Simple Summary
Bark beetles are global forest pests that feed and breed in the inner bark of trees and may transfer symbiotic fungi that cause blue staining and reduce the commercial value of timber. In the present study, we examined the effects of semiochemical treatments on deterring bark beetle populations. This population reduction was measured by a reduction in the attraction to lure/trap catches, tree mortality, and attacks on trees. Based on the analysis of 863 experiments, we found that semiochemical treatments effectively reduce the bark beetle population and, therefore, represent an eco-friendly technique for forest protection.
Abstract
Bark beetles (Coleoptera: Curculionidae: Scolytinae) are among the most damaging tree pests globally. Rising temperatures, drought, fire, storms, cyclones, and poor forest management cause stress and loss of vigour in trees, and these conditions favour bark beetle outbreaks. While research has been conducted on push–pull strategies to deter bark beetles, using attractive and deterrent semiochemicals, the potential of this strategy to reduce bark beetle populations, particularly in the genera Dendroctonus and Ips, remains uncertain. Here, we conducted a global meta-analysis of 52 research articles to quantify the effects of semiochemical treatments on managing different species of Dendroctonus and Ips for forest protection. Based on this analysis, we found that push–pull semiochemicals can significantly reduce Dendroctonus and Ips populations measured by a reduction in the attraction to lure/trap catches, tree mortality, and attacks on trees. The overall efficacy of the push–pull semiochemical treatment shows a 66% reduction for Ips compared to control and a 54% reduction compared to control for Dendroctonus, while, at the species level, there was a 69% reduction for Dendroctonus ponderosae (Hopkins) and a 94% reduction in Ips perturbatus (Eichhoff), and a 93% reduction in Ips latidens (LeConte). Interestingly, among different treatment sources, the efficacy of conspecific semiochemicals in combination with heterospecific semiochemicals and non-host volatiles showed a 92% reduction in Dendroctonus spp., and conspecific semiochemicals in combination with non-host volatiles showed a 77% significant reduction in Ips spp., while the efficacy of heterospecific semiochemicals in reducing Ips population was about 69%, and 20% in Dendroctonus. Among different ecological regions, the use of a push–pull strategy showed a 70% reduction in Dendroctonus in central-west North America, and Ips showed a 75% reduction in southwest North America. Our results demonstrate that semiochemical-based push–pull techniques have the potential to reduce Dendroctonus and Ips bark beetle populations. Furthermore, based on our analysis, the efficacy of such eco-friendly interventions could be further improved and provide a good tool for forest managers to control these pests, at least under some circumstances.
1. Introduction
Bark beetles (Coleoptera: Curculionidae: Scolytinae) can cause significant economic and environmental impacts on plantations and native forests [1]. These beetles spend much of their life in the host tree and have symbiotic associations with bacteria, fungi, viruses, and other arthropods [2]. Symbiotic fungi transferred to trees by bark beetles can block conducting tissues [3] and reduce the commercial value of timber by causing blue staining. In outbreak conditions, they can reduce harvest yield, ecological diversity, and tree carbon sequestration [4,5].
It is estimated that the western United States of America (USA) experiences an annual US$2 billion loss due to bark beetle infestations [6]. The Czech Republic experienced €260 million in economic losses during the 2018–2019 outbreak of Ips typographus (L.), while Dendroctonus frontalis Zimmerman caused a loss of about US$1 billion over the last 28 years in South Carolina alone [3]. During 1989–2004, outbreaks of Dendroctonus rufipennis (Kirby) killed 30 million trees annually in Alaska [6], while outbreaks of D. ponderosae in British Columbia increased carbon emissions by 250 megatons between 2000–2020 and tree mortality exceeded 28 million ha [7]. In China, 14,100 hectares of Jilin province were affected by I. typographus in 2020 [8]. Similarly, Ips grandicollis (Eichhoff) caused millions of dollars of economic loss in 1994 in bushfire-damaged pine trees in Australia [9].
To cope with rapid bark beetle infestations and outbreaks, effective eco-friendly control methods such as semiochemicals are required [10]. Semiochemicals are the basis of communication in which chemical compounds released by an organism [11] modify the behaviour or physiology of another organism in a particular way [12], including to attract or repel the receiver. The semiochemical-based push–pull technique is a combination of an attractant and repellent [10], in which pests are repelled from the target site (push) while an attractant (pull) lures them away [13]. The pull component can be host volatiles—signalling food resources, habitat [14], breeding sites, or refugia [15]. For instance, alpha -pinene, found in pine trees and logs, is an attractive host cue for many bark beetles [16]. Alternatively, the pull component can be an aggregation pheromone imparting signals for feeding [12] and mate location [17]. Repellents are substances that induce an inhibitory response in insects. They can be plant-derived as host repellent or non-host volatiles (NHVs); e.g., limonene, linalool, benzaldehyde, and 4-allylanisole are known plant compounds used against bark beetles [18]. Insect-based repellents produced by insects in nature are used to avoid resource competition among broods and induce a repellent response in conspecific and heterospecific receiver species [19]. Verbenone is a well-known anti-aggregation pheromone for reducing Dendroctonus and Ips damage on host trees [20,21,22]. Moreover, some semiochemicals can be attractive at a low concentration and a repellent at a high concentration, or can induce synergistic effects in a mixture [10]. Trapping studies are of large potential interest as they are steps on the way towards finding the best semiochemical blends for tree protection. Previous studies using meta-analysis to quantify anti-attractant/repellent effects have examined only forest protection outcomes, such as on attack density or tree survival. The first such study [23] had a total of only 32 data points from papers published in 2000–2011 but still found an overall effect of Cohen’s d ca = –1 less tree attack, merged for two species, D. ponderosae and I. typographus. Raffa et al. [24] complemented this dataset with data from a multi-year study by Perkins, et al. [25]. They found 33 values were significantly below zero (39%), with 31 values having a value falling below zero (94%).
Here, we use meta-analysis [26] to aggregate and analyze data from 52 individual scientific studies to compare the overall effects of push–pull semiochemical treatments on reducing bark beetle (Dendroctonus and Ips) populations measured by a reduction in trap catches, tree mortality, and attacks on trees in different regions of the world. We also examine the effect of push treatment sources (conspecific, heterospecific, and NHVs) used alone or in combination. Specifically, we hypothesized that (1) the semiochemical push–pull treatment technique effectively reduces bark beetle populations and (2) the effectiveness of the semiochemical push–pull treatment varies between Dendroctonus and Ips.
2. Materials and Methods
2.1. Database Search and Selection Criteria
We used the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) protocol to collect relevant bibliography-sourced data for this study [27]. We selected two databases, Web of Science® and SCOPUS®, from 1988 until 1 August 2022, using the following simple and standardized keywords arranged by Boolean Logic (“Semiochemical” OR “aggregation pheromone AND anti-aggregation pheromone” OR “attractant volatile AND repellent volatile” OR “attractant AND attractant disruptant” OR “attractant AND deterrent” OR “host volatile AND non-host volatile” OR “push pheromone AND pull pheromone”) AND (“Ips” OR “Dendroctonus”) [28].
2.2. Study Selection
Across both databases, 349 research articles were returned, of which 233 remained after the removal of duplicates, to which the following predefined eligibility criteria were applied:
- The study contained at least one species of Dendroctonus or Ips as the target.
- The study focused on the push–pull semiochemical technique; any study dealing with pull-alone, push-alone, insecticide use, and other control methods was excluded.
- The results were reported as means, variance (standard deviation or standard error), sample size, and other relevant statistical information to allow the calculation of the effect size.
Any study not meeting the above criteria was excluded from the analysis. Of the 233 research articles returned from the databases, 52 met these criteria (Figure 1). The selected research papers span 34 years, from 1988 to 2022 (Supplementary Materials S1). We chose to also include trapping-only data points, as these provide a much larger sample and, thus, may show for many more species the potential for the best inhibitory blends to use in direct forest protection experiments.
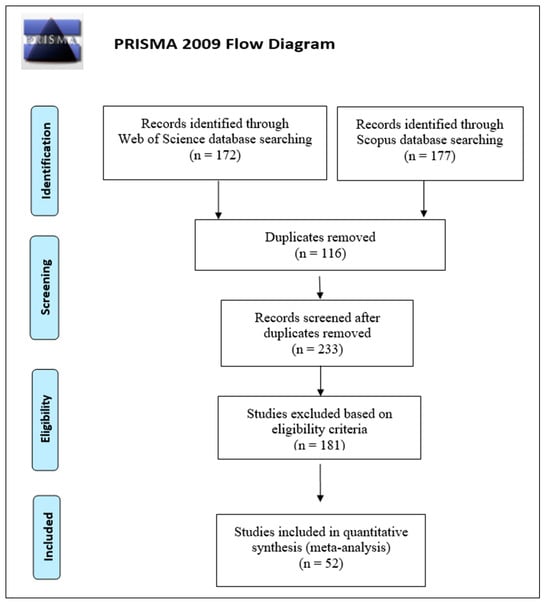
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analysis flow diagram for meta-analysis. (After [27]).
2.3. Data Extraction
Metadata were extracted from the research papers, including sample size (number of replicates, n), means, and standard deviation (SD). If the data were presented as graphs, these were digitized using Web Plot Digitizer [29] and extracted as means and SD. When the standard error (SE) was reported in the studies, it was converted to standard deviation (SD) (SD = SE × √n).
This data extraction technique enhances the power of meta-analysis [30] and has been used in many previous meta-analyses [23]. Multiple studies from one research paper do not decrease independence or analytical power [31]; therefore, different semiochemical treatment–species variants from the same research article were considered as independent data. Parameters related to different species of the bark beetle genera Dendroctonus and Ips, treatment (compound and geographic regions), and response to treatment were collected from each study.
2.4. Meta-Analysis
To estimate the “push–pull” treatment effect of semiochemicals on reducing Dendroctonus and Ips population sizes compared to control, weighted mean of the log response ratio (, also called ratio of means, was calculated [32]. We chose to use a newer effect size calculation better suited than the one used in the previous meta-analysis studies on tree experiment data [23,24] that describe effects in units of SD, Cohen’s d. Thus, data output is not the same, but significant data points are expected to be the same. The response ratio/ratio of mean (RoM) is largely unbiased, whereas the standardised mean difference, commonly known as Cohen’s d, may have non-negligible bias for small sample sizes [33] and some bias (about 5%) with sample sizes < 10. RoM also provides easier interpretation, which makes it a viable option for pooling data [34]. ( was used to estimate effect size because it maintains symmetry for variables reported in different units through log transformation [35]. Moreover, calculating the percentage (%Δ) of effect size is simple from (. Two components of variation in the sample log response ratios were calculated, sampling variation within Equation (1) and between Equation (2) experiments:
Equation (1): Standard deviation of treatment group (SDt), sample size/number of replicates in treatment group (), mean of treatment group , standard deviation of control group (SDc), sample size/number of replicates in control group (), and mean of control group .
In Equation (2), K represents the number of studies; Q represents the Q statistic (Equation (3)) used for testing the statistical significance of the between-experiment variance and is calculated by:
Here, in Equation (3), and () natural logarithm of the response ratio
Effect size as weighted mean of the log response ratio ( was calculated by Equation (4).
Here is the reciprocal of the variance
The effect size was expressed as a percentage (%Δ) and was calculated as:
and standard error (SE) of this weighted mean ( was calculated by Equation (6):
Lower (CIL) and upper (CIu) 95% confidence intervals for the mean log response ratio ( were calculated using Equations (7) and (8), respectively:
Significance level was computed using a two-tailed test. We calculated the pooled variances using the “escalc” function in the “metafor” package (version 2.4-0) in the R environment [36].
A heterogeneity test was performed before constructing the meta-analysis model to decide whether to use a fixed or random effect model. Cochran’s Q test of heterogeneity (Q) based on the full dataset (k = 863 observations) was highly significant (Cochran’s Q = 2.6 × 104, df = 862, p < 0.0001) [36]. In the random effects meta-analysis, each study’s contribution to the results was weighted based on its contribution in the data synthesis. The inverse variance methods of “meta”[37] and “metafor” [36] packages in the R environment were used to assign the weights.
Forest plots were created with ggplot2 in the R environment to present estimated pooled effect size and their 95% confidence intervals (CI) produced by the meta-analysis [38]. If the 95% CI bar did not coincide with the zero line, or if the two-tailed test returned a p-value < 0.05, the effects of push–pull semiochemical treatment were considered significant [35]. Percentage of effect sizes is denoted by (±%), where a positive value shows an increase, and a negative value indicates a reduction in bark beetle population by the semiochemical deterrent treatment compared to the control.
The dataset was further categorized into parameters related to Dendroctonus and Ips species separately, source of test treatment (conspecific, heterospecific, NHVs, and host repellent either used alone or in combination), geographic region of an experiment (countries of an experiment where push–pull treatment was studied were coded/categorized as regions (Supplementary Materials S2). To maintain heterogeneity in each observation, any variable reported in less than three studies (k < 3) was not included in the subgroup analysis.
3. Results
3.1. Metadata
Metadata were extracted from 52 research articles published in eight regions from 1988 to 2022. We obtained 863 observations (k) with push–pull semiochemical treatments using a uniform selection criterion. Most observations (72%) focused on reducing the population of nine Dendroctonus species, while the rest (28%) focused on 12 Ips species. Between-group heterogeneity existed in the sub-group analysis (Table 1).

Table 1.
Categorical variables and between-group heterogeneity (Qb) among observations (k) that affect Dendroctonus and Ips responses to push–pull semiochemical treatment.
3.2. The Overall Effect of Push–Pull Semiochemical Treatments on Dendroctonus and Ips
Our data synthesis showed a significant effect of push–pull semiochemical treatments on reducing bark beetle populations (k = 863). Significantly, semiochemical push–pull treatments reduced Dendroctonus and Ips population by 66% and 54%, respectively (Figure 2).

Figure 2.
Overall effect of push–pull semiochemical treatments on reduction in bark beetle population. The error bars show 95% CI, and (k) indicates the number of studies for each variable. The significant level is denoted as **** for **** p < 0.0001 for the effect of treatment on bark beetle population.
3.3. Effect of Push–Pull Semiochemical Treatment on Attack and Attraction of Dendroctonus and Ips Species
Of the total 624 studies identified on Dendroctonus, 506 aimed to reduce the attraction to lures in the presence of push semiochemicals, 152 focussed on the female and 153 on the male population, 103 studies examined reducing tree attacks, and 15 used the reductions in trees killed to assess efficacy (see Supplementary Materials S3, Table S1 for details of response variables from the manuscripts).
On the other hand, of 239 total studies on Ips, 233 aimed to reduce Ips attraction to lures in the presence of push semiochemicals, 161 studies were with undefined sexes, 36 focused only on the female population, and 36 focused only on the male population. Six studies assessed push–pull semiochemical treatments to reduce tree colonization. This meta-analysis shows that push–pull semiochemical treatments significantly reduced attraction towards a lure in both the Dendroctonus and Ips population. Interestingly, in the presence of push semiochemicals, attraction to lures was reduced by 39% in Dendroctonus and 69% in Ips. In Ips species, the males, and in Dendroctonus species, the females serve as the pioneer attack sex by initially boring into the tree bark and attracting both sexes for a “mass attack”. A semiochemical deterrent treatment significantly reduced the frequency of the pioneer attack sex, in both Dendroctonus and Ips. The push-pull technique obtained a significant 81% reduction in trees killed by Dendroctonus, but non-significant reduction was obtained for Ips (Figure 3).
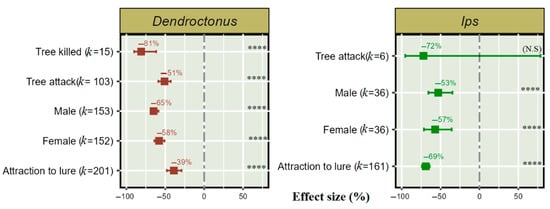
Figure 3.
Effect of push–pull semiochemical treatment on the response of Dendroctonus and Ips populations. Error bar shows 95% CI, and (k) indicates the number of studies for each species. The significant level is denoted as **** for p < 0.0001 and “N. S” in parentheses indicates a non-significant effect of treatment on bark beetle population.
3.4. Effect of Push–Pull Semiochemical Treatments on Dendroctonus and Ips Species
The effect of push–pull semiochemical treatments varied significantly (p < 0.0001) for both the Dendroctonus and Ips species (Table 1). Notably, nine Dendroctonus species (k = 624) were examined in our meta-analysis; among them, eight species significantly reduced in population, while among 12 Ips species (k = 239), there was a significant population reduction in eight species by push–pull semiochemical treatments (Figure 4).

Figure 4.
Effect of push–pull semiochemical treatments on reduction in Dendroctonus and Ips species population. The error bars show 95% CI, and (k) indicates the number of studies for each species. The significant levels are denoted as * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001, and “N. S” in parentheses indicates a non-significant effect of treatment on bark beetle population.
Two Ips species I. perturbatus and I. latidens show a 94% and 93% reduction in population by push–pull semiochemical treatments, respectively. Additionally, this semiochemical technique also showed a 86% reduction in the population of two Ips species, I. avulsus (Eichhoff) and I. shangrila (Cognato and Sun).
These results indicate an equivalent (69%) significant population reduction in D. ponderosae and I. typographus. In the studies considered where there was a reduction, the minimum was a 34% and 38% reduction for D. rufipennis (Kirby) and Ips duplicatus Sahlberg, respectively. These results demonstrate push–pull strategies’ potential effectiveness in reducing bark beetle populations, which cause significant damage to forests.
One Dendroctonus species, D. terebrans (Olivier), showed an increased population from push–pull semiochemical treatment. On the other hand, one Dendroctonus species (D. mesoamericanus Armendáriz-Toledano and Sullivan) and four Ips species (Ips paraconfusus (Lanier), Ips subelongatus (Motschulsky), I. grandicollis, and I. mexicanus (Hopkins)) did not show significant reductions with push–pull semiochemical treatments and highlight the need for further research (Figure 4).
3.5. Effect of Push–Pull Semiochemical Treatment Sources on Dendroctonus and Ips
We found seven treatments used alone or combined to protect softwood forests from Dendroctonus and Ips populations. Among them, six treatments were significantly effective for reducing both Dendroctonus and Ips populations (Figure 5).
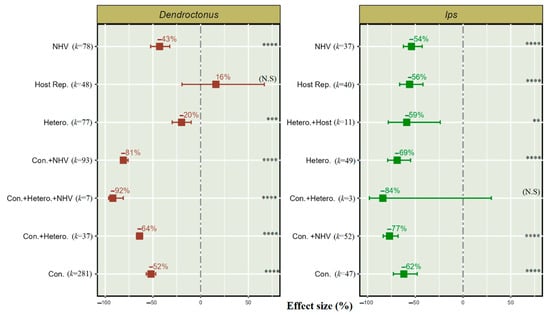
Figure 5.
Effect of push–pull semiochemical treatment sources on the Dendroctonus and Ips population. Con. (conspecific), NHV (non-host volatiles), Hetero. (heterospecific), and Host Rep. (host repellent). Error bar shows 95% CI, and (k) indicates the number of studies for each treatment source. The significant levels are denoted as ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001, and “N. S” in parentheses indicates a non-significant effect of treatment on bark beetle population.
Our results indicate that Dendroctonus and Ips have a 52% and 62% significant population reduction, respectively, when conspecific semiochemical treatment is used alone as a repellent.
The application of heterospecific semiochemicals is 69% effective for Ips and 20% for Dendroctonus when used alone as a repellent. Host repellent shows a 56% significant reduction in Ips but non-significant effects in Dendroctonus.
Interestingly, using push-pull semiochemical technique in combination provides an effective reduction in Dendroctonus and Ips population; for instance, conspecific semiochemicals combined with NHVs resulted in a 81% reduction in the Dendroctonus population and 77% in the Ips population; similarly, conspecific semiochemicals combined with heterospecific semiochemicals also result in a significant reduction in Dendroctonus, but population reduction was non-significant for Ips. These findings also indicate that, instead of using three semiochemicals, i.e., conspecific, heterospecific, and NHVs, their combination can provide an up to 92% reduction in the Dendroctonus population.
Heterospecific semiochemicals combined with host repellent were used only for Ips and provided a 59% reduction in this pest population.
3.6. Effect of Geographical Regions on Push–Pull Semiochemical Treatments in Dendroctonus and Ips
We examined data from five geographical regions for Dendroctonus and eight for Ips using a push–pull semiochemical treatment technique to reduce bark beetles’ population. Based on sub-group heterogeneity, the treatment efficacy varied significantly for Dendroctonus and Ips among geographical regions. In western Europe, Scandinavia, north-western North America, and eastern Europe, a 72–61% reduction in Ips population was obtained using push–pull semiochemical treatments. The implementation of push–pull semiochemical treatments in south-eastern North America resulted in a 75% reduction in Ips and 36% in the population of Dendroctonus (Figure 6). Similarly, in north-western North America, a 65% reduction in the Ips population and 48% in the Dendroctonus population is obtained using push–pull semiochemical treatments. There was a substantial decrease of 63% in the population of Dendroctonus in south-western North America; however, studies examining the push–pull effects in Ips in south-western North America and north-eastern North America have a small sample size and were not significant.

Figure 6.
Effect of regions on push–pull semiochemical treatment for reducing Dendroctonus and Ips population among different geographical regions. The error bar shows 95% CI, and (k) indicates the number of studies for each region. The significant levels are denoted as ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001, and "N. S" in parentheses indicates a non-significant effect of treatment on bark beetle population.
4. Discussion
The results of this meta-analysis study are consistent with the hypothesis that push–pull semiochemical methods can significantly reduce overall bark beetle (Dendroctonus and Ips) infestations. Although using the push–pull technique is challenging given the large size of forests, this technique has great potential to deter bark beetles. Indeed, soon after pheromone identification, scientists developed bark beetle control strategies using semiochemicals, particularly the push–pull techniques in experiments and field control [39].
Our findings align well with the more limited meta-analysis including only tree data for D. ponderosae and I. typographus [23] from 9 papers and 32 experiments from 2000 to 2011, where effects were moderate or strong for both species.
We also identified the different responses of the Dendroctonus and Ips species to push–pull semiochemical treatments. The same is true for the relationship between the treatment region and the bark beetle genus.
This meta-analysis found a significant reduction in the attraction of both male and female Dendroctonus and Ips beetles to the push-pull semiochemical treatments. Our results also significantly support the hypothesis of the success of push–pull semiochemical treatment in protecting trees from Dendroctonus, but a non-significant reduction in trees attacked by Ips, which could be due to the small number of Ips studies.
A significant reduction in the flight response of female Dendroctonus [40], tree attack rate [41], and tree mortality in D. ponderosae [42], and a reduced attraction to the aggregation pheromones in D. pseudotsugae barragani [43] and D. valens in the presence of push semiochemicals has been reported [44]. Likewise, an up to 60% reduction in the mortality of Pinus albicaulis by Dendroctonus, has been recorded in California using a push–pull technique [45]. These findings also indicate that semiochemical cues affect beetle orientation and flight capacity during flight.
Push–pull semiochemical treatments significantly reduced the population of Dendroctonus species, with reductions ranging from 34 to 69%. This significant reduction in the population of the Dendroctonus species is aligns with the findings of Bedard, et al. [46]; Cook et al. [14]; Liu et al. [47]; Seybold et al. [41], Seybold and Fettig [48]; and Sullivan and Clarke [49] for Dendroctonus brevicomis (LeConte), Dendroctonus pseudotsugae (Hopkins), D. valens, D. ponderosae, D. rufipennis, and D. frontalis, respectively. Moreover, using S-(-)-verbenone in combination with 1-hexanol and (Z)-3-hexanol caused about a 60% reduction in D. ponderosae attacks on trees [45]. In summary, this ecofriendly technique is significantly effective at reducing the attraction and aggregation of Dendroctonus species toward lure/pull semiochemicals in the presence of repellent/push semiochemicals.
However, for D. terebrans, this meta-analysis shows significantly more attraction to pull semiochemicals even in the presence of push semiochemicals. Likewise, Sullivan et al. [50] found that D. terebrans attraction to lures frontalin, exo-brevicomin and an alpha-pinene was significantly increased by 4-allylanisole.
Our meta-analysis indicates up to a 94% population reduction for Ips species through push–pull semiochemical treatment. This meta-analysis found a 69% reduction in the population of I. typographus, a pest that killed many healthy Picea trees in Europe in 2007 [51]. A significant reduction in the attraction of this species to 2-methyl-3-buten-2-ol and (4S)-cis-verbenol was reported using trans-conophthorin and 1-hexanol [52], and by trans-verbenol in I. shangrila [53]. Similarly, a significant population reduction in Ips pini (Say) [54], I. perturbatus [55], I. latidens (LeConte) [56], and I. avulsus [57], and an up to 83% reduction in Ips sexdentatus (Boern.) [58] were reported using push–pull treatments.
Our analysis found a non-significant effect of push–pull treatments for I. subelongatus, and this result was similar to that reported by Yejing, et al. [59], who found no significant reduction in I. subelongatus’ attraction to lure (S)-(−)-ipsenol and (S)-(+)-ipsdienol in the presence of NHVs such as myrtenol, (E)-3-hexen-1-ol, (E)-2-hexen-1-ol, and 1-hexanol, or by adding the anti-aggregation pheromone verbenone. Our findings for I. paraconfusus were inconsistent with Shea and Neustein [60], who reported that ipsdienol and verbenone successfully inhibited this bark beetle’s mass attack. The results of this metadata contradicted previous findings of Dickens, et al. [61] who found a significant reduction in I. grandicollis’ attraction to lure ipsdienol, ipsenol, and cis-verbenol in the presence of the green leaf volatile hexanal. Similarly, Birgersson, et al. [62] found a significant reduction in trap catches of I. grandicollis in the presence of the heterospecific semiochemical lanierone. These results suggest that, although push–pull strategies might be useful for deterring populations of bark beetles like I. grandicollis, more research is required.
Except for the effect size of the host repellents, which is non-significant effect, our analysis supports our hypothesis that the populations of Dendroctonus spp. can be reduced by using various push–pull semiochemical treatment sources, either alone or in combination. Among the seven treatments used to reduce the Ips population, six significantly support our hypothesis, except for the non-significant effect of conspecific semiochemicals when combined with heterospecific ones.
The findings of this metadata were aligned with the findings of previous research such as that of Sullivan [63], who reported a reduction in Ips avulsus catches toward ipsdienol and lanierone in the presence of alpha-pinene at 8 g/day. Wu, et al. [64] reported effective results in inducing repellence by NHVs from the bark and green leaves of angiosperms, and Cook et al. [14] in reducing aggregation in conifer-feeding beetles like Dendroctonus and Ips spp. on pine trees. Similarly, Zhang and Schlyter [18] reported an effective reduction in the aggregation of Dendroctonus spp. by using acetophenone, an NHV. Kandasamy, et al. [65] reported an effective reduction in the aggregation of Dendroctonus and Ips by benzyl alcohol, in combination with green leaf volatiles and other NHVs.
Jactel et al. [58] found up to an 83% reduction in attraction to aggregation pheromone by trans-conophthorin, whereas Dickens et al. [61] found the presence of 1-hexanol and hexanal effective for Ips deterrence and Fettig, et al. [66] reported 2-hexen-1-ol to significantly reduce the attraction of Dendroctonus spp. toward the lure in a trapping bioassay.
Conspecific semiochemicals can also act as anti-aggregants, e.g., Wu et al. [64] reported a repellent response in D. frontalis, D. ponderosae, I. pini, and I. sexdentatus to verbenone. Verbenone is a compound produced by oxidation of alpha-pinene by males of several Dendroctonus and Ips species as an anti-aggregation pheromone [67]. In field-trapping experiments, this semiochemical has been found to be effective in reducing the attraction of several bark beetles like D. frontalis toward lures [49] and D. ponderosae attraction to trans-verbenol, cis-verbenol, and exo-brevicomin [44].
Trans-verbenol, a conspecific anti-aggregation pheromone, has been found to significantly reduce the attraction of Ips and Dendroctonus toward aggregation pheromones [53]. Similarly, the presence of trans-verbenone and verbenone induces a push response in Dendroctonus brevicomis (LeConte) toward the pull semiochemicals (±) exo-brevicomin, (±) frontalin, and myrcene [46]. Exo-brevicomin is also a conspecific semiochemical that induced a repellent response in D. valens toward the aggregation pheromone [47]. Similarly, 3-methylcyclohex-2-en-1-one (MCH), a conspecific anti-aggregation pheromone, causes the reduction of attraction to 1-methyl-2-cyclohexen1-ol + frontalin + ethanol lures in D. pseudotsugae barragani [68].
Importantly, heterospecific semiochemicals effectively induce a deterrent response in bark beetles, as El-Ghany [15] reported that heterospecific semiochemicals serve as a chemical cue indicating that a competitor species already occupies a suitable host tree or stand. Similarly, Fettig et al. [55] reported that heterospecific semiochemicals, like trans-conophthorin, along with verbenone, is significantly effective in reducing the attraction to host trees.
Communication systems based on pheromones, like those found in bark beetle communities, play a vital role in insect interactions. The results of our meta-analysis show that the efficacy of NHVs to deter forest pests can be enhanced by incorporating the semiochemicals of a conspecific bark beetle; similarly, Seybold et al. [41] reported that using conspecific repellents with NHV reduced Dendroctonus and Ips attraction by signalling that early colonizers have previously attacked, and this is now an unsuitable host that should be avoided.
Crucially, it was observed that the combination of heterospecific semiochemicals with conspecific semiochemicals had a significantly stronger inhibitory effect in Dendroctonus than heterospecific semiochemicals alone, while the opposite was observed in Ips. Further confirmation came from Aukema and Raffa [69] and Seybold et al. [41], who found a greater pull response in Ips to frontalin, a pheromone of D. frontalis, but the exact reasons for the contradictory heterospecific effectiveness are unknown.
Our study demonstrates that semiochemical deterrent treatments are significantly effective for both Dendroctonus and Ips in various ecological conditions, and these results are aligned with those of earlier researchers. According to our findings the push-pull semiochemical technique was best in deterring Dendroctonus infestations in central-west North America by 70% and Ips infestations in south-eastern North America by 75%. In contrast, the non-significant effect of push–pull treatments from this meta-analysis for Ips in south-western North America (p > 0.05) and north-eastern North America (p > 0.05) found no support for these treatments as a deterrent, although, given the small sample size/studies, further research is needed. According to Fettig et al. [66], various studies conducted in north-western North America found acetophenone, (E)-2-hexen-1-ol + (Z)-2-hexen-1-ol, and verbenone provided significant protection to ponderosa pines from D. brevicomis attacks. Our results were aligned with the findings of Gaylord et al. [54] and Lindmark, et al. [70] for I. pini in south-western United States and I. typographus in Scandinavia, respectively.
For Ips, the population reduction in West Europe and East Europe lies between 72 and 61%, while, in East Asia, it is about 49%. It should be noted that NHV compounds naturally occurring in mixed habitats affect specialist herbivores, reducing their efficiency to locate their hosts. For effect size estimates of forest conditions on herbivore host location efficiency, see Jactel and Brockerhoff [71]. The semiochemical diversity hypothesis (SDH), suggested by Zhang and Schlyter [18], postulates a reduced searching efficiency of specialist herbivores in the face of NHVs, which may be one of the main factors for the reduction in herbivory [72] in mixed habitats.
This meta-analysis found that the effect of push-pull semiochemical treatments on Dendroctonus was highly significant and consistent with the findings of Sánchez-Martínez et al. [68] in north-western North America. It is worth noting that very limited datasets are available related to the effect of semiochemicals on Ips. Substantial geographical variation in pheromone response occurs between Ips populations [55]. Therefore, further research is required to explain the differences of the effects of semiochemicals on Ips reduction for different ecological regions.
This control technique has been used to reduce the devastation in forests caused by I. paraconfusus [14] and D. brevicomis [66], and to reduce the attraction of Ips toward lures [73], as well as protecting ponderosa pine, Pinus ponderosa var. scopulorum Engelm., trees from Ips attacks [54]. Like our findings, Lindmark et al. [70] also reported a reduction in bark beetle capture using an attractant–repellent approach and suggested using semiochemicals as an effective alternative to insecticides for reducing the bark beetle population.
5. Limitations and Suggestions
Ips spp. are recorded as serious pests in eight regions, while Dendroctonus is a pest in five regions of the world. Nine species of Dendroctonus and twelve Ips species pose a severe threat to the forest economy, but more studies focus on Dendroctonus than Ips. Therefore, more research is necessary to further comprehend the connection between the application of treatment sources and the reduction response in Ips.
The next wave of research on semiochemicals and bark beetles should take a systematic approach to other genera and examine the effects of the push–pull treatment and application mode (e.g., Specialized Pheromone & Lure Application Technology (SPLAT), aerial spray, and ground spread) on predators and parasitoids, which perform important roles in regulating bark beetle populations, at least in endemic ranges. Additionally, it is essential to quantify the impact of semiochemical push-alone and semiochemical pull-alone treatments on Dendroctonus and Ips.
6. Conclusions
The results of this meta-analysis indicate that the push–pull semiochemical technique can reduce Dendroctonus and Ips attraction to lures in forests. We conclude that (1)the use of the push–pull semiochemical technique is effective in reducing Ips (−66%) and Dendroctonus (−54%) compared to control; (2) among push–pull treatment sources, conspecific semiochemicals combined with heterospecific and NHVs provide the maximum reduction for Dendroctonus; (3) conspecific repellent semiochemicals can effectively lower the population of both genera by around half and, thus, can be used to protect the forest; (4) for Ips, heterospecific semiochemicals as the pull part of the technique is more effective when used alone rather than in combination; and (5) these deterrent techniques can diminish the pioneer attacking sex of both genera, even in the presence of an attractant bait, hence potentially halting future colonization and the attack density.
There is evidence that the push–pull semiochemical technique may be effective in preventing Ips attacks on softwood forests, but, to date, this research is quite limited; therefore, further research should evaluate the use of heterospecific semiochemicals with both NHVs and heterospecific semiochemicals in Ips. As part of ongoing Ips management plans, push–pull semiochemical treatments should be assessed, especially in native and invasive habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14100812/s1, Table S1: List of 52 research articles included in meta-analysis; Table S2: Metadata about the 863 experiments obtained from 52 studies used in this meta-analysis; Table S3: Manuscripts describing the effect of push-pull semiochemical treament on different variables.
Author Contributions
Conceptualization, S.A., R.A.H., H.F.N. and S.A.L.; methodology, S.A., R.A.H., H.F.N. and S.A.L.; software, S.A.; validation, S.A.; formal analysis, S.A.; investigation, S.A.; data curation, S.A.; writing—original draft preparation, S.A., writing—review and editing, R.A.H., H.F.N. and S.A.L.; supervision, R.A.H., H.F.N. and S.A.L., project administration, R.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by an Australian Government Research Training Program (RTP) Scholarship to S.A. This research was also funded by the Australian Government Department of Agriculture, Fisheries, and Forestry through the Pest Animals and Weeds program activity ID 4-FY9LIES.
Data Availability Statement
The data presented in this study are openly available online at Supplementary Materials (selected publication references and metadata).
Acknowledgments
The authors would like to thank Andy Howe for discussions regarding statistical analysis and Joanne de Faveri for comments on a draft version of the manuscript. The authors would like to thank the three anonymous reviewers for their comments that have improved the final version of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirkendall, L.R.; Biedermann, P.H.; Jordal, B.H. Evolution and diversity of bark and ambrosia beetles. In Bark Beetles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 85–156. [Google Scholar]
- Raffa, K.F.; Grégoire, J.-C.; Staffan Lindgren, B. Chapter 1—Natural history and ecology of Bark Beetles. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 1–40. [Google Scholar] [CrossRef]
- Gandhi, K.J.; Hofstetter, R. Introduction: Bark beetles, management, and climate change. In Bark Beetle Management, Ecology, and Climate Change; Elsevier: Amsterdam, The Netherlands, 2022; pp. xix–xxvi. [Google Scholar]
- Morris, J.L.; Cottrell, S.; Fettig, C.J.; DeRose, R.J.; Mattor, K.M.; Carter, V.A.; Clear, J.; Clement, J.; Hansen, W.D.; Hicke, J.A. Bark beetles as agents of change in social–ecological systems. Front. Ecol. Environ. 2018, 16, S34–S43. [Google Scholar] [CrossRef]
- Gillette, N.E.; Fettig, C.J. Semiochemicals for bark beetle (Coleoptera: Curculionidae) management in western North America: Where do we go from here? Can. Entomol. 2021, 153, 121–135. [Google Scholar] [CrossRef]
- Grégoire, J.-C.; Raffa, K.F.; Lindgren, B.S. Chapter 15—Economics and politics of Bark Beetles. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 585–613. [Google Scholar] [CrossRef]
- Hlásny, T.; König, L.; Krokene, P.; Lindner, M.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.F.; Schelhaas, M.-J.; Svoboda, M.; et al. Bark beetle outbreaks in Europe: State of knowledge and ways forward for management. Curr. For. Rep. 2021, 7, 138–165. [Google Scholar] [CrossRef]
- Pan, Q.; Fang, J.; Zhang, S.; He, Y.; Wang, Y. Biorhythm paralleled release of pheromone by photothermal conversion for Long-term bark beetle control. Chem. Eng. J. 2022, 440, 135933. [Google Scholar] [CrossRef]
- Wylie, F.; Peters, B.; DeBaar, M.; King, J.; Fitzgerald, C. Managing attack by bark and ambrosia beetles (Coleoptera: Scolytidae) in fire-damaged Pinus plantations and salvaged logs in Queensland, Australia. Aust. For. 1999, 62, 148–153. [Google Scholar] [CrossRef]
- Gillette, N.E.; Munson, A.S. Semiochemical sabotage: Behavioral chemicals for protection of Western conifers from Bark Beetles. In Proceedings of the Western Bark Beetle Research Group: A Unique Collaboration with Forest Health Protection—Proceedings of a Symposium at the 2007 Society of American Foresters Conference, Portland, Oregon, 23–28 October 2007; p. 85. [Google Scholar]
- Sharma, A.; Sandhi, R.K.; Reddy, G.V. A review of interactions between insect biological control agents and semiochemicals. Insects 2019, 10, 439. [Google Scholar] [CrossRef]
- Yew, J.Y.; Chung, H. Insect pheromones: An overview of function, form, and discovery. Prog. Lipid Res. 2015, 59, 88–105. [Google Scholar] [CrossRef]
- Smart, L.E.; Aradottir, G.I.; Bruce, T.J.A. Chapter 6—Role of semiochemicals in integrated pest management. In Integrated Pest Management; Abrol, D.P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 93–109. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- El-Ghany, N. Semiochemicals for controlling insect pests. J. Plant Prot. Res. 2019, 59, 1–11. [Google Scholar] [CrossRef]
- Flechtmann, C.; Dalusky, M.; Berisford, C. Bark and ambrosia beetle (Coleoptera: Scolytidae) responses to volatiles from aging loblolly pine billets. Environ. Entomol. 1999, 28, 638–648. [Google Scholar] [CrossRef]
- Verhoef, H.A.; Nagelkerke, C. Formation and ecological significance of aggregations in Collembola. Oecologia 1977, 31, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Schlyter, F. Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric. For. Entomol. 2004, 6, 1–20. [Google Scholar] [CrossRef]
- Mashaly, A.; Ali, M.; Al-Khalifa, M. Chapter 6 Trail pheromones in pest control. In New Perspectives in Plant Protection; Bandani, A.R., Ed.; IntechOpen: London, UK, 2012; pp. 121–138. [Google Scholar] [CrossRef]
- Progar, R.A.; Gillette, N.; Fettig, C.J.; Hrinkevich, K. Applied chemical ecology of the mountain pine beetle. For. Sci. 2014, 60, 414–433. [Google Scholar] [CrossRef]
- Cale, J.A.; Taft, S.; Najar, A.; Klutsch, J.G.; Hughes, C.C.; Sweeney, J.D.; Erbilgin, N. Mountain pine beetle (Dendroctonus ponderosae) can produce its aggregation pheromone and complete brood development in naïve red pine (Pinus resinosa) under laboratory conditions. Can. J. For. Res. 2016, 45, 1873–1877. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Brownie, C. The role of wind and semiochemicals in mediating switching behavior in the Southern pine beetle (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2022, 51, 340–350. [Google Scholar] [CrossRef]
- Schlyter, F. Semiochemical diversity in practice: Antiattractant semiochemicals reduce bark beetle attacks on standing trees—A first meta-analysis. Psyche 2012, 2012, 268621. [Google Scholar] [CrossRef]
- Raffa, K.F.; Andersson, M.N.; Schlyter, F. Chapter one—Host selection by bark beetles: Playing the odds in a high-stakes game. In Advances in Insect Physiology; Tittiger, C., Blomquist, G.J., Eds.; Academic Press: San Diego, CA, USA, 2016; Volume 50, pp. 1–74. [Google Scholar]
- Perkins, D.L.; Jorgensen, C.L.; Rinella, M.J. Verbenone decreases whitebark pine mortality throughout a mountain pine beetle outbreak. For. Sci. 2015, 61, 747–752. [Google Scholar] [CrossRef]
- Hunter, J.E.; Schmidt, F.L. Methods of Meta-Analysis: Correcting Error and Bias in Research Findings; Sage: Washington, DC, USA, 2004. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Grover, S.; Basu, S. Measuring student learning in introductory block-based programming: Examining misconceptions of loops, variables, and boolean logic. In Proceedings of the 2017 ACM SIGCSE Technical Symposium on Computer Science Education, Seattle, WA, USA, 8–11 March 2017; pp. 267–272. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer User Manual Version 3.4. 2014, pp. 1–18. Available online: http://Arohatgi.Info/WebPlotDigitizer/App (accessed on 27 September 2022).
- Lajeunesse, M.J.; Forbes, M.R. Variable reporting and quantitative reviews: A comparison of three meta-analytical techniques. Ecol. Lett. 2003, 6, 448–454. [Google Scholar] [CrossRef]
- Gurevitch, J.; Hedges, L.V. Statistical issues in ecological meta-analyses. Ecology 1999, 80, 1142–1149. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Lin, L.; Aloe, A.M. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat. Med. 2021, 40, 403–426. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.O.; Adhikari, N.K.J.; Beyene, J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: A simulation study. BMC Med. Res. Methodol. 2008, 8, 32. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1–37. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Schwarzer, G. meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Wickham, H. Data analysis. In ggplot2; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–201. [Google Scholar]
- Byers, J.A.; Maoz, Y.; Cohen, B.; Golani, M.; Fefer, D.; Levi-Zada, A. Protecting avocado trees from ambrosia beetles by repellents and mass trapping (push–pull): Experiments and simulations. J. Pest Sci. 2021, 94, 991–1002. [Google Scholar] [CrossRef]
- Jones, K.L.; Evenden, M.L. Effect of semiochemical exposure on flight propensity and flight capacity of Dendroctonus ponderosae in laboratory bioassays. Arthropod-Plant Interact. 2021, 15, 551–562. [Google Scholar] [CrossRef]
- Seybold, S.J.; Bentz, B.J.; Fettig, C.J.; Lundquist, J.E.; Progar, R.A.; Gillette, N.E. Management of western North American bark beetles with semiochemicals. Annu. Rev. Entomol. 2018, 63, 407–432. [Google Scholar] [CrossRef]
- Kegley, S.; Gibson, K. Individual-Tree Tests of Verbenone and Green-Leaf Volatiles to Protect Lodgepole, Whitebark and Ponderosa Pines, 2004–2007; Forest Health Protection Numbered Report 09-03; USDA Forest Service, Forest Health Protection: Missoula, MT, USA, 2009. [Google Scholar]
- Domínguez-Sánchez, B.; Macías-Sámano, J.E.; Ramírez-Marcial, N.; León-Cortés, J.L. Kairomonal response of coleopterans associated with Dendroctonus frontalis and two Ips species (Coleoptera: Curculionidae) in forest of Chiapas, Mexico. Rev. Mex. De Biodivers. 2008, 79, 175–183. [Google Scholar]
- Gillette, N.; Stein, J.; Owen, D.; Webster, J.; Fiddler, G.; Mori, S.; Wood, D. Verbenone-releasing flakes protect individual Pinus contorta trees from attack by Dendroctonus ponderosae and Dendroctonus valens (Coleoptera: Curculionidae, Scolytinae). Agric. For. Entomol. 2006, 8, 243–251. [Google Scholar] [CrossRef]
- Cardinal, E.; Shepherd, B.; Krakowski, J.; Schwarz, C.J.; Stirrett-Wood, J. Verbenone and green-leaf volatiles reduce whitebark pine mortality in a northern range-expanding mountain pine beetle outbreak. Can. J. For. Res. 2022, 52, 158–168. [Google Scholar] [CrossRef]
- Bedard, W.D.; Tilden, P.E.; Lindahl, K.Q.; Wood, D.L.; Rauch, P.A. Effects of verbenone and trans-verbenol on the response of Dendroctonus brevicomis to natural and synthetic attractant in the field. J. Chem. Ecol. 1980, 6, 997–1013. [Google Scholar] [CrossRef]
- Liu, Z.; Xin, Y.; Xu, B.; Raffa, K.F.; Sun, J. Sound-triggered production of antiaggregation pheromone limits overcrowding of Dendroctonus valens attacking pine trees. Chem. Senses 2017, 42, 59–67. [Google Scholar] [PubMed]
- Seybold, S.J.; Fettig, C.J. Managing bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) with semiochemicals. Can. Entomol. 2021, 153, 4–12. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Clarke, S.R. Semiochemicals for management of the southern pine beetle (Coleoptera: Curculionidae: Scolytinae): Successes, failures, and obstacles to progress. Can. Entomol. 2021, 153, 36–61. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Munro, H.L.; Shepherd, W.P.; Gandhi, K.J.K. 4-allylanisole as a lure adjuvant for Dendroctonus frontalis (Coleoptera: Curculionidae: Scolytinae) and two associated beetles. J. Appl. Entomol. 2022, 146, 813–822. [Google Scholar] [CrossRef]
- Stadelmann, G.; Bugmann, H.; Meier, F.; Wermelinger, B.; Bigler, C. Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. For. Ecol. Manag. 2013, 305, 273–281. [Google Scholar] [CrossRef]
- Huber, D.P.; Fettig, C.J.; Borden, J.H. Disruption of coniferophagous bark beetle (Coleoptera: Curculionidae: Scolytinae) mass attack using angiosperm nonhost volatiles: From concept to operational use. Can. Entomol. 2021, 153, 19–35. [Google Scholar] [CrossRef]
- Schlyter, F.; Jakuš, R.; Han, F.-Z.; Ma, J.-H.; Kalinová, B.; Mezei, P.; Sun, J.-H.; Ujhelyiová, L.; Zhang, Q.-H. Reproductive isolation of Ips nitidus and I. shangrila in mountain forests of Western China: Responses to chiral and achiral candidate pheromone components. J. Chem. Ecol. 2015, 41, 678–688. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Audley, J.P.; McMillin, J.D.; Fettig, C.J. Acetophenone and green leaf volatiles do not enhance the efficacy of verbenone for inhibiting attraction of Ips pini (Coleoptera: Curculionidae) to pheromone-baited traps in northern Arizona. J. Econ. Entomol. 2023, 116, 632–636. [Google Scholar] [CrossRef]
- Fettig, C.J.; Burnside, R.E.; Hayes, C.J.; Kruse, J.J.; Lisuzzo, N.J.; McKelvey, S.R.; Mori, S.R.; Nickel, S.K.; Schultz, M.E. Factors influencing northern spruce engraver colonization of white spruce slash in interior Alaska. For. Ecol. Manag. 2013, 289, 58–68. [Google Scholar] [CrossRef]
- Miller, D.; Borden, J. The use of monoterpenes as kairomones by Ips latidens (LeConte)(Coleoptera: Scolytidae). Can. Entomol. 1990, 122, 301–307. [Google Scholar] [CrossRef]
- Gitau, C.W.; Bashford, R.; Carnegie, A.J.; Gurr, G.M. A review of semiochemicals associated with bark beetle (Coleoptera: Curculionidae: Scolytinae) pests of coniferous trees: A focus on beetle interactions with other pests and their associates. For. Ecol. Manag. 2013, 297, 1–14. [Google Scholar] [CrossRef]
- Jactel, H.; Van Halder, I.; Menassieu, P.; Zhang, Q.H.; Schlyter, F. Non-host volatiles disrupt the response of the stenographer bark beetle, Ips sexdentatus (Coleoptera: Scolytidae), to pheromone-baited traps and maritime pine logs. Integr. Pest Manag. Reviews 2001, 6, 197–207. [Google Scholar] [CrossRef]
- Yejing, L.; Kong, X.; Sufang, Z.; Wang, H.; Zhang, Z.; Li, C.; Jiao, X.; Huo, T. Allelochemical effects on aggregation behaviors of Ips subelongatus (Coleoptera: Scolytidae). Sci. Silvae Sin. 2016, 52, 107–114. [Google Scholar]
- Shea, P.J.; Neustein, M. Protection of a rare stand of Torrey pine from Ips paraconfusus. In Application of Semiochemicals for Management of Bark Beetle Infestations: Proceedings of an Informal Conference; Department of Agriculture Forest Service: Ogden, UT, 1995; pp. 39–43. [Google Scholar]
- Dickens, J.; Billings, R.; Payne, T. Green leaf volatiles interrupt aggregation pheromone response in bark beetles infesting southern pines. Experientia 1992, 48, 523–524. [Google Scholar] [CrossRef]
- Birgersson, G.; Dalusky, M.J.; Espelie, K.E.; Berisford, C.W. Pheromone production, attraction, and interspecific inhibition among four species of Ips bark beetles in the Southeastern USA. Psyche 2012, 2012, 532652. [Google Scholar] [CrossRef]
- Sullivan, B.T. Host odour alpha-pinene increases or reduces response of Ips avulsus (Coleoptera: Curculionidae: Scolytinae) to its aggregation pheromone, depending on separation of release points. Can. Entomol. 2023, 155, e4. [Google Scholar] [CrossRef]
- Wu, C.X.; Liu, F.; Zhang, S.F.; Kong, X.B.; Zhang, Z. Semiochemical regulation of the intraspecific and interspecific behavior of Tomicus yunnanensis and Tomicus minor during the shoot-feeding phase. J. Chem. Ecol. 2019, 45, 227–240. [Google Scholar] [CrossRef]
- Kandasamy, D.; Gershenzon, J.; Hammerbacher, A. Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. J. Chem. Ecol. 2016, 42, 952–969. [Google Scholar] [CrossRef]
- Fettig, C.J.; Audley, J.P.; Homicz, C.S.; Progar, R.A. Applied chemical ecology of the western pine beetle, an important pest of ponderosa pine in western north America. Forests 2023, 14, 757. [Google Scholar] [CrossRef]
- Gaylord, M.L.; McKelvey, S.R.; Fettig, C.J.; McMillin, J.D. Verbenone inhibits attraction of Ips pini (Coleoptera: Curculionidae) to pheromone-baited traps in northern Arizona. J. Econ. Entomol. 2020, 113, 3017–3020. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, G.; Mehmel, C.J.; González-Gaona, E.; Mori, S.R.; López-Hernández, J.A.; Monárrez-González, J.C.; García-Rodríguez, J.L.; Mejía-Bojórquez, J.M.; Gillette, N.E. 3-Methylcyclohex-2-en-1-one reduces the aggregation of Dendroctonus pseudotsugae barragani and corresponding mortality of Pseudotsuga menziesii in northern Mexico. Agric. For. Entomol. 2022, 24, 344–354. [Google Scholar] [CrossRef]
- Aukema, B.H.; Raffa, K.F. Selective manipulation of predators using pheromones: Responses to frontalin and ipsdienol pheromone components of bark beetles in the Great Lakes region. Agric. For. Entomol. 2005, 7, 193–200. [Google Scholar] [CrossRef]
- Lindmark, M.; Ganji, S.; Wallin, E.A.; Schlyter, F.; Unelius, C.R. Semiochemicals produced by fungal bark beetle symbiont Endoconidiophora rufipennis and the discovery of an anti-attractant for Ips typographus. PLoS ONE 2023, 18, e0283906. [Google Scholar] [CrossRef]
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef]
- Jactel, H.; Birgersson, G.; Andersson, S.; Schlyter, F. Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia 2011, 166, 703–711. [Google Scholar] [CrossRef]
- Erbilgin, N.; Raffa, K.F. Opposing effects of host monoterpenes on responses by two sympatric species of bark beetles to their aggregation pheromones. J. Chem. Ecol. 2000, 26, 2527–2548. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).