Shift of Host Range for the Immature Stages of the Lanternfly, Pyrops watanabei (Matsumura) (Hemiptera, Fulgoridae) Native to Taiwan
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Insect and Plant Recording
3. Results
3.1. Oviposition Site Preference
3.2. Host Range for Nymphs
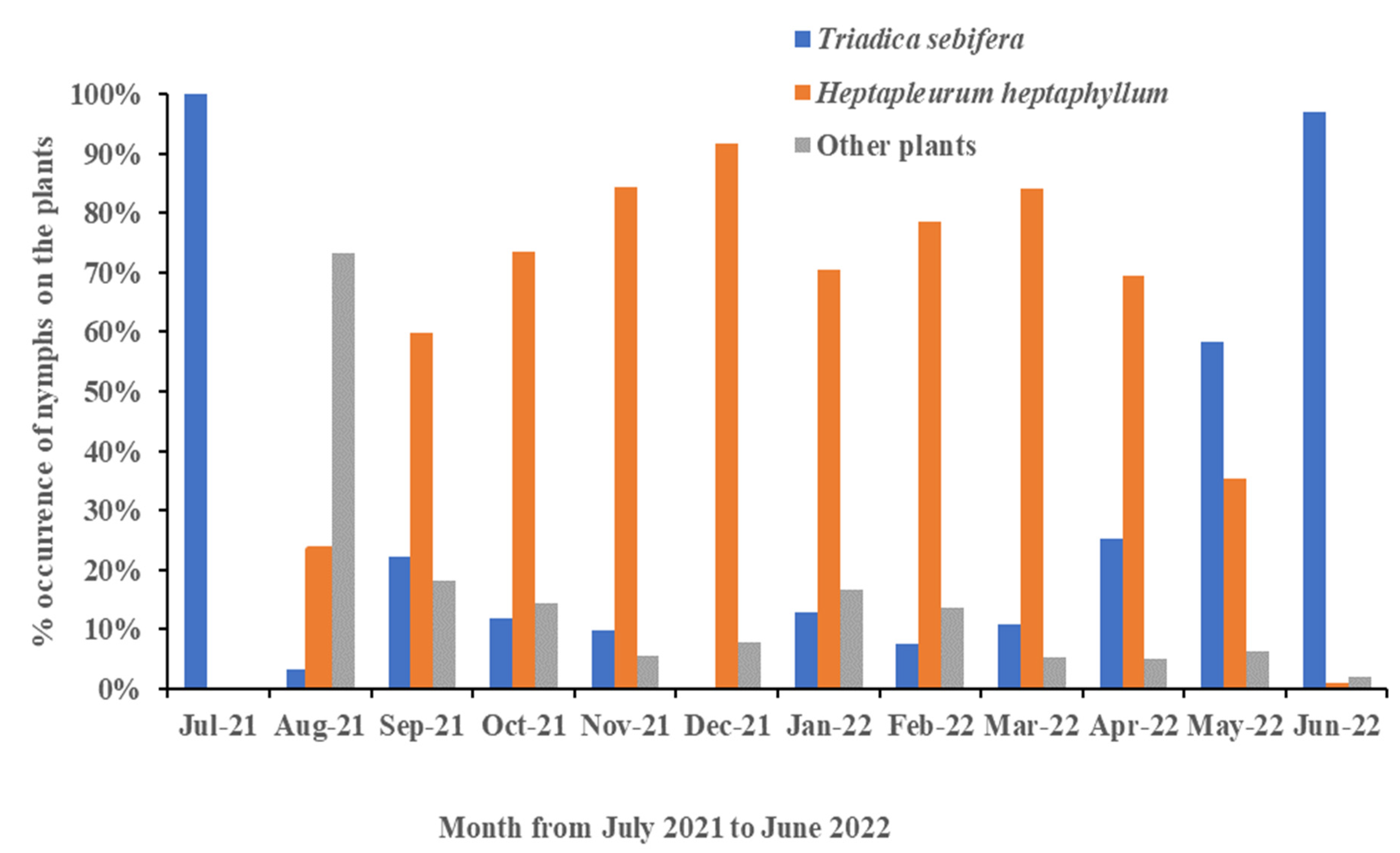
3.3. Shift in Host Range Based on Time
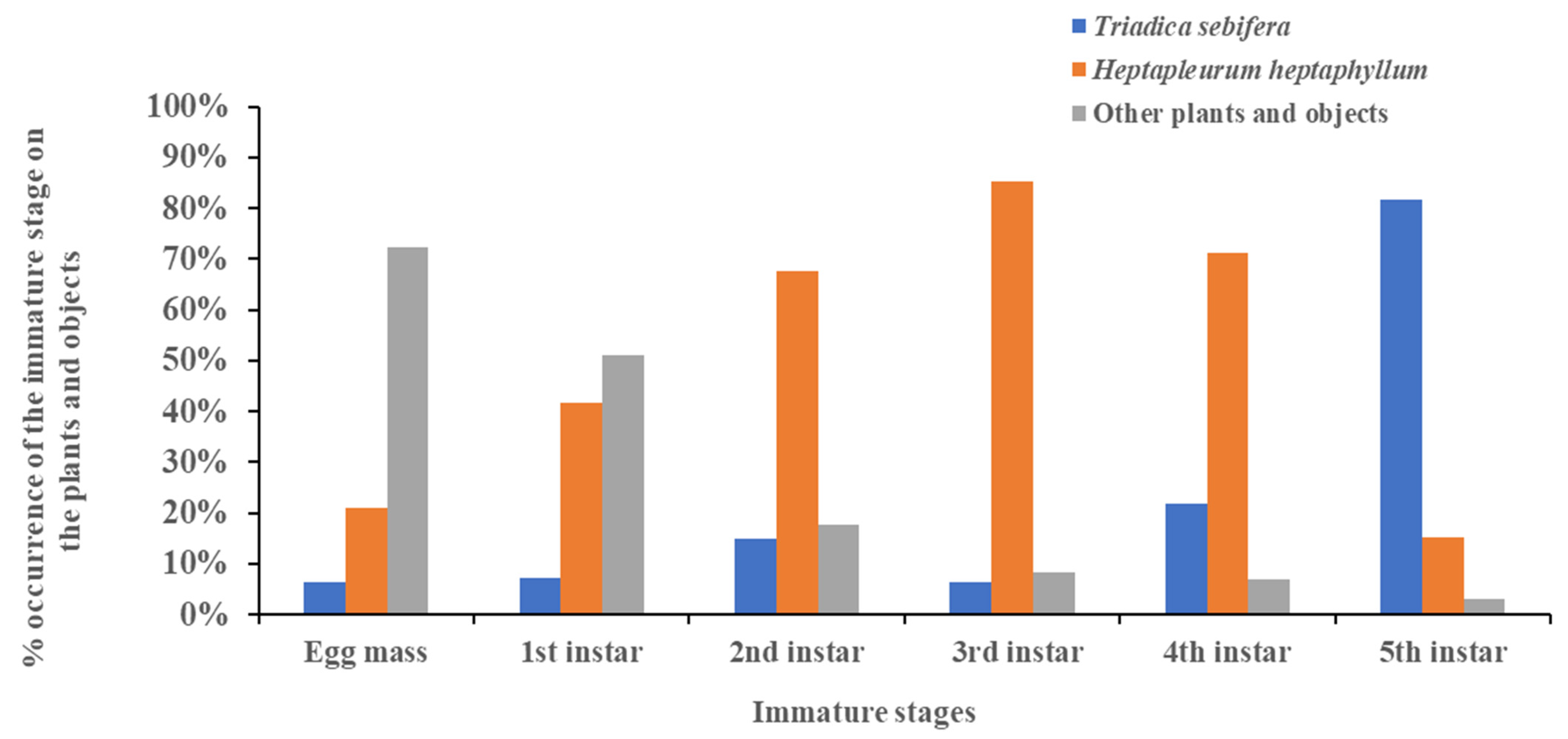
3.4. Shift in Host Range Based on Developmental Stage
3.5. Monthly Records of Egg Masses and Nymphs in Different Instars
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chao, J.-T.; Wu, W.-H.; Yang, M.-M. Criteria for the assessment of candidate taxa for inclusion in the revised schedule of protected (insect) species of Taiwan. Formosan Entomol. 2009, 13, 11–27. (In Chinese) [Google Scholar]
- Wang, C.-H.; Wu, I.-H.; Lu, C.-N. Population Monitoring of Pyrops watanabei after Its Removal from the Protected Species List; Project Report, No. 100-30; Forestry Bureau: Taipei, Taiwan, 2011; 83p. (In Chinese) [Google Scholar]
- Constant, J.; Pham, H.T. Review of the clavatus group of the lanternfly genus Pyrops (Hemiptera: Fulgoromorpha: Fulgoridae). Eur. J. Taxon. 2017, 305, 1–26. [Google Scholar] [CrossRef]
- Chen, W.-C. Studies of the Conservation Biology of Pyrops watanabei in Taipei Area. Master’s Thesis, University of Taipei, Taipei, Taiwan, 2006; 103p. (In Chinese). [Google Scholar]
- Wang, W.; Xu, S.; Qin, D. The lanternfly genus Pyrops Spinola (Hemiptera: Fulgoridae) from China with description of a new species. Entomotaxonomia 2018, 40, 296–309. [Google Scholar]
- Hsu, M.-H.; Yang, Y.-L.; Wu, M.-L.; Wang, L.-J. Host plants of the immature stages of the invasive longan lanternfly, Pyrops candelaria (L.) (Hemiptera, Fulgoridae) in Taiwan. Insects 2021, 12, 1022. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liao, J.-R.; Shiao, S.-F.; Ko, C.-C. Origin and potential expansion of the invasive longan lanternfly, Pyrops candelaria (Hemiptera: Fulgoridae) in Taiwan. Biology 2021, 10, 678. [Google Scholar] [CrossRef]
- Chung, K.F.; Shao, K.T. Catalogue of Life in Taiwan. Web Electronic Publication. Version 2022. Available online: http://taibnet.sinica.edu.tw/ (accessed on 11 August 2022).
- Ohashi, H. Araliaceae. In Flora of Taiwan, 2nd ed.; Huang, T.-C., Ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1993; Volume 3, pp. 1003–1004. [Google Scholar]
- Sun, Z.-L. Experiment on the Lumbercore Plywood Manufacturing of “Schefflera octophylla (Lour.) Harms” in Taiwan; Bulletin of Taiwan Forestry Research Institute, No. 198; Taiwan Forestry Research Institute: Taipei, Taiwan, 1970; 14p. (In Chinese) [Google Scholar]
- Kanehira, R. Formosa Trees: Indigenous to the Islands (Revised); Department of Forestry, Government Research Institute: Formosa, Taiwan, 1936; 805p. (In Japanese) [Google Scholar]
- Hsu, C.-K.; Chen, F.-H.; Wu, C.-C.; Wang, L.-J. Business Opportunities in Blooming Trees: A Preliminary Study on Honey Plants in Lienhuachih Research Center; Forestry Research Newsletter, No. 1; Taiwan Forestry Research Institute: Taipei, Taiwan, 2018; Volume 25, pp. 28–35. (In Chinese) [Google Scholar]
- Wu, Y.-H.; Yi, Y.-C.; Lee, S.-Y.; Chung, C.-C.; Hsu, M.-H.; Chuang, Y.-Y.; Tzeng, H.-Y. Habitat preference of the invasive pest Tessaratoma papillosa (Hemiptera: Tessaratomidae) during overwintering. Q. J. Forest Res. 2021, 43, 21–34. (In Chinese) [Google Scholar]
- Kim, J.G.; Lee, E.-H.; Seo, Y.-M.; Kim, N.-Y. Cyclic behavior of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae) on host plants. J. Insect Behav. 2011, 24, 423–435. [Google Scholar]
- Jung, M.; Kho, J.-W.; Gook, D.-H.; Young, S.L.; Lee, G.-H. Dispersal and oviposition patterns of Lycorma delicatula (Hemiptera: Fulgoridae) during the oviposition period in Ailanthus altissima (Simaroubaceae). Sci. Rep. 2022, 12, 9972. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chen, A.-C.; Tseng, C.-J.; Hwang, S.-G. An Investigation and Study of Chinese Tallow Tree in Taiwan (Sapium sebiferum, Roxb.); Bulletin of Taiwan Forestry Research Institute, No. 57; Taiwan Forestry Research Institute: Taipei, Taiwan, 1958; 37p. [Google Scholar]
- Wu, S.-H.; Aleck Yang, T.-Y.; Teng, Y.-C.; Chang, C.-Y.; Yang, K.-C.; Hsieh, C.-F. Insights of the latest naturalized flora of Taiwan: Change in the past eight years. Taiwania 2010, 55, 139–159. [Google Scholar]
- Hsieh, C.-F.; Chaw, S.-M.; Wang, J.-C. Euphobiaceae. In Flora of Taiwan, 2nd ed.; Huang, T.-C., Ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1993; Volume 3, pp. 494–496. [Google Scholar]
- Ma, T.-P. The Mechanical and Physical Properties of Elaeocarpus Sylvestrix, Alnipyllum Pterospermum, Aleurites Montana, Sapium Discolor and Paulownia Kawakamii Grown in Taiwan; Bulletin of Taiwan Forestry Research Institute, No. 138; Taiwan Forestry Research Institute: Taipei, Taiwan, 1966; 10p. (In Chinese) [Google Scholar]



| Study Sites | Profile | Elevation | Main Plants | GPS Coordinates |
|---|---|---|---|---|
| Mt. Danfeng, Beitou, Taipei City | A trail along the mountain ridge | 120 m | T. cochinchinensis and H. heptaphyllum | 25.133, 121.513 |
| Guizikeng, Beitou, Taipei City | A greenspace on a hill | 139 m | T. sebifera and H. heptaphyllum | 25.152, 121.493 |
| Mt. Tatung, Shulin, New Taipei City | A trail along the mountain ridge | 237 m | T. sebifera and H. heptaphyllum | 24.998, 121.409 |
| Qinglongling, Shulin, New Taipei City | A trail on a hill | 245 m | T. sebifera and H. heptaphyllum | 24.999, 121.402 |
| Shuidui Park, Wugu, New Taipei City | A park on a hillside | 150 m | T. sebifera and H. heptaphyllum | 25.074, 121.428 |
| Dashuinan Rd., Linkou, New Taipei City | Green areas by the road near a golf course | 255 m | T. sebifera and H. heptaphyllum | 25.084, 121.341 |
| Sinlin Trails, Linkou, New Taipei City | Trails on hills | 250 m | T. cochinchinensis and H. heptaphyllum | 25.066, 121.396 |
| Mt. Dagu, Linkou, New Taipei City | Green areas by the roads near a golf course | 120 m | T. sebifera and H. heptaphyllum | 25.108, 121.300 |
| Qionzaihu Temple, Taishan, New Taipei City | A trail near the temple on a hill | 150 m | T. sebifera and H. heptaphyllum | 25.051, 121.422 |
| Yangchou Trails, Luzhu, Taoyuan City | Trails on hills | 225 m | T. sebifera and H. heptaphyllum | 25.051, 121.310 |
| Mt. Wujiutong, Luzhu, Taoyuan City | Trails on the mountain | 155 m | T. sebifera and H. heptaphyllum | 25.067, 121.294 |
| Sioucai Trails, Yangmei, Taoyuan City | Trails on hills | 255 m | T. sebifera and H. heptaphyllum | 24.885, 121.145 |
| Tung Tree Trails, Guishan, Taoyuan City | Trails on hills | 240 m | T. sebifera and H. heptaphyllum | 25.053, 121.343 |
| Plant Species or Objects | Family | n a | No. Egg Masses |
| Heptapleurum heptaphyllum | Araliaceae | 38 | 39 |
| Acacia confusa | Fabaceae | 24 | 26 |
| Machilus thunbergii | Lauraceae | 15 | 15 |
| Wood b | 12 | 12 | |
| Mallotus paniculatus | Euphorbiaceae | 10 | 10 |
| Triadica sebifera | Euphorbiaceae | 9 | 12 |
| Macaranga tanarius | Euphorbiaceae | 6 | 6 |
| Machilus zuihoensis | Lauraceae | 5 | 5 |
| *Psychotria rubra | Rubiaceae | 5 | 5 |
| Triadica cochinchinensis | Euphorbiaceae | 4 | 4 |
| Rhus succedanea | Anacardiaceae | 4 | 4 |
| *Murraya exotica | Rutaceae | 3 | 3 |
| Liquidambar formosana | Altingiaceae | 3 | 3 |
| Semecarpus gigantifolius | Anacardiaceae | 3 | 3 |
| Ardisia sieboldii | Primulaceae | 2 | 3 |
| Lagerstroemia subcostata | Lythraceae | 2 | 4 |
| Bridelia balansae | Phyllanthaceae | 2 | 2 |
| *Ilex asprella | Aquifoliaceae | 2 | 2 |
| Pouteria campechiana | Sapotaceae | 2 | 2 |
| Diospyros morrisiana | Ebenaceae | 2 | 2 |
| Dimocarpus longan | Spindaceae | 2 | 2 |
| Ficus elastica | Moraceae | 1 | 2 |
| Meliosma rigida | Sabiaceae | 1 | 3 |
| Gordonia axillaris | Theaceae | 1 | 1 |
| Elaeocarpus decipiens | Elaeocarpaceae | 1 | 1 |
| *Clerodendrum cyrtophyllum | Lamiaceae | 1 | 1 |
| Ficus erecta | Moraceae | 1 | 1 |
| *Magnolia coco | Magnoliaceae | 1 | 1 |
| *Duranta erecta | Verbenaceae | 1 | 1 |
| Daphniphyllum glaucescens | Daphniphyllaceae | 1 | 1 |
| Morus australis | Moraceae | 1 | 1 |
| Randia cochinchinensis | Rubiaceae | 1 | 1 |
| Koelreuteria henryi | Spindaceae | 1 | 1 |
| Cement pot | 1 | 1 | |
| Elaeocarpus japonicus | Elaeocarpaceae | 1 | 1 |
| *Lantana camara | Verbenaceae | 1 | 1 |
| Syzygium jambos | Myrtaceae | 1 | 1 |
| Cinnamomum camphora | Lauraceae | 1 | 1 |
| Juniperus chinensis | Cupressaceae | 1 | 1 |
| Prunus campanulata | Rosaceae | 1 | 1 |
| Sum | 174 | 186 |
| Plant Species | Family | na | No. Nymphs | Mean | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Instar | 2nd Instar | 3rd Instar | 4th Instar | 5th Instar | Total b | ||||
| Heptapleurum heptaphyllum | Araliaceae | 1072 | 231 | 345 | 1085 | 454 | 50 | 2165 | 2.0 |
| Triadica sebifera | Euphorbiaceae | 188 | 40 | 76 | 83 | 139 | 270 | 608 | 3.2 |
| *Callicarpa formosana | Lamiaceae | 43 | 9 | 44 | 50 | 13 | 0 | 116 | 2.7 |
| Triadica cochinchinensis | Euphorbiaceae | 16 | 0 | 2 | 7 | 16 | 8 | 33 | 2.1 |
| *Maesa perlaria | Primulaceae | 15 | 2 | 11 | 14 | 2 | 0 | 29 | 1.9 |
| Acacia confusa | Fabaceae | 10 | 0 | 0 | 9 | 7 | 2 | 18 | 1.8 |
| *Breynia officinalis | Phyllanthaceae | 8 | 20 | 12 | 1 | 1 | 0 | 34 | 4.3 |
| *Psychotria rubra | Rubiaceae | 5 | 43 | 1 | 1 | 1 | 0 | 46 | 9.2 |
| Lagerstroemia subcostata | Lythraceae | 5 | 18 | 9 | 0 | 0 | 0 | 27 | 5.4 |
| *Viburnum parvifolium | Adoxaceae | 4 | 0 | 1 | 1 | 2 | 0 | 4 | 1.0 |
| Ilex ficoidea | Aquifoliaceae | 3 | 0 | 0 | 7 | 0 | 0 | 7 | 2.3 |
| Machilus thunbergii | Lauraceae | 2 | 0 | 0 | 8 | 1 | 0 | 9 | 4.5 |
| Ficus erecta | Moraceae | 2 | 0 | 3 | 0 | 0 | 0 | 3 | 1.5 |
| *Melicope pteleifolia | Rutaceae | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1.0 |
| *Murraya exotica | Rutaceae | 1 | 5 | 0 | 0 | 0 | 0 | 5 | 5.0 |
| †Mussaenda parviflora | Rubiaceae | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1.0 |
| Symplocos chinensis | Symplocaceae | 1 | 4 | 0 | 0 | 0 | 0 | 4 | 4.0 |
| Elaeocarpus decipiens | Elaeocarpaceae | 1 | 1 | 2 | 0 | 0 | 0 | 3 | 3.0 |
| Psidium guajava | Myrtaceae | 1 | 0 | 1 | 3 | 0 | 0 | 4 | 4.0 |
| Bischofia javanica | Phyllanthaceae | 1 | 5 | 0 | 0 | 0 | 0 | 5 | 5.0 |
| †Piper kadsura | Piperaceae | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 2.0 |
| *Clerodendrum ohwii | Lamiaceae | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1.0 |
| Randia cochinchinensis | Rubiaceae | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1.0 |
| Pouteria campechiana | Sapotaceae | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1.0 |
| Archidendron lucidum | Fabaceae | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 3.0 |
| †Zanthoxylum nitidum | Rutaceae | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1.0 |
| Ilex rotunda | Aquifoliaceae | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1.0 |
| *Ilex aspella | Aquifoliaceae | 1 | 3 | 0 | 0 | 0 | 0 | 3 | 3.0 |
| *Tibouchina semidecandra | Melastomataceae | 1 | 10 | 0 | 0 | 0 | 0 | 10 | 10.0 |
| *Itea parviflora | Iteaceae | 1 | 10 | 0 | 0 | 0 | 0 | 10 | 10.0 |
| Mallotus paniculatus | Euphorbiaceae | 1 | 32 | 0 | 0 | 0 | 0 | 32 | 32.0 |
| Diospyros morrisiana | Ebenaceae | 1 | 50 | 0 | 0 | 0 | 0 | 50 | 50.0 |
| Dimocarpus longan | Spindaceae | 1 | 70 | 0 | 0 | 0 | 0 | 70 | 70.0 |
| Sum | 1393 | 554 | 511 | 1274 | 638 | 330 | 3307 | ||
| Time | No. Egg Masses | No. Nymphs | ||||||
|---|---|---|---|---|---|---|---|---|
| Year | Month | 1st Instar | 2nd Instar | 3rd Instar | 4th Instar | 5th Instar | Total a | |
| 2021 | July | 8 | 0 | 0 | 0 | 2 | 15 | 17 |
| August | 139 | 306 | 5 | 0 | 0 | 0 | 311 | |
| September | 34 | 184 | 125 | 12 | 0 | 0 | 321 | |
| October | 4 | 42 | 125 | 107 | 1 | 0 | 275 | |
| November | 0 | 20 | 76 | 127 | 8 | 0 | 231 | |
| December | 0 | 0 | 61 | 262 | 65 | 0 | 388 | |
| 2022 | January | 0 | 2 | 55 | 224 | 81 | 0 | 362 |
| February | 0 | 0 | 43 | 293 | 93 | 0 | 429 | |
| March | 0 | 0 | 19 | 203 | 118 | 4 | 344 | |
| April | 0 | 0 | 2 | 46 | 189 | 94 | 331 | |
| May | 0 | 0 | 0 | 0 | 47 | 49 | 96 | |
| June | 1 | 0 | 0 | 0 | 34 | 168 | 202 | |
| Sum | 186 | 554 | 511 | 1274 | 638 | 330 | 3307 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-H.; Yang, Y.-L.; Wu, M.-L.; Wang, L.-J. Shift of Host Range for the Immature Stages of the Lanternfly, Pyrops watanabei (Matsumura) (Hemiptera, Fulgoridae) Native to Taiwan. Insects 2022, 13, 826. https://doi.org/10.3390/insects13090826
Hsu M-H, Yang Y-L, Wu M-L, Wang L-J. Shift of Host Range for the Immature Stages of the Lanternfly, Pyrops watanabei (Matsumura) (Hemiptera, Fulgoridae) Native to Taiwan. Insects. 2022; 13(9):826. https://doi.org/10.3390/insects13090826
Chicago/Turabian StyleHsu, Meng-Hao, Yueh-Lin Yang, Meng-Ling Wu, and Liang-Jong Wang. 2022. "Shift of Host Range for the Immature Stages of the Lanternfly, Pyrops watanabei (Matsumura) (Hemiptera, Fulgoridae) Native to Taiwan" Insects 13, no. 9: 826. https://doi.org/10.3390/insects13090826
APA StyleHsu, M.-H., Yang, Y.-L., Wu, M.-L., & Wang, L.-J. (2022). Shift of Host Range for the Immature Stages of the Lanternfly, Pyrops watanabei (Matsumura) (Hemiptera, Fulgoridae) Native to Taiwan. Insects, 13(9), 826. https://doi.org/10.3390/insects13090826






