Ann. Appl. Biol. 2005, 145, 157–164. [Google Scholar] [CrossRef]
- Nokkala, C.; Kuznetsova, V.G.; Nokkala, S. Meiosis in rare males in parthenogenetic Cacopsylla myrtilli (Wagner, 1947) (Hemiptera, Psyllidae) populations from northern Europe. Comp. Cytogenet. 2013, 7, 241–251. [Google Scholar] [CrossRef][Green Version]
- Oman, P. A new Agallia from the Western United States. J. Kans. Entomol. 1971, 44, 325–328. [Google Scholar]
- Xu, Y.; Dietrich, C.H.; Zhang, Y.-L.; Dmitriev, D.A.; Zhang, L.; Wang, Y.-M.; Lu, S.-H.; Qin, D.-Z. Phylogeny of the tribe Empoascini (Hemiptera: Cicadellidae: Typhlocybinae) based on morphological characteristics, with reclassification of the Empoasca generic group. Syst. Entomol. 2021, 46, 266–286. [Google Scholar] [CrossRef]
- Southern, P.S.; Dietrich, C.H. Eight new species of Empoasca (Hemiptera: Cicadellidae: Typhlocybinae: Empoascini) from Peru and Bolivia. Zootaxa 2010, 2524, 1–23. [Google Scholar] [CrossRef]
- Aguin-Pombo, D.; Freitas, N. Empoasca fabalis (Hemiptera: Cicadellidae): First report of an invasive pest of sweet potatoes in Portugal (Madeira Island). Zootaxa 2020, 4838, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Böll, S.; Herrmann, J.V. A long-term study on the population dynamics of the grape leafhopper (Empoasca vitis) and antagonistic mymarid species. J. Pest Sci. 2004, 77, 33–42. [Google Scholar] [CrossRef]
- Rassoulian, G.R.; Sardarbandeh, H.; Kharazi-Pakdel, A. Study of soybean leafhoppers fauna and an investigation on biology of dominant species Empoasca decipiens Paoli in Karaj area. Commun. Agric. Appl. Biol. Sci. 2005, 70, 671–675. [Google Scholar]
- Nikolova, I. Sex ratio of Empoasca pteridis Dhlb. (Hemiptera: Cicadellidae) and its seasonal and annual variation in Alfalfa Field. Asian J. Res. Rev. Agric. 2019, 1, 45–52. [Google Scholar]
- Parh, I.A. Studies on the Bionomics of Empoasca Species (Hemiptera, Cicadellidae) associated with Cowpea in Southern Nigeria. Ph.D. Thesis, University of Ibadan, Ibadan, Nigeria, 1979. [Google Scholar]
- Akingbohungbe, A.E. Nomenclatural problems, biology, host plant and possible vector status of Auchenorrhyncha associated with crop plants in Nigeria. In Ist International Workshop on Leafhoppers and Planthoppers of Economic Importance; Knight, W.J., Pant, N.C., Robertson, T.S., Wilson, M.R., Eds.; CAB/CIE: London, UK, 1983; pp. 365–370. [Google Scholar]
- Parh, I.A.; Taylor, T.A. Studies on the life cycle of the cicadellid bug Empoasca dolichi Paoli, in Southern Nigeria. J. Nat. Hist. 1981, 15, 829–835. [Google Scholar] [CrossRef]
- Akingbohungbe, A.E. Seasonal variation in cowpea crop performance at Ile-Ife, Nigeria and the relationship to insect damage. Int. J. Trop. Insect Sci. 1982, 3, 287–296. [Google Scholar] [CrossRef]
- Lorenzo-Carballa, M.O.; Hassall, C.; Encalada, A.C.; Sanmartín-Villar, I.; Torres-Cambas, Y.; Cordero-Rivera, A. Parthenogenesis did not consistently evolve in insular populations of Ischnura hastata (Odonata, Coenagrionidae). Ecol. Entomol. 2017, 1, 67–76. [Google Scholar] [CrossRef]
- Magro, A.; Lecompte, E.; Hemptinne, J.L.; Soares, A.O.; Dutrillaux, A.M.; Murienne, J.; Fürsch, H.; Dutrillaux, B. First case of parthenogenesis in ladybirds (Coleoptera: Coccinellidae) suggests new mechanisms for the evolution of asexual reproduction. J. Zool. Syst. Evol. Res. 2020, 58, 194–208. [Google Scholar] [CrossRef]
- Adolfsson, S.; Michalakis, Y.; Paczesniak, D.; Bode, S.N.; Butlin, R.K.; Lamatsch, D.K.; Martins, M.J.F.; Schmit, O.; Vandekerkhove, J.; Jokela, J. Evaluation of elevated ploidy and asexual reproduction as alternative explanations for geographic parthenogenesis in Eucypris virens ostracods. Evolution 2010, 64, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Engelstädter, J.; Sandrock, C.; Vorburger, C. Contagious parthenogenesis, automixis, and a sex determination meltdown. Evolution 2011, 65, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Nokkala, S.; Kuznetsova, V.G.; Nokkala, C. Characteristics of parthenogenesis in Cacopsylla ledi (Flor, 1861) (Hemiptera, Sternorrhyncha, Psylloidea): Cytological and molecular approaches. Comp. Cytogenet. 2017, 11, 807–817. [Google Scholar] [CrossRef][Green Version]
- Delmotte, F.; Leterme, N.; Bonhomme, J.; Rispe, C.; Simon, J.-C. Multiple routes to asexuality in an aphid species. Proc. R. Soc. Lond. B 2001, 268, 2291–2299. [Google Scholar] [CrossRef]
- Ghauri, M.S.K. The identity of Empoasca dolichi Paoli (Hemiptera: Cicadellidae) and descriptions of new species of Empoasca from light-traps near crops in Malawi and Nigeria. Bull. Entomol. Res. 1979, 69, 343–355. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Asche, M.; Hoch, H. Contribution to the planthopper fauna of Greece (Homoptera, Auchenorrhyncha, Fulgoromorpha, Delphacidae). Ann. Inst. Phytopathol. Benaki 1983, 14, 19–68. [Google Scholar]
- Asche, M.; Remane, R. Zur Problematik von Delphacodes mulsanti (Fieber 1866) und zur Kenntnis einiger benachbarter Taxa (Homoptera Auchenorrhyncha Fulgoromorpha Delphacidae) (Vorlaufige Mitteilung). Marbg. Entomol. Publ. 1983, 1, 25–56. [Google Scholar]
- Holzinger, W.E.; Kammerlander, I.; Nickel, H. The Auchenorrhyncha of Central Europe. Die Zikaden Mitteleuropas, Volume 1: Fulgoromorpha, Cicadomorpha excl. Cicadellidae; Brill Academic Publishers: Leiden, The Netherlands; Boston, MA, USA, 2003; pp. 1–673. [Google Scholar]
- Nickel, H. The Leafhoppers and Planthoppers of Germany (Hemiptera Auchenorrhyncha): Patterns and Strategies in a Highly Diverse Group of Phytophagous Insects; Goecke & Evers Publishing House: Keltern, Germany; Pensoft Publishers: Sofia, Bulgary; Moscow, Russia, 2003; pp. 1–460. [Google Scholar]
- Ossiannilsson, F. The Auchenorrhyncha (Homoptera) of Fennoscandia and Denmark. In Part 1: Introduction, Infraorder Fulgoromorpha; Fauna Entomologica Scandinavica; Scandinavian Science Press: Klampenborg, Denmark, 1978; Volume 7, pp. 1–222. [Google Scholar]
- Lauterer, P. New and interesting records of leafhoppers from Czechoslovakia (Homoptera, Auchenorrhyncha). Acta Mus. Morav. 1980, 65, 117–140. [Google Scholar]
- De Vrijer, P.W.F. The role of acoustic signals in the reproductive isolation and speciation of planthoppers (Homoptera, Delphacidae). Proc. Exper. Appl. Entomol. N.E.V. Amst. 1992, 3, 110–115. [Google Scholar]
- Müller, H.-J. Die Wirkung exogener Faktoren auf die zyklische Formenbildung der Insekten, insbesondere der Gattung Euscelis (Hom., Auchenorrhyncha). (Zugleich 5. Beitrag zur Biologie mitteleuropaischer Zikaden.). Zool. Jahrb. Abt. Syst. Oekol. Geogr. Tiere 1957, 85, 317–430. [Google Scholar]
- Claridge, M.F.; de Vrijer, P.W.F. Reproductive behavior: The role of acoustic signals in species recognition and speciation. In Planthoppers, Their Ecology and Management; Denno, R.F., Perfect, T.J., Eds.; Chapman & Hall: New York, NY, USA, 1994; pp. 216–233. [Google Scholar]
- Claridge, V.F. Acoustic signals in the Homoptera: Behavior, taxonomy, and evolution. Annu. Rev. Entomol. 1985, 30, 297–317. [Google Scholar] [CrossRef]
- Drosopoulos, S. Acoustic communication, mating behaviour and hybridisation in closely related, pseudogamic and parthenogenetic species of planthoppers. In Insect Sounds and Communication: Physiology, Behaviour, Ecology and Evolution; Drosopoulos, S., Claridge, M.F., Eds.; CRC Press, Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2005; pp. 239–253. [Google Scholar]
- Nast, J. Palaearctic Auchenorrhyncha (Homoptera), an Annotated Checklist; Polish Scientific Publishers: Warsaw, Poland, 1972; pp. 1–550. [Google Scholar]
- Tishechkin, D.Y. Review of species of the genus Laburrus (Homoptera, Cicadellidae) from European Russia. Entomol. Rev. 2002, 82, 593–606. [Google Scholar]
- Carver, M.; Gross, G.F.; Woodward, T.E. Hemiptera (bugs, leafhoppers, cicada, aphids, scale insects, etc.). In The Insects of Australia, a Textbook for Students and Research Workers, 2nd ed.; Naumann, I.D., Crane, P.B., Lawrence, J.F., Neilsen, E.S., Spradbery, J.P., Taylor, R.W., Whitten, M.J., Littlejohn, M.J., Eds.; Melbourne Univ. Press: Melbourne, Australia, 1991; Volume 1, pp. 429–509. [Google Scholar]
- Day, M.F.; Fletcher, M.J. An annotated catalogue of the Australian Cicadelloidea (Hemiptera: Auchenorrhyncha). Invertebr. Taxon 1994, 8, 1117–1288. [Google Scholar] [CrossRef]
- ALA (Australian Living Australina). Species Ledromorpha planirostris (Donovan, 1805). Available online: https://bie.ala.org.au/species/https://biodiversity.org.au/afd/taxa/0e83d2e7-815b-4cb8-a9af-fe60f164f09e (accessed on 26 July 2023).
- Fletcher, M.J.; Day, M.F.; Humphrey, M. The discovery of the holotype of Ledromorpha planirostris (Donovan) (Cicadellidae: Ledrinae), with notes on other Australian Auchenorrhyncha species described by Edward Donovan. Gen. Appl. Entomol. 2003, 32, 23–30. Available online: https://www.researchgate.net/publication/361053504 (accessed on 8 October 2023).
- Evans, J.W. The leafhoppers and froghoppers of Australia and New Zealand (Homoptera: Cicadelloidea and Cercopoidea). Aust. Mus. Mem. 1966, 12, 1–347. [Google Scholar] [CrossRef]
- Donovan, E. An Epitome of the Natural History of the Insects of New Holland, New Zealand, New Guinea, Otaheite, and other islands in the Indian, Southern, and Pacific Oceans: Including the Figures and Descriptions of One Hundred and Fifty-Three Species of the More Splendid, Beautiful, and Interesting Insects, Hitherto Discovered in Those Countries, and Which for the Most Part Have Not Appeared in the Works of Any Preceding Author. The Figures Are Correctly Delineated from Specimens of the Insects; and with the Descriptions Are Arranged According to the Linnæan System, with Reference to the Writings of Fabricius and Other Entomologists; Privately Published: London, UK, 1805. [Google Scholar]
- Jones, J.; Deitz, L. Phylogeny and systematics of the leafhopper subfamily Ledrinae (Hemiptera: Cicadellidae). Zootaxa 2009, 2186, 1–120. [Google Scholar] [CrossRef]
- Gnezdilov, V.M.; Holzinger, W.E.; Wilson, M.R. The Western Palaearctic Issidae (Hemiptera, Fulgoroidea): An illustrated checklist and key to genera and subgenera. Proc. ZIN 2014, 318 (Suppl. 1), 1–124. [Google Scholar]
- Dlabola, J. Die Zikaden von Zentralasien, Dagestan und Transkaukasien (Homopt. Auchenorrhyncha). Acta Entomol. Mus. Natl. Pragae 1961, 34, 241–358. [Google Scholar]
- Novikov, D.V.; Novikova, N.V.; Anufriev, G.A.; Dietrich, C.H. Auchenorrhyncha (Hemiptera) of Kyrgyz Grasslands. Russ. Entomol. J. 2006, 15, 303–310. [Google Scholar]
- Mozaffarian, F.; Gnezdilov, V.M. First record of Scorlupella montana (Hem.: Fulgoroidea: Issidae) from Iran. J. Entmol. Soc. Iran 2011, 30, 93–95. Available online: https://www.researchgate.net/publication/261515613 (accessed on 8 October 2023).
- Kearney, M.; Blacket, M.J. The evolution of sexual and parthenogenetic Warramaba: A window onto Plio–Pleistocene diversification processes in an arid biome. Mol. Ecol. 2008, 17, 5257–5275. [Google Scholar] [CrossRef]
- Liegeois, M.; Sartori, M.; Schwander, T. Extremely widespread parthenogenesis and a trade-off between alternative forms of reproduction in mayflies (Ephemeroptera). J. Hered. 2021, 112, 45–57. [Google Scholar] [CrossRef]
| Term | Definition |
|---|---|
| Allopolyploidy | A form of polyploidy characterized by organisms possessing more than two sets of chromosomes originating from different species. |
| Apomixis | A reproductive process without fertilization in which meiosis and the fusion of gametes are partially or completely suppressed. Also known as ameiotic, apomictic parthenogenesis, or apomictic thelytoky. |
| Automixis | A form of reproduction without fertilization in which normal meiosis and the reduction of chromosome numbers occur. This is followed by the subsequent restoration of diploidy. Also known as meiotic, automictic parthenogenesis, or automictic thelytoky. |
| Arrhenotoky | A parthenogenetic mode of reproduction in which females exclusively produce male offspring from unfertilized eggs. Sometimes also referred to as androgenesis. |
| Autopolyploidy | A form of polyploidy in which organisms have more than two sets of chromosomes, all derived from the same parental species. |
| Contagious parthenogenesis | The reproductive process by which males, which periodically occur in a unisexual lineage, fertilize closely related “sexual” females. Subsequently, these fertilized females give rise to a new unisexual lineage through parthenogenesis. |
| Cyclical parthenogenesis | A life cycle with both sexual and asexual phases. This term is typically applied to cases in which the alternation between sexual and asexual reproduction is more or less regular and predictable. |
| Cytoplasmic incompatibility | A phenomenon in which the sperm and eggs of organisms are unable to form viable offspring due to modifications induced by intracellular parasites, such as Wolbachia, that reside within the cytoplasm of host cells. |
| Deuterotoky | A parthenogenetic mode of reproduction in which both male and female offspring are produced from unfertilized eggs. |
| Facultative parthenogenesis | A reproductive phenomenon in which sexual reproduction is the normal mode of reproduction for an organism; however, under certain conditions, a high percentage of the eggs have the capability to develop without fertilization. Also known as facultative thelytoky. |
| Haplodiploidy | A sex-determination system in which unfertilized haploid eggs develop into males while fertilized eggs develop into females. |
| Hermaphroditism | A condition in which an individual has both male and female reproductive organs. |
| Hybridization | The biological process or act of mating of individuals from different species resulting in the formation of hybrid offspring. |
| Geographical parthenogenesis | A phenomenon in which unisexual organisms, or parthenoforms, exhibit a different geographic distribution compared to their bisexual relatives. Parthenoforms tend to colonize regions characterized by higher latitudes, islands, and areas that were previously covered by glaciers. |
| Karyotype | The characterization of the chromosome complements of a species (such as the shape, type, number, etc.). |
| Obligatory parthenogenesis | A form of reproduction in which parthenogenesis is the sole mode of reproduction of an organism. |
| Parthenogenesis | A mode of reproduction characterized by the absence of fertilization (no fusion of sperm and egg nuclei). Also known as unisexual, uniparental, or asexual reproduction. |
| Paternal Genome Elimination (PGE) | A mode of reproduction in which males develop from fertilized eggs. However, during a certain stage of development, the complete haploid set of chromosomes inherited from their fathers is selectively eliminated and excluded from the sperm, resulting in males that are somatically haploid. |
| Paternal Genome Heterochromatinization (PGH) | A mode of reproduction characterized by the complete inactivation of the paternal genome through chromatin condensation. This process renders males functionally haploid, a condition known as parahaploidy. |
| Polyploidy | A genetic condition characterized by the presence of more than two sets of homologous chromosomes in the cells of an organism. |
| Pseudogamy | A reproductive phenomenon in which parthenogenetic development is initiated by the penetration of a sperm cell into an ovum. However, the sperm genome does not contribute to the genetic information of the resulting zygote. Also known as sperm-dependent parthenogenesis and gynogenesis. |
| Thelytoky | A form of reproduction in which female individuals produce exclusively female progeny by a process that does not involve fertilization. Thelytoky may be the sole mode of reproduction for a species or biotype (complete thelytoky), or it may alternate with sexual reproduction (i.e., cyclical thelytoky or heterogony) depending on environmental factors such as seasonal changes, temperature, or photoperiod. |
| Species/Genera | Known Populations | Type of Parthenogenesis | Host Plants (Unisexuals/Bisexuals) | Chromosome Numbers (Unisexuals/Bisexuals) | Meiosis Type | Suggested Origin | Similarities to Bisexuals | Distribution of Bisexuals | Distribution of Unisexuals |
|---|---|---|---|---|---|---|---|---|---|
| Delphacodes cf capnodes * | Bisexual !/Unisexual | Thelytokous | Carex riparia/Carex spp. Eriophorum angustifolium | 3n = 44/ 2n = 30 | Apomictic | _ | Acoustic signals Morphology Mating behavior | Europe | Greece/ Germany !/Czeck Republic! |
| Agallia quadripunctata | Bisexual !/Unisexual | Thelytokous | Trifolium ! Medicago !, Beta !, Ulmus !, Acer ! | Unknown/ Unknown | Unknown | _ | Unknown | United States *, Cuba ! Mexico | Canada, Eastern United States Ø |
| Empoasca A | Unisexual | Thelytokous | Polyphagous | 3n = 31 ● | Apomictic | Hybrid (?) | _ | _ | Madeira Island |
| Empoasca B | Unisexual | Thelytokous | Polyphagous | 3n = 27 (?) | Apomictic | Autopolyploidy (?) | _ | _ | Madeira Island |
| Empoasca C | Unisexual/Bisexual ! | Thelytokous | Polyphagous/Ricinus, Phaseolus, Capsicum frutesceni, Gossypium | 3n = 24 ● | Apomictic | Autopolyploidy (?) | Morphology | Africa, Israel, Pakistan | Madeira Island |
| Empoasca confusania (?) | Unisexual/Bisexual ! | Thelytokous | Vigna unguiculata, Amaranthus, Fabaceae spp./Vigna unguiculata, Fabaceae spp. | Unknown/ Unknown | Unknown | _ | Morphology | Africa, Nigeria | Nigeria |
| Family | Species | Type of Populations | Host Plants of Unisexuals | Distribution of Bisexuals | Distribution of Unisexuals |
|---|---|---|---|---|---|
| Issidae | Scorlupella montana | Bisexual !/ Unisexual ! | Carex spp., Eriophorum angustifolium | United States *, Cuba ! and Mexico ! | Greece/Germany !/Czeck Republic ! |
| Cicadellidae | Anaceratagallia kerzhneri | Bisexual !/ Unisexual ! | _ | Eastern Russia | Mongolian steppe |
| Ledromorpha planirostris | Unisexual ! | Eucalyptus spp. Ø | Unknown | Eastern Australia from Queensland to Victoria and Tasmania Ø | |
| Laburrus amazon | Bisexual !/ Unisexual ! | Artemisia pauciflora, A. nitrosa Ø | Lower Volga! | Southern European Russia, Kazakhstan, Mongolia Ø |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguín-Pombo, D.; Kuznetsova, V.G. True Parthenogenesis and Female-Biased Sex Ratios in Cicadomorpha and Fulgoromorpha (Hemiptera, Auchenorrhyncha). Insects 2023, 14, 820. https://doi.org/10.3390/insects14100820
Aguín-Pombo D, Kuznetsova VG. True Parthenogenesis and Female-Biased Sex Ratios in Cicadomorpha and Fulgoromorpha (Hemiptera, Auchenorrhyncha). Insects. 2023; 14(10):820. https://doi.org/10.3390/insects14100820
Chicago/Turabian StyleAguín-Pombo, Dora, and Valentina G. Kuznetsova. 2023. "True Parthenogenesis and Female-Biased Sex Ratios in Cicadomorpha and Fulgoromorpha (Hemiptera, Auchenorrhyncha)" Insects 14, no. 10: 820. https://doi.org/10.3390/insects14100820
APA StyleAguín-Pombo, D., & Kuznetsova, V. G. (2023). True Parthenogenesis and Female-Biased Sex Ratios in Cicadomorpha and Fulgoromorpha (Hemiptera, Auchenorrhyncha). Insects, 14(10), 820. https://doi.org/10.3390/insects14100820







