Intraguild Interactions of Three Biological Control Agents of the Fall Armyworm Spodoptera frugiperda (JE Smith) in Florida
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Colony Establishment
2.2. Biological Control Agents’ Interactions
2.2.1. Predator vs. Predator (without Prey)
2.2.2. Predator vs. Predator (with Prey)
2.2.3. Predators’ Behavior toward Parasitized and Non-Parasitized Larvae
2.3. Predator–Prey Preference
2.3.1. Olfactometer
2.3.2. Petri Dish Arena
2.4. Data Analysis
3. Results
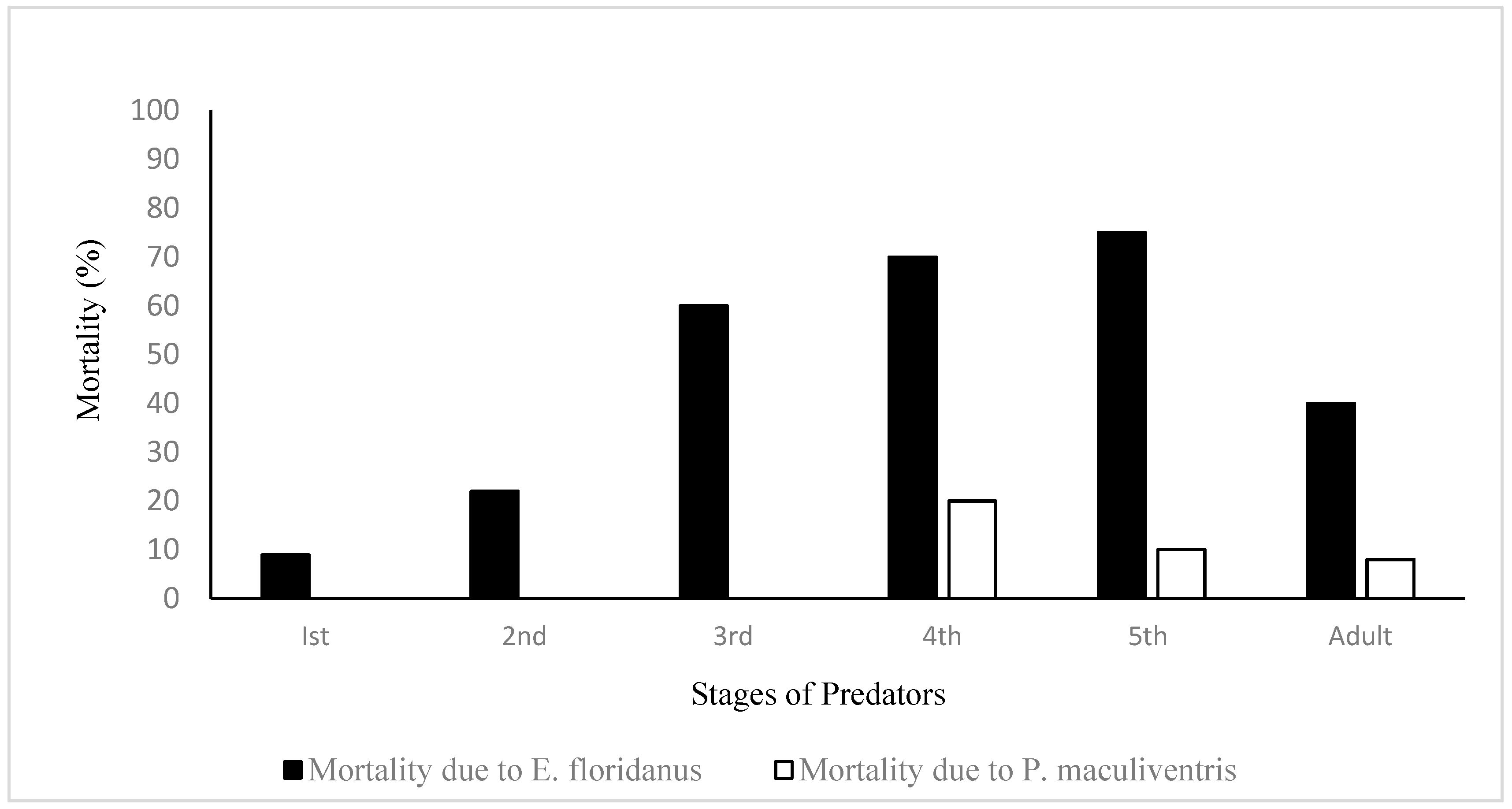
3.1. Predator vs. Predator, Intraguild Predation
3.1.1. Without Fall Armyworm
3.1.2. With Fall Armyworm
3.2. Predator vs. Parasitoid
3.3. Predator–Prey Preference
4. Discussion
4.1. Predator Competition
4.2. Prey Preference
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capinera, J.L. Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae), 2020. IFAS Extension. Available online: https://edis.ifas.ufl.edu/publication/in255 (accessed on 12 December 2020).
- Foster, R.E. Strategies for protecting sweet corn ears from damage by fall armyworms (Lepidoptera: Noctuidae) in Southern Florida. Fl. Entomol. 1989, 72, 146–151. [Google Scholar] [CrossRef]
- Meagher, R.L., Jr.; Gallo-Meagher, M. Identifying host strains of fall armyworm (Lepidoptera: Noctuidae) in Florida using mitochondrial markers. Fl. Entomol. 2003, 84, 450–455. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) host strains in agricultural and turfgrass habitats. Environ. Entomol. 2004, 33, 881–889. [Google Scholar] [CrossRef]
- Pashley, D.P. Current status of fall armyworm host strains. Fl. Entomol. 1988, 71, 227–234. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.D.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afri. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Sparks, A.N. A Review of the biology of the fall armyworm. Fl. Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- García-Lara, S.; Serna-Saldivar, S.O. Corn History and Culture. Corn: Chemistry and Technology; Serna-Saldivar, S.O., Ed.; Woodhead Publishing and AACC International: Elsevier, The Netherlands, 2019; pp. 1–18. [Google Scholar]
- Li, Y.C.; Klassen, W.; Lamberts, M.; Olczyk, T. Sweet Corn Production in Miami-Dade County, Florida. Available online: https://edis.ifas.ufl.edu/publication/TR013 (accessed on 20 February 2019).
- Florida Department of Agriculture and Consumer Service. Florida agriculture by numbers. Proc. Fla. Agric. Stat. Bull. 2016, 178. [Google Scholar]
- Anonymous. The Biology of Zea mays L. ssp mays (maize or corn). In DoHa Aging; Office of the Gene Technology Regulator, Australian Government: Phillip, ACT, Australia, 2008; pp. 1–80. Available online: https://www.ogtr.gov.au/resources/publications/biology-zea-mays-l-ssp-mays-maize-or-corn (accessed on 5 September 2022).
- Nagoshi, R.N.; Koffi, D.; Agboka, K.; Tounou, K.A.; Banerjee, R.; Jurat-Fuentes, J.L.; Meagher, R.L. Comparative molecular analyses of invasive fall armyworm in Togo reveal strong similarities to populations from the eastern United States and the Greater Antilles. PLoS ONE 2017, 12, e0181982. [Google Scholar] [CrossRef]
- Koffi, D.; Kyerematen, R.; Eziah, V.Y.; Agboka, K.; Adom, M.; Meagher, R.L. Natural enemies of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Ghana. Fl. Entomol. 2020, 103, 85–90. [Google Scholar] [CrossRef]
- Juárez, M.L.; Murúa, M.G.; García, M.G.; Ontivero, M.; Vera, M.T.; Groot, A.T.; Castagnaro, A.P.; Gastaminza, G.; Willink, E. Host association of Spodoptera frugiperda (Lepidoptera: Noctuidae) corn and rice strains in Argentina, Brazil, and Paraguay. J. Econ. Entomol. 2012, 105, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Murúa, G.; Molina-Ochoa, J.; Coviella, C. Population dynamics of the fall armyworm, Spodoptera frugiperda (lepidoptera: Noctuidae) and its parasitoids in northwestern argentina. Fla. Èntomol. 2006, 89, 175–182. [Google Scholar] [CrossRef]
- Santos, L.M.; Redaelli, L.R.; Diefenbach, L.M.G.; Efrom, C.F.S. Larval and pupal stage of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in sweet and field corn genotypes. Braz. J. Biol. 2003, 63, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Marenco, R.J.; Foster, R.E.; Sanchez, C.A. Sweet Corn Response to Fall Armyworm (Lepidoptera: Noctuidae) Damage During Vegetative Growth. J. Econ. Èntomol. 1992, 85, 1285–1292. [Google Scholar] [CrossRef]
- Nuessly, G.S.; Webb, S.E. Insect Management for Sweet Corn. UF/IFAS Extension. 2017. Available online: https://edis.ifas.ufl.edu/ig158 (accessed on 5 May 2018).
- Ozores-Hampton, M.; Kanissery, R.; Mcavoy, E.J.; Raid, R.N.; Beuzelin, J. Sweet corn production, Gainesville. In IFAS Extension; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2016; p. HS737. [Google Scholar]
- Wiseman, B.R.; Davis, F.M. Plant Resistance to the Fall Armyworm. Fla. Èntomol. 1979, 62, 123. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [PubMed]
- Togola, A.; Meseka, S.; Menkir, A.; Badu-Apraku, B.; Boukar, O.; Tamò, M.; Djouaka, R. Measurement of Pesticide Residues from Chemical Control of the Invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) in a Maize Experimental Field in Mokwa, Nigeria. Int. J. Environ. Res. Public Health 2018, 15, 849. [Google Scholar] [CrossRef]
- Belfroid, A.C.; Van Drunen, M.; Beek, M.A.; Schrap, S.M.; Van Gestel, C.A.M.; Van Hattum, B. Relative risks of transformation products of pesticides for aquatic ecosystems. Sci. Total Environ. 1998, 222, 167–183. [Google Scholar] [CrossRef]
- Cock, M.J.W.; Biesmeijer, J.C.; Cannon, R.J.C.; Gerad, P.J.; Gillespie, D.; Jimenez, J.J.; Lavelle, P.M.; Raina, S.K. The positive contribution of invertebrates to sustainable agriculture and food security. CABI Rev. 2012, 2012, 1–27. [Google Scholar] [CrossRef]
- Gross, H.R.; Pair, S.D. The Fall Armyworm: Status and Expectations of Biological Control with Parasitoids and Predators. Fla. Èntomol. 1986, 69, 502. [Google Scholar] [CrossRef]
- De Clercq, P. Dark clouds and their silver linings: Exotic generalist predators in augmentative biological control. Neotropical Èntomol. 2002, 31, 169–176. [Google Scholar] [CrossRef]
- McLain, D.K. Responses of predatory pentatomid bugs to the excrement of the predator Euthyrhynchus floridanus (Hemiptera: Pentatomidae). J. Ga. Entomol. Soc. 1981, 16, 535–537. [Google Scholar]
- Perier, J.D. Integration of two predaceous stinkbugs and a larval parasitoid to manage the fall armyworm Spodoptera frugi-perda (Lepidoptera: Noctuidae). Doctoral Dissertation, Florida Agricultural and Mechanical University, Tallahassee, FL, USA, 2019. [Google Scholar]
- Mead, F.W.; Richman, D.B. Florida Predatory Stink Bug, Euthyrhynchus floridanus (Linnaeus) (Insecta: Hemiptera: Pentatomidae). 2019. Available online: https://edis.ifas.ufl.edu/publication/IN314 (accessed on 13 June 2022).
- Herrick, J.N.; Reitz, S.T.; Carpenter, J.E.; O’Brien, C.W. Predation by Podisus maculiventris (Hemiptera: Pentatomidae) on Plutella xylostalla (Lepidoptera: Plutellidae) parasitized by Cotesia plutellae (Hymenoptera: Braconidae) and its impact on cabbage. Biol. Control 2008, 45, 386–395. [Google Scholar] [CrossRef]
- Meagher, R.L., Jr.; Nuessly, G.S.; Nagoshi, R.N.; Hay-Roe, M.M. Parasitoids attacking fall armyworm (Lepidoptera: Noctuidae) in sweet corn habitats. Biol. Control 2016, 95, 66–72. [Google Scholar] [CrossRef]
- Richman, D.B.; Mead, F.W. Common Name: Spined Soldier Bug, Scientific Name: Podisus maculiventris (Say) (Insecta: Hemip-tera: Petatomidae). 2020. Available online: https://entnemdept.ufl.edu/creatures/beneficial/podisus_maculiventris.htm (accessed on 30 August 2022).
- Legaspi, J.C.; Legaspi, B.C. Does a Polyphagous Predator Prefer Prey Species That Confer Reproductive Advantage?: Case Study ofPodisus maculiventris. Environ. Èntomol. 2004, 33, 1401–1409. [Google Scholar] [CrossRef]
- Perkins, W.D. Laboratory Rearing of the Fall Armyworm. Fla. Èntomol. 1979, 62, 87. [Google Scholar] [CrossRef]
- Hedeker, D. A mixed-effects multinomial logistic regression model. Stat. Med. 2003, 22, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- S.A.S. Institute. SAS 9.2 for Windows; S.A.S. Institute: Cary, NC, USA, 2013. [Google Scholar]
- A Rosenheim, J.; Kaya, H.K.; Ehler, L.; Marois, J.; Jaffee, B. Intraguild Predation Among Biological-Control Agents: Theory and Evidence. Biol. Control 1995, 5, 303–335. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Wilhoit, L.R.; Armer, C. Influence of intraguild predation among generalist insect predators on the sup-pression of an herbivore population. Oecologia 1993, 96, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Polis, G.A.; Myers, C.A.; Holt, R.D. The ecology and evolution of intraguild predation. Potential competitors that eat each other. Annu. Rev. Ecol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- De Clercq, P.; Peeters, I.; Vergauwe, G.; Thas, O. Interaction between Podisus maculiventris and Harmonia axyridis, two preda-tors used in augmentative biological control in greenhouse crops. BioControl 2003, 48, 39–55. [Google Scholar] [CrossRef]
- Mukerji, M.K.; LeRoux, E.J. A quantitative study of food consumption and growth of Podisus maculiventris (Hemiptera: Pen-tatomidae). Can. Entomol. 1969, 101, 387–403. [Google Scholar] [CrossRef]
- Welch, K.D.; Harwood, J. Temporal dynamics of natural enemy–pest interactions in a changing environment. Biol. Control 2014, 75, 18–27. [Google Scholar] [CrossRef]
- Yasuda, T.; Wakamura, S. Behavioral responses in prey location of the predatory stink bug, Eocanthecona furcellata, to chemical cues in the larvae of Spodoptera litura. Èntomol. Exp. Appl. 1996, 81, 91–96. [Google Scholar] [CrossRef]
- McLain, D.K. Terrestrial Trail-Following by Three Species of Predatory Stink Bugs. Fla. Èntomol. 1979, 62, 152. [Google Scholar] [CrossRef]
- Snyder, W.E.; Ives, A.R. Generalist predators disrupt biological control by a specialist parasitoid. Ecology 2001, 82, 705–716. [Google Scholar] [CrossRef]
- Oliveira, H.N.; De Clercq, P.; Zanuncio, J.C.; Pratissoli, D.; Pedruzzi, E.P. Nymphal development and feeding preference of Podisus maculiventris (Heteroptera: Pentatomidae) on eggs of Ephestia kuehniella (Lepidoptera: Pyralidae) parasitised or not by Trichogramma brassicae (Hymenoptera: Trichogrammatidae). Braz. J. Biol. 2004, 64, 459–463. [Google Scholar] [CrossRef] [Green Version]
| Life Stages | Life Stages | ||
|---|---|---|---|
| P. maculiventris | E. floridanus | P. maculiventris | E. floridanus |
| adult | instar 1 | instar 1 | adult |
| adult | instar 2 | instar 2 | adult |
| adult | instar 3 | instar 3 | adult |
| adult | instar 4 | instar 4 | adult |
| adult | instar 5 | instar 5 | adult |
| adult | adult | adult | adult |
| Variables | Parameter Estimates (β) ± S.E | df | p | |
|---|---|---|---|---|
| Species | E. floridanus | −1.5184 ± 0.2227 | 1 | <0.0001 *** |
| P. maculiventris | Reference point | |||
| Life stage | ||||
| First instars | −2.1636 ± 0.6421 | 1 | 0.0008 *** | |
| Second instars | −1.0872 ± 0.4650 | 1 | 0.0194 *** | |
| Third instars | Reference point | |||
| Fourth instars | 1.2705 ± 0.4000 | 1 | 0.0015 *** | |
| Fifth instars | 1.1025 ± 0.3952 | 1 | 0.0053 *** | |
| Adult | 0.6045 ± 0.3887 | 1 | 0.1198 ns | |
| Variables | Counts 2 | Parameter Estimates (β) ± S.E | df | p |
|---|---|---|---|---|
| Observed behavior | 60 | |||
| One fed alone on the pray | 23 | −0.5878 ± 0.5578 | 1 | 0.2920 ns |
| Fed on each other | 11 | −0.5878 ± 0.5578 | 1 | 0.2920 ns |
| No feeding | 20 | Reference point | ||
| Both fed on prey | 6 | −2.1972 ± 1.0541 | 1 | 0.0371 * |
| Variables | Counts 1 | Parameter Estimates (β) ± S.E. | df | p | |
|---|---|---|---|---|---|
| Species | E. floridanus | 23 | −0.2821 ± 0.2396 | 1 | 0.2390 ns |
| P. maculiventris | 28 | Reference point | |||
| Treatment (larval type) | Non-parasitized | 29 | 0.3913 ± 0.2403 | 1 | 0.1034 ns |
| Parasitized | 22 | Reference point | |||
| Test Method | Variables | Parameter Estimates (β) ± S.E. | df | p | |
|---|---|---|---|---|---|
| Olfactometer | Species | E. floridanus | −1.1677 ± 0.3997 | 1 | 0.0035 *** |
| P. maculiventris | Reference point | ||||
| Treatment (larval type) | Non-parasitized | 0.1826 ± 0.3036 | 1 | 0.5474 ns | |
| Parasitized | Reference point | ||||
| Petri dish arena | Species | E. floridanus | −1.0918 ± 0.4093 | 1 | 0.0076 *** |
| P. maculiventris | Reference point | ||||
| Treatment (larval type) | Non-parasitized | 0.6163 ± 0.3395 | 1 | 0.0695 ns | |
| Parasitized | Reference point | ||||
| Variables | Parameter Estimates (β) ± S.E. | df | p | |
|---|---|---|---|---|
| Species | E. floridanus | −1.1276 ± 0.2855 | 1 | <0.0001 *** |
| P. maculiventris | Reference point | |||
| Method | Olfactometer | 0.0964 ± 0.2198 | 1 | 0.6611 ns |
| Petri dish | Reference point | |||
| Treatment | Non-parasitized larvae | 0.3833 ± 0.2235 | 1 | 0.0864 ns |
| Parasitized larvae | Reference point | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perier, J.D.; Haseeb, M.; Kanga, L.H.B.; Meagher, R.L.; Legaspi, J.C. Intraguild Interactions of Three Biological Control Agents of the Fall Armyworm Spodoptera frugiperda (JE Smith) in Florida. Insects 2022, 13, 815. https://doi.org/10.3390/insects13090815
Perier JD, Haseeb M, Kanga LHB, Meagher RL, Legaspi JC. Intraguild Interactions of Three Biological Control Agents of the Fall Armyworm Spodoptera frugiperda (JE Smith) in Florida. Insects. 2022; 13(9):815. https://doi.org/10.3390/insects13090815
Chicago/Turabian StylePerier, Jermaine D., Muhammad Haseeb, Lambert H. B. Kanga, Robert L. Meagher, and Jesusa C. Legaspi. 2022. "Intraguild Interactions of Three Biological Control Agents of the Fall Armyworm Spodoptera frugiperda (JE Smith) in Florida" Insects 13, no. 9: 815. https://doi.org/10.3390/insects13090815
APA StylePerier, J. D., Haseeb, M., Kanga, L. H. B., Meagher, R. L., & Legaspi, J. C. (2022). Intraguild Interactions of Three Biological Control Agents of the Fall Armyworm Spodoptera frugiperda (JE Smith) in Florida. Insects, 13(9), 815. https://doi.org/10.3390/insects13090815










