Simple Summary
Stored product pests cause significant losses to agricultural products every year. Their control depends heavily on the use of fumigants and other insecticides, which have many negative consequences for humans and the environment. Nowadays, the use of fungal entomopathogens is one of the most promising alternatives to reduce the use of chemicals in storage facilities. We tested new wild strains of entomopathogenic fungi from the genera Cladosporium, Condenascus, Lecanicillium, and Penicillium in laboratory bioassays on various storage beetles. All strains caused remarkable mortality in adult beetles, reaching 80% in some cases after 21 days. The results of our study show that insect-pathogenic fungi can be effective biological tools for the protection of stored agricultural products. Research to discover new strains with high pathogenicity and to develop new methods for mass production and standardization of entomopathogens should be continued to enable their practical application in the future.
Abstract
There is ample evidence that entomopathogenic fungi can be used as alternative biological control agents for the management of insect pests in storage facilities. As the market demands more environmentally friendly methods and chemical insecticides become increasingly obsolete, more studies are being conducted to evaluate new strains of entomopathogenic fungi for their efficacy in storage facilities. In this context, we tested ten species of fungi isolated from soil, belonging to the genera Cladosporium, Condenascus, Lecanicillium, and Penicillium, for their long-term effects on economically important beetle species. Whole wheat was directly sprayed with a conidial suspension of 108 spores/Ml of each of the tested fungi and then adults of Sitophilus granarius, S. oryzae, S. zeamais, Rhyzopertha dominica, and Trogoderma granarium were placed on the sprayed medium to study the mortality effects. Significantly higher mortality than the control was observed in all treatments. The lowest LT50 (9.164 days) was observed in T. granarium infected with Penicillium goetzii. The isolate with the strongest results was L. dimorphum, which recorded remarkably low LT50 values in S. oryzae (~11 days), R. dominica (~12 days), T. granarium (~10 days), and S. granarius (~13 days). However, for S. zeamais, it was more than 16 days. Our results confirm the existing literature on the efficacy of EPF on storage beetles, suggest the possible virulence of wild untested strains, and also highlight the importance of EPF specificity.
1. Introduction
In agriculture, financial losses due to pest infestations are not limited to the field but continue into storage. It is estimated that pest infestations of stored products cause annual economic losses of 10% worldwide [1]. Storage pests are mainly beetles and moths [2], which contribute to the spoilage of stored goods not only by feeding on them but also by transmitting harmful microorganisms and contaminating products with their frass and exuviae, which can be harmful to human health [1,3]. Storages offer favorable environmental conditions for the rapid development of these pests, offering ideal temperatures, humidity, and abundant food. Infestation can lead to enormous economic losses as the quality, quantity, and commercial value of stored commodities are affected [4].
Control of these pests is usually based on the use of synthetic insecticides and fumigants, a practice which, despite its effectiveness, is increasingly problematic, as many of these substances are banned due to the health risks associated with their use, as well as the contamination of products with chemical residues that lead to deterioration of nutritional quality and the development of resistance [5]. Recently, over-reliance on phosphine, especially after the restriction of methyl bromide, has already led to increased frequency, prevalence, and severity of resistance in numerous stored-product pests, and the lack of suitable alternatives is worsening these effects day by day [6]. In addition, phosphine can have erosive effects and damage equipment when used repeatedly at high concentrations [7].
Entomopathogenic fungi (EPFs) have been shown to have significant potential to control insects while minimizing the negative effects of insecticides, and they are used in pest management worldwide [8,9,10,11,12]. EPFs exclusively infect insects, the mycelium penetrates the cuticle and grows in the hemocoel, resulting in death, and then sporulation follows on the external surfaces of insects’ cadavers, promoting epizootics [13]. The wide distribution of EPF in a variety of habitats is evidence of its safety, low environmental impact, and low toxicity to mammals [14,15].
Although high mortality rates in various storage insects due to fungal pathogens have been reported in the literature, little attention has been paid to the practical use of such pathogens as biological control agents in storage facilities [16,17,18]. In this regard, three commercially available species have been tested for their potential for protecting stored products from severe pests: Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae) [19,20], Metarhizium anisopliae (Metschinkoff) Sorokin (Hypocreales: Clavicipitaceae) [21,22], and Cordyceps fumosorosea (Wize) (formerly Isaria fumosorosea) (Hypocreales: Cordycipitaceae) [23,24,25], In addition to commercially available EPFs, extensive research is being carried out on wild strains collected from nature (from the soil or infected dead insects), isolated in the laboratory and evaluated for their insecticidal ability. This results in the enrichment of our biological «arsenal» for the control of insect pests.
Following this strategy we studied, the long-term efficacy of ten wild fungal isolates of the genera Cladosporium, Condenascus, Lecanicillium, and Penicillium isolated from soil in Greece. The effect was determined by measuring the survival time of adults of the granary weevil Sitophilus granarius, the rice weevil S. oryzae, the maize weevil S. zeamais Motsch. (Coleoptera: Curculionoidea), the lesser grain borer Rhyzopertha dominica (Coleoptera: Bostrychidae), and the khapra beetle Trogoderma granarium Everts (Coleoptera: Dermestidae). The results of our study were analyzed in the context of the intended use of insect pathogens as a key component of integrated pest management in storage facilities.
2. Materials and Methods
2.1. Collection, Isolation, and Identification of Fungi
Soil samples were collected in 2019 in Patras, Achaia, Greece. Samples were collected from a depth of 10 cm below the top soil layer and placed in sealed polyethylene bags after excavation. The isolation of the fungal samples was performed according to the bait—methods of Mantzoukas et al. 2019 [25]. The mycelium present on the dead insect baits was inoculated onto a new medium to purify the fungal cultures. The purification of the cultures was performed until the growth of a single colony on Sabouraud Dextrose Agar (SDA) plates was achieved. The morphological characteristics of the strains were observed by inoculating a fungal mycelial plug (1 cm) onto an SDA plate for 10 days. At the end of the growth period, the sporulation structure was taped from the edge with transparent tape and then stained with Phenol cotton blue reagent. Spore morphology was observed under a phase contact microscope (×100) (ZEISS Primo Star, Carl Zeiss Microscopy GmbH, Munich, Germany). DNA sequencing was also performed using the method described by Mantzoukas et al. 2019 [25].
2.2. Insects
Mortality bioassays were performed on five important beetle pests: S. granarius, S. oryzae, S. zeamais, R. dominica, and T. granarium. These species are globally common storage pests that cause severe losses and damage to a variety of commodities. Adults of mixed sexes aged < 2 weeks were collected and transferred to uninfested wheat grains. The adults were left there for 1 week to lay eggs and then removed so that individuals of standardized age could be obtained. All species were reared on durum wheat at 27.5 °C and 75% relative humidity (RH).
2.3. Bioassays
Individual batches of 500 g wheat were filled into cylindrical 0.45 L glass jars. The product was sprayed directly with 1 mL of conidial suspension containing 108 spores/mL of the fungus using a Potter spray tower (Burkard Manufacturing Co. Ltd., Rickmansworth, Hertfordshire, UK) at 1 kgf/cm2. After the application of EPF, the wheat batches were placed back into the jars and shaken by hand for 30 s to achieve uniform distribution of the fungi. A separate set of batches was sprayed with distilled water only and served as control. Twenty 10 g samples were taken from each jar and placed in a 9 cm Petri dish. The inner “neck” side of each Petri dish was covered with Fluon (Northern Products, Woonsocket, RI, USA) to prevent insect escape. Adults were starved for 24 h. Ten individuals of each beetle species were placed in each Petri dish and then placed in plastic boxes containing saturated sodium chloride solutions to maintain 75% r.h. Petri dishes were then placed in incubators at 27.5 °C and 75% r.h. After 7, 14, and 21 days, all Petri dishes were opened and dead adults were counted. All dead adults were immediately removed and immersed in 95% ethanol for 1 min, washed in sterile distilled water for 5 min, dried, and then placed on moistened filter paper. The above procedure was performed in a laminar flow chamber. The cadavers were kept in the dark at 25 °C for 5–7 days, and those that showed hyphal growth characteristics of entomopathogenic fungi were classified as infected. The fungal species was first identified by microscopic observation based on the shape and size of the hyphal growth and confirmed by PCR analysis. In the present work, the DNA sequences were matched using the Basic Local Alignment Search Tool (NCBI BLAST) [25].
The whole procedure was repeated ten times by preparing new batches of treated and untreated grains for each replicate (10 × 1 × 10 × 5 = 500 Petri dishes for each replicate × dose × fungal strains × insect species).
2.4. Data Analysis
All values were arcsine transformed before analysis. Mortality data were subjected to a two-way analysis of variance (ANOVA) to evaluate the main effects and interactions of fungal isolate and exposure time on insect mortality. In the case of significant F values, means were compared using the Bonferroni test. The median lethal time (LT50) of tested adults was calculated by probit analysis with a 95% confidence interval (CI). All statistical tests were performed using SPSS (SPSS, Inc., Chicago, IL, USA, version 23).
3. Results
The fungal species recovered from the soil and tested for pathogenicity belonged to the genera Cladosporium, Condenascus, Lecanicillium, and Penicillium (Table 1).

Table 1.
Isolates of several EPF species that were tested in the present study. All collected fungal isolates were lab cultured and stored at 25 °C in SDA plates.
Mycelial and conidial growth on cadavers suggested that almost all deaths were due to a pathogen. Observation of the cadavers showed that external mycelium appeared within the first 72 h after they were placed on moist filter paper.
The average mortality (%) of adult beetles experimentally exposed to EPF in the present study is shown in Figure 1. After 7 days of exposure, the number of dead insects was relatively low (<50%), but then increased significantly and exceeded 80% on day 21 of the experiment (Figure 1).
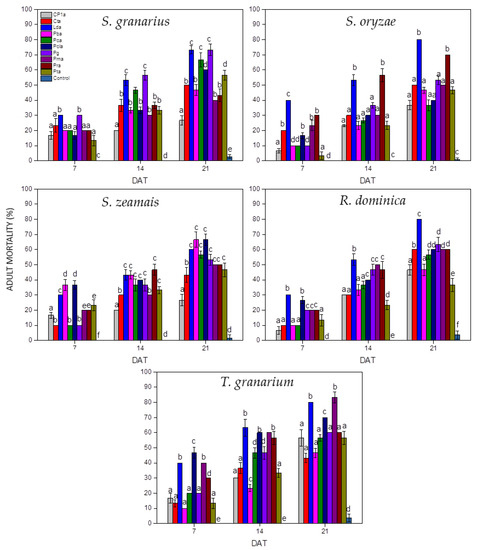
Figure 1.
Mean mortalities (%) of experimental adult beetles treated with EPF in the present study (grains were sprayed with conidial suspension at 108 conidia/mL DAT: days after treatment, columns of the same DAT marked with the same letter did not differ significantly, error bars represent standard deviation of the mean).
Probit analysis was used to estimate the median lethal time LT50. The probit mortality regression data along with the confidence limits (CL) and other estimated probit parameters are presented in Table 2. These data indicate that S. zeamais and T. granarium were the most resistant and susceptible to EPF isolates, respectively. However, the differences in LT50 among various beetle species and EPF isolates were insignificant (Table 2).

Table 2.
Lethal time (LT50) and associated probit parameters of tested adults treated with conidial suspension at 108 conidia/mL by several species of entomopathogenic fungi for a period of 7, 14, and 21 days.
The main effects and interaction of fungal isolate and exposure time on beetle mortality proved to be significant in all cases (p < 0.001) (Table 3).

Table 3.
Two-way ANOVA results for main effects and interactions for adult mortality of stored product beetle pests exposed to EPF.
4. Discussion
As mentioned earlier, eliminating the use of chemical pesticides and replacing them with alternative methods of pest control in storage is of great importance. The use of fungal strains as biopesticides is not a new approach. Many studies have demonstrated their effectiveness against destructive pests. There is extensive literature that provides results supporting the use of entomopathogenic fungi as a means of integrated pest management in storage facilities [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
Kavallieratos et al. tested the effect of M. anisopliae on adult S. oryzae, resulting in critical mortality at doses of 1.77 × 107 and 1.77 × 108 conidia/mL [24]. Khashaveh et al. reported that B. bassiana can be successfully used to control storage pests in wheat [26]. Wakefield et al. achieved 100% mortality of Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) after 10 days of treatment with a dose of 108 conidia/mL of some B. bassiana isolates [27]. Wakil et al. examined several isolates of B. bassiana and M. anisopliae against R. dominica, S. granarius, T. castaneum, and T. granarium, and found that the first one was the most susceptible, whereas the last one was the most resistant to fungal infection [28]. Finally, Batta treated newly emerged adults R. dominica with M. anisopliae conidia formulated in wheat flour (86.7%) and inverted emulsion (93.3%) and found high mortality after 7 days [29]. An analytical list with data from relevant lab bioassays is provided by Rumbos and Athanassiou [15].
There have been very few field studies to assess the effectiveness of EPF in storage facilities, despite the abundance of laboratory data that is accumulated from numerous investigations. Stathers provided information from research projects carried out in Africa with contradictory conclusions [30]. In a field trial, stored maize was treated with B. bassiana for protection against P. truncatus; however, despite the pest densities being substantially lower in treated grains, the losses were significant [31]. Beauveria bassiana combined with electrostatic powder was tested as a surface treatment in an empty store, and this produced a sufficient level of control against O. surinamensis [32]. Combined application of B. bassiana with synthetic insecticides to wheat stored in polypropylene bags provided satisfactory protection for 30 days but after 180 days, grain damage was above acceptable levels despite significantly increased pest mortality [33,34].
For this experiment, ten different fungal species from soil were evaluated for long-term entomopathogenicity in five important storage beetles. All isolates caused significantly higher mortality in treated beetles compared to the control. Most of the species found and tested in this study have not been previously examined against insects.
The genus Lecanicillium is known for its entomopathogenic properties and infects a wide range of insects [35,36,37,38,39,40,41,42,43,44]. Strains of Lecanicillium (Verticillium) lecanii Zimmermann are not only commercially available to control severe agricultural pests [36], but have also been shown to be effective against storage pests such as S. oryzae [37], S. zeamais [38], and the red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) [39]. Extracts containing secondary metabolites from L. attenuatum exhibited the highest insecticidal activity against the Asian tiger mosquito Aedes albopictus (Skuse) (Diptera: Culicidae) and the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) [40]. Larvae of the latter species fed with cabbage leaves sprayed with isolates of L. muscarium recorded mortality greater than 80% [41]. The same Lecanicillium species caused high mortality in the silverleaf whitefly Bemisia tabaci (Gennadius) and the greenhouse whitefly Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) under both controlled laboratory and glasshouse conditions [42,43]. In addition, L. longisporum succeeded in controlling populations of the green peach aphid Myzus persicae (Sulzer) (Hemiptera: Aphididae [44].
Although many Lecanicillium species are known for their insecticidal action, L. dimorphum, the species that had the highest mortality in this study, has been poorly evaluated for its entomopathogenic activity in only a few studies. It has been reported to colonize Phoenicococcus marlatti Cockerell (Hemiptera: Phoenicococcidae) resulting in 100% parasitism [45], while it caused 56.5% mortality in nymphs of M. persicae after 7 days [44]. On the other hand, it caused significant fecundity reduction in the predatory minute pirate bug Orius laevigatus (Fieber) (Hemiptera: Anthocoridae) an important and widely distributed biocontrol agent [46]. The present study enriches our knowledge about the potential of this EPF species as the pest control agent.
The genus Cladosporium is also known for its insecticidal potential against whiteflies [47], spidermites [48], moths [49], and aphids [47,50], but our report is the first to investigate C. puyae, and although its performance was not as successful as other strains, possible virulence to other insects cannot be excluded. The same is true for Condenascus tortuosus.
In our study, seven different Penicillium species were evaluated for their efficacy against beetles. Some species of the genus Penicillium are known for their entomopathogenic activity but most of them are not thoroughly studied. Extracts from Penicillium sp. were effective for the control of the tobacco cutworm Spodoptera litura (F.) (Lepidoptera: Noctuidae) and the southern house mosquito Culex quinquefasciatus Say (Diptera: Culicidae) larvae [51] and caused significant mortality levels in the confused flour beetle Tribolium confusum Jacquelin Du Val (Coleoptera: Tenebrionidae) [52]. Moreover, Da Costa et al. investigated the efficacy of Penicillium sp. against various mosquitoes, and mortality rates ranged from 0 to 100%, depending on the concentration of conidia [53]. Penicillium citrinum and P. chrysogenum have also been reported to have insect-pathogenic properties, and P. chrysogenum in particular is pathogenic to the African malaria mosquito Anopheles gambiae Giles (Diptera: Culicidae) [54]. In a study conducted on C. quinquefasciatus larvae, strain P. citrinum CM-010 was by far more effective than strains of M. anisopliae and B. bassiana [55]. In another study, P. citrinum CTD-24 caused the highest cumulative mortality in eggs and neonates of the fall armyworm Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) [56].
Due to several constraints, including unpredictable environmental conditions, probable chemical residues, the presence of other species, and the implementation of other control measures, the practical application of EPF against storage pests is hindered [27,30]. Basically, the greatest obstacle is the hostile dry environment that makes it difficult for fungi to survive. However, advancements in formulation technology may turn EPF into a useful IPM tool for stored product protection. Additionally, it should be noted that an EPF-based biopesticide may exhibit greater persistence than a chemical pesticide since the entomopathogen may cycle on a dead insect body (cadaver), reintroducing further inoculum into the ecosystem. This internal sporulation may occur even in the dry storage environment [30].
5. Conclusions
Apart from the pathogenic activity of the tested fungal species on insects, our study once again confirmed that the efficacy of a given fungal species is always strain-specific and virulence may vary depending on the host. Successful infection and germination depend on both biotic and abiotic factors, such as host susceptibility, host life stage, length of the incubation period, temperature, and humidity [57,58]. From the above literature and our results, it appears that a particular EPF species may be virulent against a particular host while having little to no effect on others. For this reason, extensive screening should be performed to determine the level of virulence acceptable for further development of a fungus-based formulation.
The use of entomopathogenic fungi to control stored-product pests is a promising IPM tool. Despite multiple reports of effective laboratory tests using EPF on storage pests, this has not yet been successfully translated into practice. This would necessitate further development of conidia formulation and a thorough investigation into whether internal sporulation is taking place in infected insects in the store [30]. For EPF to be used as part of an IPM approach, research must be conducted under as realistic field conditions as possible. Knowledge of their effectiveness when paired with other storage IPM strategies (such as insecticides, heat or cooling treatment, other biocontrol agents, inert dust, modified atmospheres, etc.) is also crucial. Apart from that, further experimentation should be conducted to isolate new strains and thoroughly investigate the level of virulence and specificity. Characterization of new species using molecular tools and DNA sequencing can contribute to a better understanding of these organisms by clarifying taxonomic relationships and mechanisms involved in pathogenicity.
Author Contributions
Conceptualization, S.M. and I.L.; methodology, S.M.; software, S.M.; validation, S.M., I.L. and P.A.E.; formal analysis, S.M.; investigation, S.M.; resources, S.M.; data curation, S.M.; writing—original draft preparation, S.M., I.L., F.K. and P.A.E.; writing—review and editing, S.M., I.L., F.K. and P.A.E.; visualization, S.M.; supervision, S.M.; project administration, P.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harein, P.; Meronuck, R. Stored grain losses due to insects and molds and the importance of proper grain management. In Stored Product Management; Krischik, V., Cuperus, G., Galliart, D., Eds.; Oklahoma State University: Stillwater, OK, USA, 1995; pp. 29–31. [Google Scholar]
- Pathipati, U.R.; Kanuparthi, P.L. Silver Nanoparticles for Insect Control: Bioassays and Mechanisms. In Silver Nanomaterials for Agri-Food Applications; Abd-Elsalam, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 471–494. [Google Scholar] [CrossRef]
- Jakubas-Zawalska, J.; Asman, M.; Kłyś, M.; Solarz, K. Sensitization to Sitophilus granarius in Selected Suburban Population of South Poland. J. Stored. Prod. Res. 2016, 69, 1–6. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, H.N. Ecofriendly Nonchemical/Nonthermal Methods for Disinfestation and Control of Pest/Fungal Infestation during Storage of Major Important Cereal Grains: A Review. Food Front. 2021, 2, 93–105. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the Fumigant Phosphine and Its Management in Insect Pests of Stored Products: A Global Perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Harush, A.; Quinn, E.; Trostanetsky, A.; Rapaport, A.; Kostyukovsky, M.; Gottlieb, D. Integrated Pest Management for Stored Grain: Potential Natural Biological Control by a Parasitoid Wasp Community. Insects 2021, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Biopesticide: An Environment Friendly Pest Management Strategy. J. Biofertil. Biopestic. 2015, 6, 127. [Google Scholar] [CrossRef]
- Butt, T.M.; Jackson, C.; Magan, N. Fungi as Biocontrol Agents. Progress, Problems and Potential; CABI Publishing: Wallingford, UK, 2001; 390p. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Mark Tatchell, G.; Davidson, G.; Greaves, J.; Grant, W.P. The Development, Regulation and Use of Biopesticides for Integrated Pest Management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Hunter, D.M. Mycopesticides as Part of Integrated Pest Management of Locusts and Grasshoppers. J. Orthoptera Res. 2005, 14, 197–201. [Google Scholar] [CrossRef]
- Manivel, S.B.; Rajkumar, G.S. Mycopesticides: Fungal Based Pesticides for Sustainable Agriculture. In Fungi and Their Role in Sustainable Development: Current Perspective; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 183–203. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, J.; Ning, J.; Qin, P.; Zhou, J.; Zou, Z.; Wang, Y.; Jiang, H.; Ahmad, F.; Zhao, L.; et al. Differential Immune Responses of Monochamus alternatus against Symbiotic and Entomopathogenic Fungi. Sci. China Life Sci. 2017, 60, 902–910. [Google Scholar] [CrossRef]
- Batta, Y.A. Recent Advances in Formulation and Application of Entomopathogenic Fungi for Biocontrol of Stored-Grain. Insects 2016, 26, 1171–1183. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. Use of Entomopathogenic Fungi for the Control of Stored-Product Insects: Can Fungi Protect Durable Commodities? J. Pest Sci. 2017, 90, 839–854. [Google Scholar] [CrossRef]
- Schöller, M.E.; Flinn, P.W.; Grieshop, M.J.; Zdárková, E. Biological control of stored product pests. In Insect Management for Food Storage and Processing, 2nd ed.; Heaps, J.W., Ed.; AACC International: Eagan, MN, USA, 2006; pp. 67–87. [Google Scholar]
- Subramanyam, B.; Hagstrum, D.W. Alternatives to Pesticides in Stored-Product IPM; Kluwer Academic Publishers: Boston, MA, USA, 2000; 437p. [Google Scholar]
- Phillips, T.W.; Throne, J.E. Biorational Approaches to Managing Stored-Product Insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef]
- Rice, W.C.; Cogburn, R.R. Activity of the Entomopathogenic Fungus Beauveria bassiana (Deuteromycota: Hyphomycetes) against Three Coleopteran Pests of Stored Grain. J. Econ. Entomol. 1999, 92, 691–694. [Google Scholar] [CrossRef]
- Lord, J.C. Desiccant Dusts Synergize the Effect of Beauveria bassiana (Hyphomycetes: Moniliales) on Stored-Grain Beetles. J. Econ. Entomol. 2001, 94, 367–372. [Google Scholar] [CrossRef]
- Batta, Y.A.; Abu Safieh, D.I. A study of treatment effect with Metarhizium anisopliae and four types of dusts on wheat grain infestation with red flour beetles (Tribolium castaneum Herbs, Coleoptera: Tenebrionidae). Islam. Univer. Gaza J. 2005, 13, 11–22. [Google Scholar]
- Athanassiou, C.G.; Steenberg, T. Insecticidal Effect of Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreaes) in Combination with Three Diatomaceous Earth Formulations against Sitophilus granarius (L.) (Coleoptera: Curculionidae). Biol. Control 2007, 40, 411–416. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A Phylogenetically-Based Nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Aountala, M.M.; Kontodimas, D.C. Evaluation of the Entomopathogenic Fungi Beauveria bassiana, Metarhizium anisopliae, and Isaria fumosorosea for Control of Sitophilus oryzae. J. Food Prot. 2014, 77, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Zikou, A.; Triantafillou, V.; Lagogiannis, I.; Eliopoulos, P.A. Interactions between Beauveria bassiana and Isaria fumosorosea and Their Hosts Sitophilus granarius (L.) and Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Insects 2019, 10, 362. [Google Scholar] [CrossRef]
- Khashaveh, A.; Ghosta, Y.; Safaralizadeh, M.H.; Ziaee, M. The Use of Entomopathogenic Fungus, Beauveria bassiana (Bals.) Vuill. in Assays with Storage Grain Beetles. J. Agr. Sci. Technol. 2011, 13, 35–43. [Google Scholar]
- Wakefield, M.E.; Cox, P.D.; Moore, D.; De Muro, M.A.; Bell, B.A. Mycopest: Results and perspectives. In Proceedings of the VI meeting of COST Action, Working Group IV, Biocontrol of Arthropod Pests in Stored Products, Locorotondo, Italy, 23–29 June 2005; Volume 842, pp. 17–27. [Google Scholar]
- Wakil, W.; Kavallieratos, N.G.; Ghazanfar, M.U.; Usman, M.; Habib, A.; El-Shafie, H.A. Efficacy of different entomopathogenic fungal isolates against four key stored-grain beetle species. J. Stored Prod. Res. 2021, 93, 101845. [Google Scholar] [CrossRef]
- Batta, Y.A. Control of the Lesser Grain Borer (Rhyzopertha dominica (F.), Coleoptera: Bostrichidae) by Treatments with Residual Formulations of Metarhizium anisopliae (Metschnikoff) Sorokin (Deuteromycotina: Hyphomycetes). J. Stored Prod. Res. 2005, 41, 221–229. [Google Scholar] [CrossRef]
- Stathers, T. Entomopathogenic fungi in grain storage-any lessons for Europe from elsewhere? In Proceedings of the VI meeting of COST Action, Working Group IV, Biocontrol of Arthropod Pests in Stored Products, Locorotondo, Italy, 23–29 June 2005; Volume 842, pp. 100–109. [Google Scholar]
- Meikle, W.G.; Cherry, A.J.; Holst, N.; Hounna, B.; Markham, R.H. The effects of an entomopathogenic fungus, Beauveria bassiana (Balsamo) Vuillemin (Hyphomycetes), on Prostephanus truncatus (Horn) (Col.: Bostrichidae), Sitophilus zeamais Motschulsky (Col.: Curculionidae), and grain losses in stored maize in the Benin Republic. J. Invertebr. Pathol. 2001, 77, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Rumbos, C.I.; Sakka, M.; Potin, O.; Storm, C.; Dillon, A.B. Delivering Beauveria bassiana with electrostatic powder for the control of stored-product beetles. Pest Manag. Sci. 2017, 73, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Wakil, W.; Kavallieratos, N.G.; Nika, E.P.; Qayyum, M.A.; Yaseen, T.; Ghazanfar, M.U.; Yasin, M. Combinations of Beauveria bassiana and spinetoram for the management of four important stored-product pests: Laboratory and field trials. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Wakil, W.; Kavallieratos, N.G.; Ghazanfar, M.U.; Usman, M. Laboratory and field studies on the combined application of Beauveria bassiana and fipronil against four major stored-product coleopteran insect pests. Environ. Sci. Pollut. Res. 2022, 29, 34912–34929. [Google Scholar] [CrossRef]
- Broumandnia, F.; Rajabpour, A. Efficacies of Some Isolates of Lecanicillium lecanii to Control Tribolium castaneum (Col., Tenebrionidae). J. Plant Dis. Prot. 2020, 127, 625–631. [Google Scholar] [CrossRef]
- Arakere, U.C.; Jagannath, S.; Krishnamurthy, S.; Chowdappa, S.; Konappa, N. Microbial Bio-Pesticide as Sustainable Solution for Management of Pests: Achievements and Prospects. In Biopesticides: Volume 2: Advances in Bio-Inoculants; Rakshit, A., Singh Meena, V., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Kumar Singh, A., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2022; pp. 183–200. [Google Scholar] [CrossRef]
- Dal Bello, G.; Padin, S.; López Lastra, C.; Fabrizio, M. Laboratory Evaluation of Chemical-Biological Control of the Rice Weevil (Sitophilus oryzae L.) in Stored Grains. J. Stored Prod. Res. 2000, 37, 77–84. [Google Scholar] [CrossRef]
- Ahmed, B.I. Potentials of Entomopathogenic Fungi in Controlling the Menace of Maize Weevil Sitophilus zeamais Motsch (Coleoptera: Curculionidae) on Stored Maize Grain. Arch. Phytopath. Plant Prot. 2010, 43, 107–115. [Google Scholar] [CrossRef]
- Sabbour, M.M. Efficacy of Some Microbial Control Agents and Inorganic Insecticides against Red Flour Beetle Tribolium castaneum and Confused Flour Beetle, Tribolium confusum (Coleoptera: Tenebrionidae) Integrated Protection of Stored Products. IOBC-WPRS Bull. 2014, 98, 193–201. [Google Scholar]
- Woo, R.M.; Park, M.G.; Choi, J.Y.; Park, D.H.; Kim, J.Y.; Wang, M.; Kim, H.J.; Woo, S.D.; Kim, J.S.; Je, Y.H. Insecticidal and Insect Growth Regulatory Activities of Secondary Metabolites from Entomopathogenic Fungi, Lecanicillium attenuatum. J. Appl. Entomol. 2020, 144, 655–663. [Google Scholar] [CrossRef]
- Kuchár, M.; Glare, T.R.; Hampton, J.G.; Dickie, I.A.; Christey, M.C. Virulence of the Plant-Associated Endophytic Fungus Lecanicillium muscarium to Diamondback Moth Larvae. N. Z. Plant Prot. 2019, 72, 253–259. [Google Scholar] [CrossRef]
- Moyo, D.; Ishikura, S.; Rakotondrafara, A.; Clayton, M.; Kinoshita, R.; Tani, M.; Koike, M.; Aiuchi, D. Behavioral Change of Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) Infected by Lecanicillium muscarium (Hypocreales: Cordycipitaceae). Appl. Entomol. Zool. 2021, 56, 327–336. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Walters, K.F.A. Pathogenicity of the Entomopathogenic Fungus, Lecanicillium muscarium, against the sweetpotato whitefly Bemisia tabaci under Laboratory and Glasshouse Conditions. Mycopathologia 2005, 160, 315–319. [Google Scholar] [CrossRef]
- Mitina, G.V.; Stepanycheva, E.A.; Choglokova, A.A.; Cherepanova, M.A. Features of Behavioral Reactions of the Peach Aphid Myzus persicae (Sulzer, 1776) (Hemiptera, Aphididae) to Volatile Organic Compounds of Entomopathogenic Fungi of the Genus Lecanicillium. Entomol. Rev. 2021, 101, 1015–1023. [Google Scholar] [CrossRef]
- Asensio, L.; Lopez-Llorca, L.V.; López-Jiménez, J.A. Use of Light, Scanning Electron Microscopy and Bioassays to Evaluate Parasitism by Entomopathogenic Fungi of the Red Scale Insect of Palms (Phoenicococcus marlatti Ckll., 1899). Micron 2005, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Pazyuk, I.; Choglokova, A.; Mitina, G. Effect of Entomopathogenic Fungi of the Genus Lecanicillium on Behavioral Reactions and Average Per-Day Fecundity of the Predatory Bug Orius laevigatus Fieber (Heteroptera, Anthocoridae). BIO Web. Conf. 2022, 43, 02003. [Google Scholar] [CrossRef]
- Abdel-Baky, N.F. Cladosporium Spp. An Entomopathogenic Fungus for Controlling Whiteflies and Aphids in Egypt. Pak. J. Biol. Sci. 2000, 3, 1662–1667. [Google Scholar] [CrossRef]
- Eken, C.; Hayat, R. Preliminary Evaluation of Cladosporium cladosporioides (Fresen.) de Vries in Laboratory Conditions, as a Potential Candidate for Biocontrol of Tetranychus urticae Koch. World J. Microbiol. Biotechnol. 2009, 25, 489–492. [Google Scholar] [CrossRef]
- Habibullah Bahar, M.; Backhouse, D.; Gregg, P.C.; Mensah, R. Biocontrol Science and Technology Efficacy of a Cladosporium sp. Fungus against Helicoverpa armigera (Lepidoptera: Noctuidae), Other Insect Pests and Beneficial Insects of Cotton. Biocontrol. Sci. Technol. 2011, 21, 1387–1397. [Google Scholar] [CrossRef]
- Shaker, N.; Ahmed, G.M.M.; Ibrahim, H.; El-sawy, M.M.; Mostafa, M.; Ismail, H. Secondary Metabolites of the Entomopathogenic Fungus, Cladosporium cladosporioides and Its Relation to Toxicity of Cotton Aphid, Aphis gossypii (Glov.). Int. J. 2019, 5, 115–120. [Google Scholar]
- Arunthirumeni, M.; Vinitha, G.; Shivakumar, M.S. Antifeedant and Larvicidal Activity of Bioactive Compounds Isolated from Entomopathogenic Fungi Penicillium sp. for the Control of Agricultural and Medically Important Insect Pest (Spodoptera litura and Culex quinquefasciatus). Parasitol. Int. 2023, 92, 102688. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Al-Keridis, L. Application of Penicillium sp. as Entomopathogenic Fungi to Control the Red Rust Beetle Tribolium castaneum (Hbst.) (Coleoptera:Tenebrionidae). Biosci. Biotechnol. Res. Asia 2015, 12, 7–12. [Google Scholar] [CrossRef]
- da Costa, G.L.; de Oliveira, P.C. Penicillium Species in Mosquitoes from Two Brazilian Regions. J. Basic Microbiol. 1998, 38, 343–347. [Google Scholar] [CrossRef]
- Malassigné, S.; Moro, C.V.; Luis, P. Mosquito Mycobiota: An Overview of Non-Entomopathogenic Fungal Interactions. Pathogens 2020, 9, 564. [Google Scholar] [CrossRef]
- Maketon, M.; Amnuaykanjanasin, A.; Kaysorngup, A. A Rapid Knockdown Effect of Penicillium citrinum for Control of the Mosquito Culex quinquefasciatus in Thailand. World J. Microbiol. Biotechnol. 2014, 30, 727–736. [Google Scholar] [CrossRef]
- Idrees, A.; Qadir, Z.A.; Akutse, K.S.; Afzal, A.; Hussain, M.; Islam, W.; Waqas, M.S.; Bamisile, B.S.; Li, J. Effectiveness of Entomopathogenic Fungi on Immature Stages and Feeding Performance of Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae. Insects 2021, 12, 1044. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G. Biocontrol Science and Technology Review on Safety of the Entomopathogenic Fungi Beauveria Bassiana and Beauveria Brongniartii. Biocontrol. Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Kitsiou, F.; Natsiopoulos, D.; Eliopoulos, P.A. Entomopathogenic Fungi: Interactions and Applications. Encyclopedia 2022, 2, 646–656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).