Synthesis of Piperine-Based Ester Derivatives with Diverse Aromatic Rings and Their Agricultural Bioactivities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot, and Eriosoma lanigerum Hausmann
Abstract
Simple Summary
Abstract
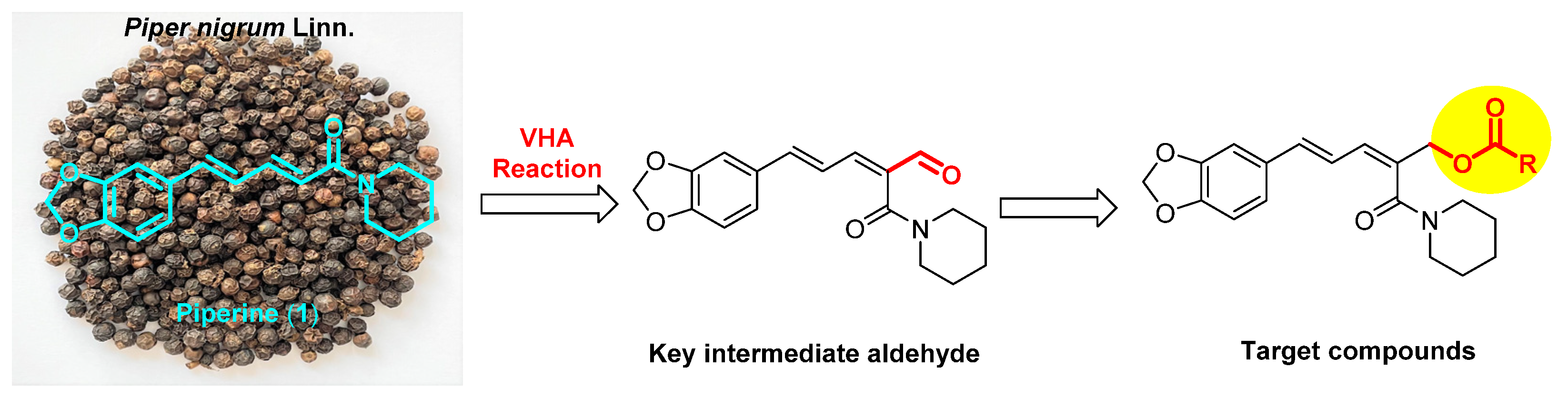
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Reagents and Instruments
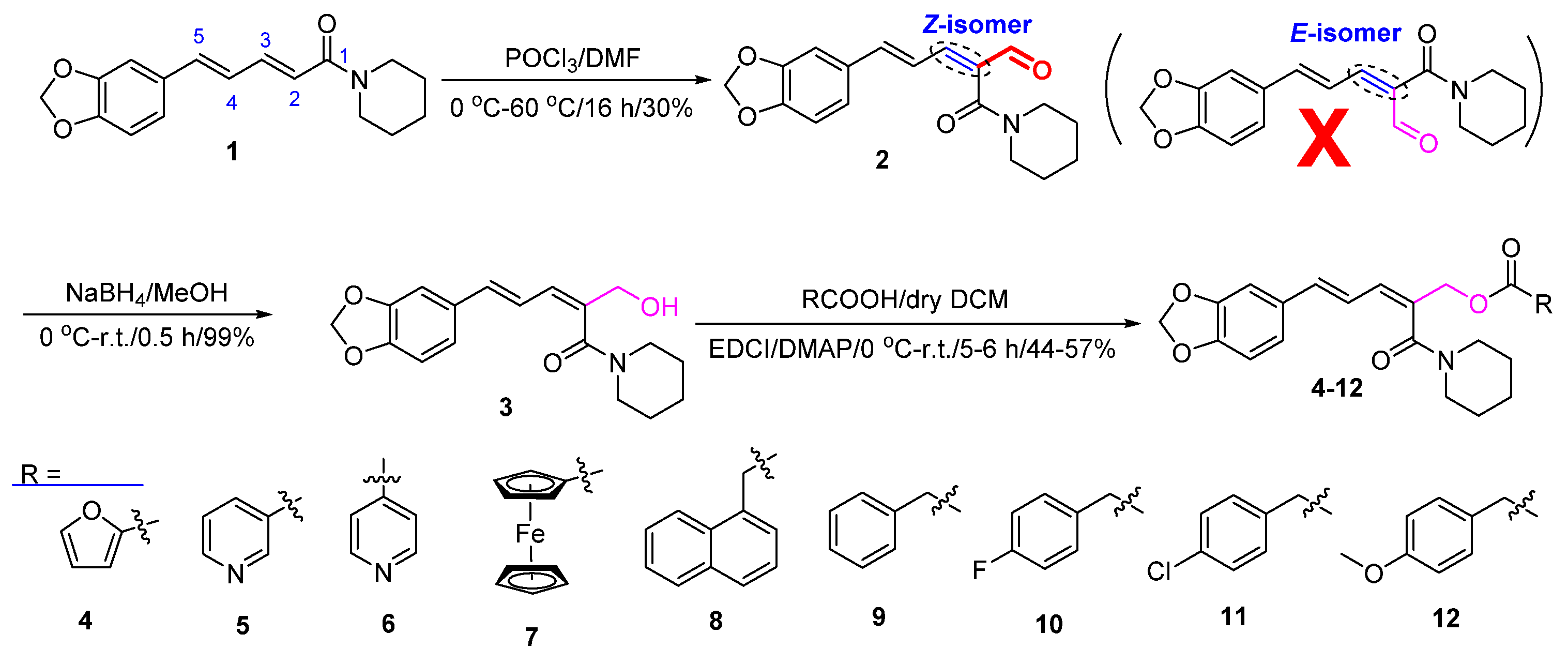
2.3. General Procedure for the Synthesis of Aldehyde 2
- Data for 2: Yield: 30%; yellow solid; mp 184–186 °C; IR cm–1 (KBr): 3052, 2937, 2858, 2733, 1678, 1607, 1445, 1256, 1188, 812, 707; 1H NMR (500 MHz, CDCl3) δ: 9.49 (s, 1H, –CHO), 7.15 (d, J = 11.0 Hz, 1H), 6.97–7.03 (m, 3H), 6.80–6.89 (m, 2H), 6.01 (s, 2H, –OCH2O–), 3.74 (s, 2H, –NCH2–), 3.26 (t, J = 6.0 Hz, 2H, –NCH2–), 1.66 (s, 4H), 1.54 (s, 2H); 13C NMR (125 MHz, CDCl3) δ: 189.7, 163.8, 149.6, 149.4, 148.4, 144.5, 137.4, 129.9, 124.4, 121.5, 108.6, 106.3, 101.6, 47.9, 42.5, 26.6, 25.8, 24.4. HRMS [ESI]: calcd for C18H19NO4Na ([M + Na]+), 336.1206; found, 336.1206.
2.4. General Procedure for the Synthesis of Compound 3
- Data for3: Yield: 99%; yellow solid; mp 123–125 °C; IR cm–1 (KBr): 3270, 2939, 2866, 1618, 1441, 1249, 1190, 811, 719; 1H NMR (500 MHz, CDCl3) δ: 6.87 (d, J = 0.5 Hz, 1H), 6.77–6.79 (m, 1H), 6.74 (d, J = 8.0 Hz, 1H), 6.48–6.55 (m, 2H), 6.32–6.34 (m, 1H), 5.95 (s, 2H, –OCH2O–), 4.30 (s, 2H, –CH2OH), 3.67–3.69 (m, 2H, –NCH2–), 3.45 (t, J = 5.5 Hz, 2H, –NCH2–), 1.63–1.64 (m, 4H), 1.52 (t, J = 5.5 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ: 168.8, 148.1, 147.6, 136.8, 134.9, 131.2, 127.4, 122.2, 121.8, 108.4, 105.5, 101.2, 63.9, 47.8, 42.4, 26.6, 25.8, 24.4. HRMS [ESI]: calcd for C18H21NO4Na ([M + Na]+), 338.1363; found, 338.1354.
2.5. General Procedure for the Synthesis of Target Compounds 4–12
- Data for 4: Yield: 53%; yellow solid; mp 106–107 °C; IR cm–1 (KBr): 3429, 3051, 2932, 2353, 1723, 1588, 1484, 1447, 1241, 1183, 1029, 958, 764, 598; 1H NMR (400 MHz, CDCl3) δ: 7.58 (d, J = 0.8 Hz, 1H), 7.19 (d, J = 2.8 Hz, 1H), 6.90 (d, J = 1.2 Hz, 1H), 6.82–6.84 (m, 1H), 6.77 (d, J = 8.0 Hz, 1H), 6.51–6.60 (m, 3H), 6.46 (d, J = 10.4 Hz, 1H), 5.97 (s, 2H), 5.04 (s, 2H), 3.71 (s, 2H), 3.46 (t, J = 5.2 Hz, 2H), 1.64 (s, 4H), 1.48 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 167.3, 158.2, 148.2, 148.0, 146.5, 144.3, 136.7, 131.7, 130.9, 130.5, 122.2, 121.8, 118.2, 111.9, 108.5, 105.5, 101.2, 66.5, 47.8, 42.5, 26.5, 25.8, 24.5.
- Data for 5: Yield: 53%; yellow oil; IR cm–1 (KBr): 3399, 3307, 3075, 2373, 1723, 1579, 1491, 1450, 1368, 1284, 1121, 1026, 931, 741, 701, 605; 1H NMR (400 MHz, CDCl3) δ: 9.21 (d, J = 1.6 Hz, 1H), 8.79 (dd, J1 = 4.8 Hz, J2 = 1.6 Hz, 1H), 8.32 (dt, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H), 7.43 (dd, J1 = 8.0 Hz, J2 = 5.2 Hz, 1H), 6.91 (d, J = 1.2 Hz, 1H), 6.83–6.85 (m, 1H), 6.78 (d, J = 8.0 Hz, 1H), 6.53–6.66 (m, 2H), 6.50 (d, J = 10.4 Hz, 1H), 5.97 (s, 2H), 5.11 (s, 2H), 3.72 (s, 2H), 3.47 (t, J = 5.2 Hz, 1H), 1.64 (s, 4H), 1.46 (s, 2H); 13C NMR (100 MHz, CDCl3) δ: 167.2, 164.8, 153.5, 150.7, 148.2, 148.1, 137.2, 137.0, 132.0, 130.7, 130.2, 125.9, 123.4, 122.3, 121.7, 108.5, 105.5, 101.3, 67.2, 47.7, 42.4, 26.6, 25.8, 24.4.
2.6. Biological Assay
2.6.1. Acaricidal Activity against T. cinnabarinus
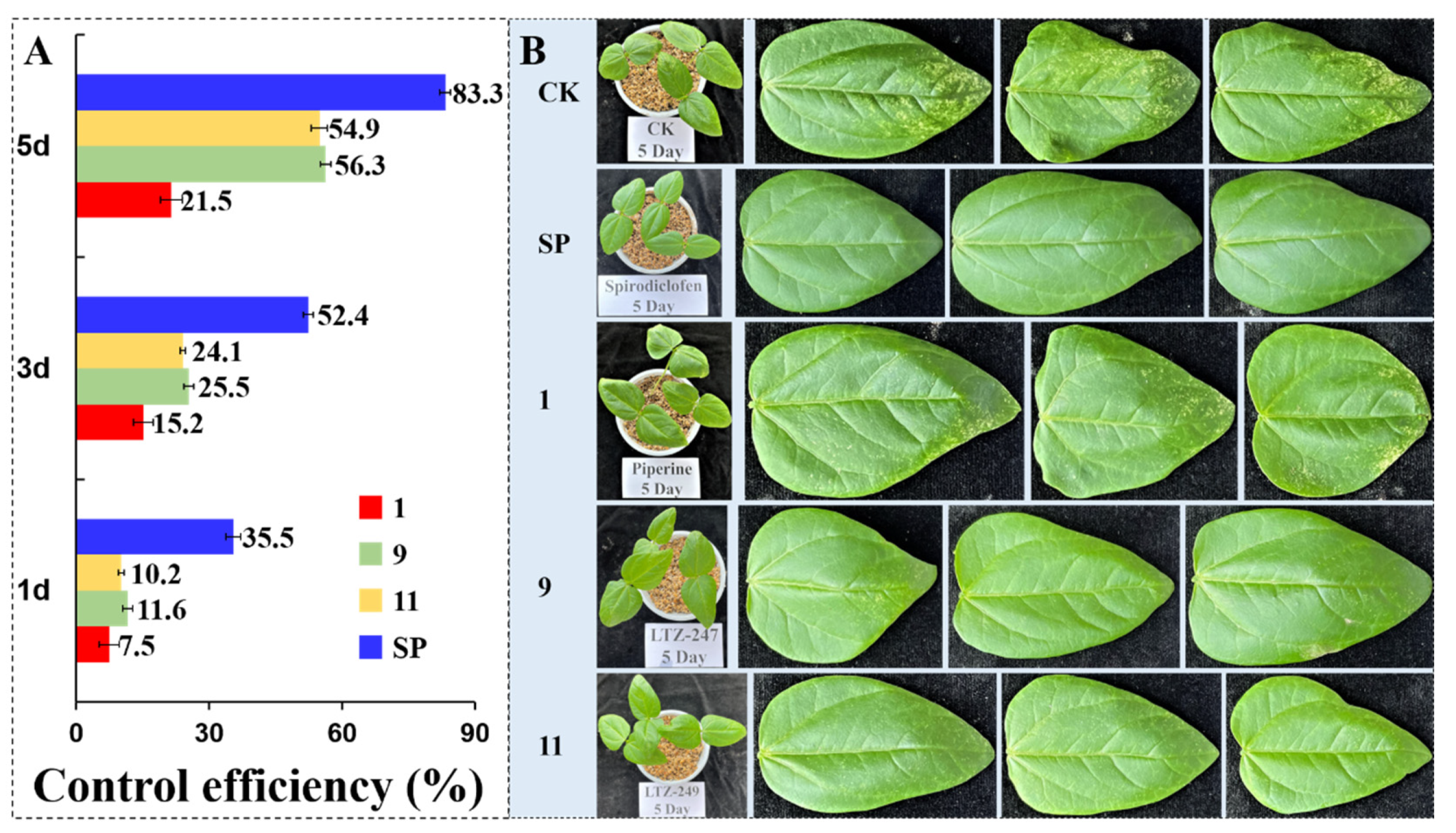
2.6.2. Control Efficiency of Some Compounds against T. cinnabarinus in the Greenhouse
2.6.3. Aphicidal Activity against A. citricola
2.6.4. Aphicidal Activity against E. lanigerum
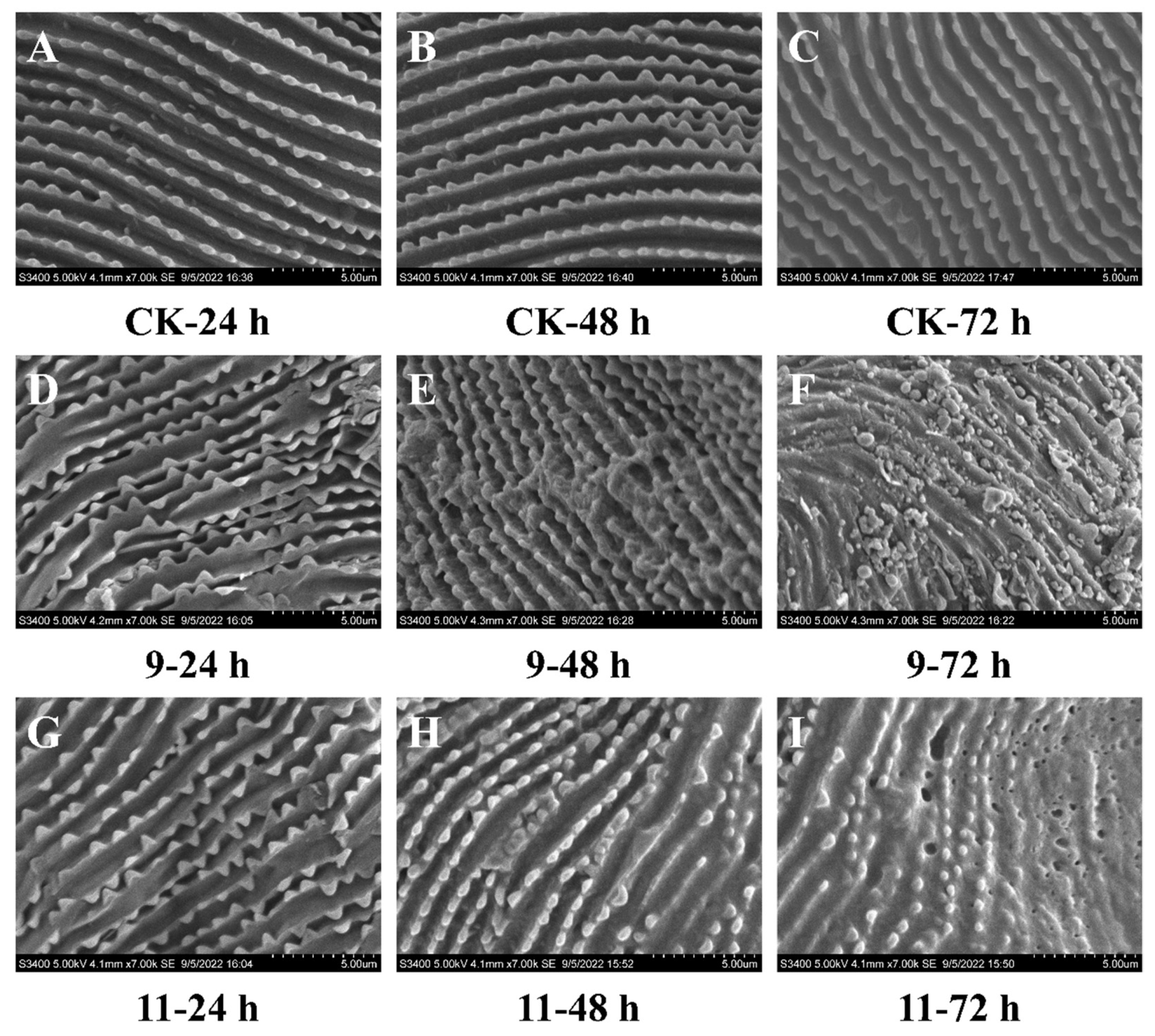
2.6.5. The Effects of Piperine Derivatives 9 and 11 on the Features of Mite Cuticles
2.7. Statistical Analysis
3. Results and Discussion
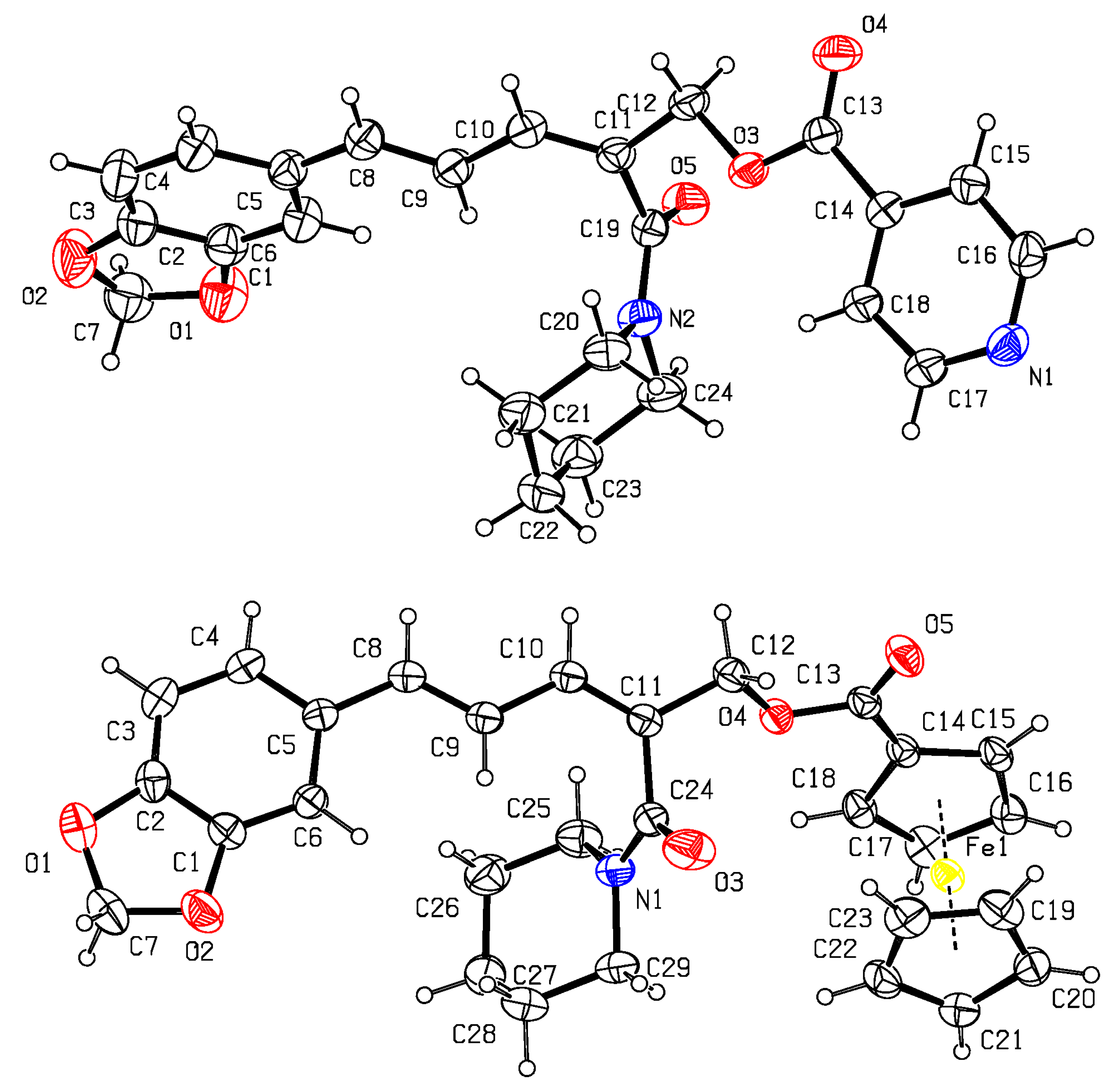
3.1. Synthesis
3.2. Pesticidal Activities
3.2.1. Acaricidal Activity against T. cinnabarinus
3.2.2. Aphicidal Activity against A. citricola
3.2.3. Aphicidal Activity against E. lanigerum
3.2.4. The Effects on the Features of Mite Cuticles by SEM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, B.J.; Zhang, X.L.; Bai, X.M.; Fu, B.J.; Chen, D.L. Four steps to food security for swelling cities. Nature 2019, 566, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Hilliou, F.; Chertemps, T.; Maibeche, M.; Le Goff, G. Resistance in the genus Spodoptera: Key insect detoxification genes. Insects 2021, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lv, M.; Ma, Q.; Zhang, Y.; Xu, H. High value–added application of natural products in crop protection: Semisynthesis and acaricidal activity of limonoid–type derivatives and investigation of their biocompatible O/W nanoemulsions as agronanopesticide candidates. J. Agric. Food Chem. 2021, 69, 14488–14500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.Q.; Lai, T.; Liu, X.J.; Guo, F.Y.; Guo, T.; Ding, W. Acaricidal mechanism of scopoletin against Tetranychus cinnabarinus. Front. Physiol. 2019, 10, 164. [Google Scholar] [CrossRef]
- Scott, I.M.; McDowell, T.; Renaud, J.B.; Krolikowski, S.W.; Chen, L.; Dhaubhadel, S. Investigation of metabolic resistance to soybean aphid (Aphis glycines Matsumura) feeding in soybean cultivars. Insects 2022, 13, 356. [Google Scholar] [CrossRef]
- Barczak, T.; Bennewicz, J.; Korczynski, M.; Blazejewicz-Zawadzinska, M.; Piekarska-Boniecka, H. Aphid assemblages associated with urban park plant communities. Insects 2021, 12, 173. [Google Scholar] [CrossRef]
- O’Hara, F.M.; Davis, J.A.; Swale, D.R. Profile of commercialized aphicides on the survivorship and feeding behavior of the cotton aphid, Aphis gossypii. Pestic. Biochem. Physiol. 2022, 186, 105174. [Google Scholar] [CrossRef]
- Smirnova, E.; Firth, A.E.; Miller, W.A.; Scheidecker, D.; Brault, V.; Reinbold, C.; Rakotondrafara, A.M.; Chung, B.Y.W.; Ziegler–Graff, V. Discovery of a small non–AUG–initiated ORF in poleroviruses and luteoviruses that is required for long–distance movement. PLoS Pathog. 2015, 11, e1004868. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, R.; He, J.; Ma, Z.; Zhang, X. Chemical compositions of Ligusticum chuanxiong oil and lemongrass oil and their joint action against Aphis citricola Van Der Goot (Hemiptera: Aphididae). Molecules 2016, 21, 1359. [Google Scholar] [CrossRef]
- Liu, X.; Wu, D.; Zhang, Y.; Zhou, H.; Lai, T.; Ding, W. RNA–seq analysis reveals candidate targets for curcumin against Tetranychus cinnabarinus. Biomed. Res. Int. 2016, 2016, 2796260. [Google Scholar]
- Kumar, A.; Gill, J.P.S.; Bedi, J.S.; Kumar, A. Pesticide residues in Indian raw honeys, an indicator of environmental pollution. Environ. Sci. Pollut. Res. Int. 2018, 25, 34005–34016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Z.; Feng, K.; Lu, W.; Wen, X.; Sun, J.; Li, J.; Liu, J.; He, L. Transcriptome analysis revealed that multiple genes were related to the cyflumetofen resistance of Tetranychus cinnabarinus (Boisduval). Pestic. Biochem. Physiol. 2021, 173, 104799. [Google Scholar] [CrossRef] [PubMed]
- Granados–Echegoyen, C.; Loera–Alvarado, G.; Miranda-Salcedo, M.A.; Hernández–Cruz, J.; Luna–Cruz, A.; Loera–Alvarado, E. Field efficacy of synthetic and botanical-derived insecticides against Melanaphis sacchari1, and non-target and beneficial species associated with cultivated sorghum. Southwest. Entomol. 2021, 46, 33–46. [Google Scholar] [CrossRef]
- de Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β–catenin pathway and has anti–cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, V.; Elangovan, K.; Niranjali Devaraj, S. Targeting hepatocellular carcinoma with piperine by radical–mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study. Food Chem. Toxicol. 2017, 105, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Tawani, A.; Amanullah, A.; Mishra, A.; Kumar, A. Evidences for piperine inhibiting cancer by targeting human G–quadruplex DNA sequences. Sci. Rep. 2016, 6, 39239. [Google Scholar] [CrossRef]
- Viswanadha, V.P.; Dhivya, V.; Beeraka, N.M.; Huang, C.Y.; Gavryushova, L.V.; Minyaeva, N.N.; Chubarev, V.N.; Mikhaleva, L.M.; Tarasov, V.V.; Aliev, G. The protective effect of piperine against isoproterenol–induced inflammation in experimental models of myocardial toxicity. Eur. J. Pharmacol. 2020, 885, 173524. [Google Scholar] [CrossRef]
- Nazifi, M.; Oryan, S.; Esfahani, D.E.; Ashrafpoor, M. The functional effects of piperine and piperine plus donepezil on hippocampal synaptic plasticity impairment in rat model of Alzheimer’s disease. Life Sci. 2021, 265, 118802. [Google Scholar] [CrossRef]
- Wansri, R.; Lin, A.C.K.; Pengon, J.; Kamchonwongpaisan, S.; Srimongkolpithak, N.; Rattanajak, R.; Wilasluck, P.; Deetanya, P.; Wangkanont, K.; Hengphasatporn, K.; et al. Semi–synthesis of N–aryl amide analogs of piperine from Piper nigrum and evaluation of their antitrypanosomal, antimalarial, and anti–SARS-CoV-2 main protease activities. Molecules 2022, 27, 2841. [Google Scholar] [CrossRef]
- Arcaro, C.A.; Gutierres, V.O.; Assis, R.P.; Moreira, T.F.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Piperine, a natural bioenhancer, nullifies the antidiabetic and antioxidant activities of curcumin in streptozotocin–diabetic rats. PLoS ONE 2014, 9, e113993. [Google Scholar] [CrossRef]
- Hu, X.; Wu, D.; Tang, L.; Zhang, J.; Zeng, Z.; Geng, F.; Li, H. Binding mechanism and antioxidant activity of piperine to hemoglobin. Food Chem. 2022, 394, 133558. [Google Scholar] [CrossRef]
- Philipova, I.; Valcheva, V.; Mihaylova, R.; Mateeva, M.; Doytchinova, I.; Stavrakov, G. Synthetic piperine amide analogs with antimycobacterial activity. Chem. Biol. Drug Des. 2018, 91, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Xiong, H.; Song, D.; Cao, X. Natural phenolic derivatives based on piperine scaffold as potential antifungal agents. BMC Chem. 2020, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yu, X.; Zhi, X.Y.; Lv, M.; Xu, H. Natural-product-based insecticidal agents 14. semisynthesis and insecticidal activity of new piperine-based hydrazone derivatives against Mythimna separata Walker in vivo. Bioorg. Med. Chem. Lett. 2013, 23, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Lv, M.; Xu, H. Piperine: Bioactivities and structural modifications. Mini-Rev. Med. Chem. 2015, 15, 145–156. [Google Scholar] [CrossRef]
- Qu, H.; Lv, M.; Yu, X.; Lian, X.; Xu, H. Discovery of some piperine-based phenylsulfonylhydrazone derivatives as potent botanically narcotic agents. Sci. Rep. 2015, 5, 13077. [Google Scholar] [CrossRef]
- Nesterkina, M.; Bernier, U.R.; Tabanca, N.; Kravchenko, I. Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti. Open Chem. 2018, 16, 95–98. [Google Scholar] [CrossRef]
- Yu, X.; Che, Z.; Xu, H. Recent advances in the chemistry and biology of podophyllotoxins. Chem.-Eur. J. 2017, 23, 4467–4526. [Google Scholar] [CrossRef]
- Sun, G.S.; Xu, X.; Jin, S.H.; Lin, L.; Zhang, J.J. Ovicidal and insecticidal activities of pyriproxyfen derivatives with an oxime ester group. Molecules 2017, 22, 958. [Google Scholar] [CrossRef]
- Huang, X.B.; Li, T.; Shan, X.; Lu, R.; Hao, M.; Lv, M.; Sun, Z.; Xu, H. High value-added use of citrus industrial wastes in agriculture: Semisynthesis and anti-tobacco mosaic virus/insecticidal activities of ester derivatives of limonin modified in the B ring. J. Agric. Food Chem. 2020, 68, 12241–12251. [Google Scholar] [CrossRef]
- Tharamak, S.; Yooboon, T.; Pengsook, A.; Ratwatthananon, A.; Kumrungsee, N.; Bullangpoti, V.; Pluempanupat, W. Synthesis of thymyl esters and their insecticidal activity against Spodoptera litura (Lepidoptera: Noctuidae). Pest Manag. Sci. 2020, 76, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, X.; Li, S.; Ma, J.; Lv, M.; Xu, H. Semisynthesis of esters of fraxinellone C4/10-oxime and their pesticidal activities. J. Agric. Food Chem. 2016, 64, 5472–5478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Xu, H. Recent progress in the chemistry and biology of limonoids. RSC Adv. 2017, 7, 35191–35220. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Yang, Y.; Fan, L.; Su, F.; Ye, M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2019, 33, 1924–1930. [Google Scholar] [CrossRef]
- Huang, M.; Duan, W.G.; Lin, G.S.; Li, K.; Hu, Q. Synthesis and antifungal activity of novel 3–caren–5–one oxime esters. Molecules 2017, 22, 1538. [Google Scholar] [CrossRef]
- Xu, X.; Cai, X.; Wang, B.; Min, W.; Wang, Q.; Lai, C.; Hu, H.; Xu, D. Synthesis and herbicidal activities of novel substituted acetophenone oxime esters of pyrithiobac. Chem. Sel. 2020, 5, 69–74. [Google Scholar] [CrossRef]
- Herbivo, C.; Comel, A.; Kirsch, G.; Raposo, M.M.M. Synthesis of 5–aryl–5′–formyl–2,2′–bithiophenes as new precursors for nonlinear optical (NLO) materials. Tetrahedron 2009, 65, 2079–2086. [Google Scholar] [CrossRef]
- Hao, M.; Sun, Z.; Xu, J.; Lv, M.; Xu, H. Semisynthesis and pesticidal activities of derivatives of the diterpenoid andrographolide and investigation on the stress response of Aphis citricola Van der Goot (Homoptera: Aphididae). J. Agric. Food Chem. 2020, 68, 4131–4143. [Google Scholar] [CrossRef]
- Li, S.; Lv, M.; Li, T.; Hao, M.; Xu, H. Spirodiclofen ether derivatives: Semisynthesis, structural elucidation, and pesticidal activities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot and Mythimna separata Walker. Pest Manag. Sci. 2021, 77, 2395–2402. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, K.; Lv, M.; Hao, M. Construction of cholesterol oxime ether derivatives containing isoxazoline/isoxazole fragments and their agricultural bioactive properties/control efficiency. J. Agric. Food Chem. 2021, 69, 8098–8109. [Google Scholar] [CrossRef]
- Li, T.; Lv, M.; Xu, H. Construction of new oxime esters of cholesterol containing piperic acid-like fragments as insecticidal agents against Aphis citricola Van der Goot (Homoptera: Aphididae) and Plutella xylostella Linnaeus (Lepidoptera: Plutellidae), Bioorganic Med. Chem. Lett. 2022, 62, 128634. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Jiang, W.; Li, Q.; Li, T.; Wu, W.; Bai, H.; Shi, B. Design, synthesis, and study of the insecticidal activity of novel steroidal 1,3,4–oxadiazoles. J. Agric. Food Chem. 2021, 69, 11572–11581. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; LaJeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef]
- Netz, N.; Opatz, T. A modular formal total synthesis of (±)–cycloclavine. J. Org. Chem. 2016, 81, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Lv, M.; Wang, J.; Qin, Y.; Xu, H. Acaricidal and insecticidal efficacy of new esters derivatives of a natural coumarin osthole. Ind. Crops Prod. 2022, 182, 114855. [Google Scholar] [CrossRef]
- Hao, M.; Lv, M.; Zhou, L.; Li, H.; Xu, J.; Xu, H. Construction, pesticidal activities, control effects, and detoxification enzyme activities of osthole ester/amide derivatives. J. Agric. Food Chem. 2022, 70, 9337–9345. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lv, M.; Sun, Z.; Hao, M.; Xu, H. Optimization of osthole in the lactone ring: Structural elucidation, pesticidal activities, and control efficiency of osthole ester derivatives. J. Agric. Food Chem. 2021, 69, 6465–6474. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lv, M.; Wen, H.; Wang, J.; Wang, Z.; Xu, J.; Fang, S.; Xu, H. High value-added application of natural plant products in crop protection: Construction and pesticidal activities of piperine-type ester derivatives and their toxicology study. J. Agric. Food Chem. 2022. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
| Compound | Corrected Mortality Rate (Mean ± SE, %) a | |
|---|---|---|
| 48 h | 72 h | |
| 1 | 8.32 ± 2.3 | 25.2 ± 3.1 i b |
| 2 | 16.1 ± 3.9 | 30.9 ± 0.6 hi |
| 3 | 13.7 ± 2.2 | 33.0 ± 3.2 gh |
| 4 | 18.2 ± 2.8 | 41.7 ± 1.2 ef |
| 5 | 13.5 ± 2.7 | 30.4 ± 3.8 hi |
| 6 | 15.3 ± 3.2 | 45.0 ± 0.8 def |
| 7 | 22.1 ± 3.1 | 46.3 ± 0.2 de |
| 8 | 18.7 ± 2.8 | 39.1 ± 0.7 fg |
| 9 | 39.1 ± 3.2 | 59.4 ± 0.9 b |
| 10 | 32.0 ± 3.6 | 50.8 ± 2.4 cd |
| 11 | 25.0 ± 1.1 | 56.3 ± 2.6 bc |
| 12 | 27.4 ± 2.0 | 41.7 ± 2.5 ef |
| spirodiclofen | 50.4 ± 1.8 | 88.6 ± 0.9 a |
| Compound | LC50 (mg/mL) | Confidence Interval 95% (mg/mL) | Regression Equation a | r |
|---|---|---|---|---|
| 1 | 14.198 | 9.545–25.947 | Y = –0.735 + 0.638X | 0.990 |
| 9 | 0.298 | 0.243–0.368 | Y = 0.656 + 1.247X | 0.996 |
| 11 | 0.313 | 0.252–0.395 | Y = 0.614 + 1.216X | 0.997 |
| spirodiclofen | 0.115 | 0.093–0.141 | Y = 1.147 + 1.220X | 0.990 |
| Compound | Corrected Mortality Rate (Mean ± SE, %) a | |
|---|---|---|
| 24 h | 48 h | |
| 1 | 7.8 ± 1.1 | 25.0 ± 1.9 e b |
| 2 | 6.7 ± 1.9 | 18.2 ± 1.9 f |
| 3 | 8.9 ± 1.1 | 27.3 ± 3.0 e |
| 4 | 16.7 ± 1.9 | 29.5 ± 2.2 de |
| 5 | 15.6 ± 1.1 | 28.4 ± 1.9 de |
| 6 | 35.6 ± 1.1 | 55.7 ± 1.9 b |
| 7 | 15.6 ± 2.2 | 35.2 ± 3.4 cd |
| 8 | 20.0 ± 1.9 | 29.5 ± 3.0 de |
| 9 | 11.1 ± 1.1 | 35.2 ± 1.9 cd |
| 10 | 16.7 ± 1.9 | 37.5 ± 3.0 c |
| 11 | 14.4 ± 1.1 | 39.8 ± 1.1 c |
| 12 | 30.0 ± 1.9 | 42.0 ± 1.9 c |
| methomyl c | 50.0 ± 1.9 | 88.6 ± 1.1 a |
| Compound | LD50 (µg/nymph) | Confidence interval 95% (µg/nymph) | Regression Equation a | r |
|---|---|---|---|---|
| 1 | 0.308 | 0.217–0.491 | Y = 0.419 + 0.821X | 0.997 |
| 6 | 0.030 | 0.024–0.037 | Y = 2.366 + 1.549X | 0.982 |
| Compound | Corrected Mortality Rate (Mean ± SE, %) a | |
|---|---|---|
| 48 h | 72 h | |
| 1 | 16.7 ± 1.4 | 24.5 ± 1.0 e b |
| 2 | 11.0 ± 1.8 | 22.9 ± 2.3 e |
| 3 | 10.7 ± 2.0 | 29.9 ± 0.5 cd |
| 4 | 23.9 ± 2.2 | 38.1 ± 0.6 b |
| 5 | 11.1 ± 3.3 | 33.4 ± 2.1 c |
| 6 | 12.3 ± 1.8 | 24.8 ± 1.8 e |
| 7 | 13.2 ± 2.1 | 22.8 ± 0.7 e |
| 8 | 15.2 ± 1.9 | 32.4 ± 2.6 c |
| 9 | 17.7 ± 1.4 | 32.9 ± 0.5 c |
| 10 | 17.3 ± 2.5 | 25.9 ± 2.2 de |
| 11 | 13.7 ± 2.8 | 29.7 ± 1.6 cd |
| 12 | 12.7 ± 1.1 | 22.7 ± 0.6 e |
| thiamethoxam | 50.2 ± 0.2 | 90.9 ± 1.3 a |
| chlorpyrifos | 48.8 ± 0.8 | 91.3 ± 0.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Lv, M.; Wen, H.; Wang, Y.; Thapa, S.; Zhang, S.; Xu, H. Synthesis of Piperine-Based Ester Derivatives with Diverse Aromatic Rings and Their Agricultural Bioactivities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot, and Eriosoma lanigerum Hausmann. Insects 2023, 14, 40. https://doi.org/10.3390/insects14010040
Li T, Lv M, Wen H, Wang Y, Thapa S, Zhang S, Xu H. Synthesis of Piperine-Based Ester Derivatives with Diverse Aromatic Rings and Their Agricultural Bioactivities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot, and Eriosoma lanigerum Hausmann. Insects. 2023; 14(1):40. https://doi.org/10.3390/insects14010040
Chicago/Turabian StyleLi, Tianze, Min Lv, Houpeng Wen, Yanyan Wang, Sunita Thapa, Shaoyong Zhang, and Hui Xu. 2023. "Synthesis of Piperine-Based Ester Derivatives with Diverse Aromatic Rings and Their Agricultural Bioactivities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot, and Eriosoma lanigerum Hausmann" Insects 14, no. 1: 40. https://doi.org/10.3390/insects14010040
APA StyleLi, T., Lv, M., Wen, H., Wang, Y., Thapa, S., Zhang, S., & Xu, H. (2023). Synthesis of Piperine-Based Ester Derivatives with Diverse Aromatic Rings and Their Agricultural Bioactivities against Tetranychus cinnabarinus Boisduval, Aphis citricola Van der Goot, and Eriosoma lanigerum Hausmann. Insects, 14(1), 40. https://doi.org/10.3390/insects14010040






