Simple Summary
The papaya mealybug, Paracoccus marginatus, is a polyphagous invasive pest that causes severe damage in China. To improve our understanding of the expansion and prevalence of P. marginatus individuals on host plants, it is important to explore the fitness changes of insects after host plant shifting. In this study, we measured the development, fecundity, and population parameters in P. marginatus individuals over a span of three consecutive generations after being transferred from potato (Solanum tuberosum) to papaya (Carica papaya), sweet potato (Ipomoea batatas), and alligator weed (Alternanthera philoxeroides). Further, the population growth rates of insects on C. papaya, I. batatas, and S. tuberosum in the F2 generation were projected. We found that P. marginatus individuals transferred to C. papaya had higher fitness. When transferred to I. batatas, the fitness of P. marginatus initially decreased in F0 and then rebounded in F1 and F2. Paracoccus marginatus individuals could rapidly expand their populations on the above host plants. However, P. marginatus individuals were unable to complete their development on A. philoxeroides. Our findings provide new insights into the host plant fitness, prevalence, and potential pest control of P. marginatus.
Abstract
The papaya mealybug, Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Pseudococcidae), is a polyphagous invasive pest in China. The effect that the shifting of the host plant has on the fitness of a polyphagous pest is critical to its prevalence and potential pest control. In order to assess the fitness changes of P. marginatus after transferal from potato (Solanum tuberosum (Tubiflorae: Solanaceae)) to papaya (Carica papaya (Parietales: Caricacea)), sweet potato (Ipomoea batatas (Tubiflorae: Convolvulaceae)), and alligator weed (Alternanthera philoxeroides (Centrospermae: Amaranthaceae)), the life table data of three consecutive generations were collected and analyzed using the age-stage, two-sex life table method. The results showed that when P. marginatus was transferred from S. tuberosum to papaya, a higher intrinsic rate of increase (r) and finite rate of increase (λ) were observed. Paracoccus marginatus individuals transferred to I. batatas had the significantly lower population parameters than those on C. papaya; however, the fitness recovered for those on I. batatas after two generations. Paracoccus marginatus individuals were unable to complete development on A. philoxeroides. Our results conclusively demonstrate that P. marginatus individuals can readily adapt to C. papaya and I. batatas even after host plant shifting, and are capable of causing severe damage to these hosts.
1. Introduction
The papaya mealybug, Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Pseudococcidae), is a globally invasive pest, which attacks host plants by sucking sap from the leaves, stems, and other plant parts [1]. Since the 1990s, P. marginatus had spread rapidly through the Americas, Africa, and most provinces of southern China [2,3]. Paracoccus marginatus is a polyphagous pest of many economic crops and weeds included in more than 64 families, such as Euphorbiaceae, Rubiaceae, and Caricaceae [4]. In our preliminary field investigation, P. marginatus was found on important field crops including potato (Solanum tuberosum (Tubiflorae: Solanaceae)), papaya (Carica papaya (Parietales: Caricacea)), and sweet potato (Ipomoea batatas (Tubiflorae: Convolvulaceae)). It has also been observed on alligator weed (Alternanthera philoxeroides (Centrospermae: Amaranthaceae)). Most of the insects were observed on the leaves of the above plants. Solanum tuberosum has been used for a number of years as a host for the mass-rearing of P. marginatus in the laboratory.
Polyphagous insects, which characteristically have a wide range of host plants in nature, are often associated with host shifting. Huang et al. (2014) demonstrated that the rapid host shifting of Phenacoccus solenopsis (Tinsley) (Hemiptera: Pseudococcidae) was due to its efficient host plant fitness [5]. The fitness of insects on a new host plant can be evaluated from the population growth rate on the new host plant versus their old host plant [6,7]. Therefore, it is important to understand how invasive pests adapt on the new host plant using demographic characteristics.
Many studies have shown that significant effects may occur in an insect species after host plant shifting, such as changes in their development and fecundity. For example, Milanović et al. (2016) reported that when Lymantria dispar L. (Lepidoptera: Lymantriidae) transferred from Hungarian oak to Turkey oak, the developmental time shortened while the efficiency of food utilization increased [8]. Mody et al. (2007) demonstrated that host plant shifting had a strong effect on Chrysopsyche imparilis (Lepidoptera: Lasiocampidae), especially in adult fecundity and the mean body mass of second-instar larvae [9]. Furthermore, when assessing the effects of host plant shifting based on the life table and population dynamics of Aphis gossypii (Glover) (Hemiptera: Aphididae), Fan et al. (2018) showed that the fecundity (F), intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R0) significantly increased when transferred from wheat to cotton [10]. Amarasekare et al. (2008) reported the survival rates of P. marginatus on different host plants [11]. Nisha and Kennedy (2017), using the female age-specific life table, reported the life table results for P. marginatus on different host plants [12]. However, the results were limited by ignoring the males in the population [13].
We hypothesized that the fitness of P. marginatus would change after host plant shifting. We also predicted that P. marginatus can readily adapt to some new host plants within a few generations. To test these hypotheses, we measured the development, fecundity, and population parameters in P. marginatus over a span of three consecutive generations after being transferred from S. tuberosum to C. papaya, I. batatas, and A. philoxeroides. Further, we projected the population growth of the insects on C. papaya, I. batatas, and S. tuberosum in the F2 generation.
2. Materials and Methods
2.1. Cultivation of Host Plants
Carica papaya (Parietales: Caricaceae), I. batatas (Tubiflorae: Convolvulaceae), S. tuberosum (Tubiflorae: Solanaceae), and A. philoxeroides (Centrospermae: Amaranthaceae) were obtained from the Institute of Plant Protection, Fujian Academy of Agricultural Sciences. The host plants were cultivated (40.0 cm in length, 30.0 cm in width, and 15.0 cm in height) with nutrient soil (Cuijun, Fuzhou, China) and kept in growth chambers (PRX-450D, Haishu Safe Apparatus, Ningbo, China) at 28 ± 1 °C, 70 ± 5% RH, with a photoperiod of 14: 10 (L: D) h. Young leaves (<30-d old) were used for the study.
2.2. Paracoccus marginatus
The eggs of P. marginatus were originally obtained from a papaya orchard in Fuzhou city (Fujian Province, China, 25°15′~26°39′ N, 118°08′~120°31′ E), and reared on the leaves of S. tuberosum in a growth chamber for 20 generations to allow P. marginatus to adapt to S. tuberosum as a host. The female life cycle (which differs from that of the male) consists of the egg, three larval instars, and the adult stage, while the male life cycle includes the egg, three larval instars, a pupal stage, and the adult stage.
2.3. Life Table Study of P. marginatus
Egg masses laid within a 24 h period on potato leaves were randomly selected for the life table study. In order to accurately observe the lifespan of each insect, the eggs were placed on leaves of C. papaya, I. batatas, S. tuberosum, or A. philoxeroides in plastic dishes (3.5 cm in diameter and 2.0 cm in height) containing agar (3%). After hatching, each 1st instar was transferred into a fresh dish containing leaves of the same plant and reared individually. Following the advice of Mou et al. (2015), only hatched eggs were used in the life table studies to accurately estimate the life table parameters [14]. Newly emerged adult males and females were paired. The daily fecundity and survival were recorded until the death of all individuals. The life table data for three consecutive generations (F0, F1, and F2) were recorded. Paracoccus marginatus reared on A. philoxeroides only survived for a single generation (F0); therefore, only one life table could be constructed for insects on this host.
2.4. Life Table Data Analysis
The raw life history data of all individuals of P. marginatus, including the developmental duration, longevity, and female fecundity, were analyzed according to the age-stage, two-sex life table procedure [15,16] using the program TWOSEX-MSChart [17]. The variances and standard errors of parameters were estimated using the bootstrap technique [18,19]. The differences between treatments were assessed using paired bootstrap tests [20]. The age-stage-specific survival rate (sxj) is the probability that each hatched egg will survive to age x and stage j. The age-specific survival rate (lx) was calculated as:
where k is the number of stages. The age-specific fecundity (mx) was calculated as:
The intrinsic rate of increase (r) was estimated using the Euler–Lotka equation [21,22] with the age indexed from 0 [23]:
The finite rate of increase (λ), net reproductive rate (R0), and mean generation time (T) were calculated as follows:
The age-stage-specific life expectancy (exj), i.e., the length of time that an individual of age x and stage j is expected to survive, was calculated according to Chi and Su (2006) [24]:
where is the probability that an individual of age x and stage j can survive to age i and stage y by assuming that . The age-stage reproductive value (vxj), which represents the contribution of each individual in age x and stage j makes to the future population [25,26], was calculated as:
2.5. Population Projection
The population growth of P. marginatus was simulated according to Chi (1990) [27] by using the computer program TIMING-MSChart [28]. An initial population of 10 newly laid eggs was used for the simulation. The stage growth rate of stage j was calculated according to Huang et al. (2018) [29].
As the population approaches a stable age-stage distribution, the number of individuals of each stage (nj,t) and the total population size (ntotal,t) will increase at the finite rate of increase (λ) and the intrinsic rate of increase (r). These can be expressed as:
3. Results
3.1. Development and Fecundity of P. marginatus after Host Plant Shifting
There were no significant differences in egg duration among the four host plants in the F0 generation. However, the developmental times of female and male nymphs fed on C. papaya were significantly shorter than those on the three other hosts. Extremely long developmental times occurred in both female and male nymphs when fed on A. philoxeroides. The detailed development durations for each instar are contained in Supplementary Table S1. The female adults reared on C. papaya lived significantly longer than those fed on the other three plants, although there was no significant difference between those fed on C. papaya and S. tuberosum. The egg duration was, however, significantly longer in the F1 and F2 generations when reared on I. batatas and S. tuberosum. The durations of male nymphs on I. batatas and S. tuberosum were shortened in the F1 and F2 generations. The durations of the female nymphs were unchanged when reared on the three host plants. The female adult longevities were unchanged on C. papaya and I. batatas, but were shortened on S. tuberosum. The adult longevities of the males were shortened on I. batatas and S. tuberosum, but unchanged on C. papaya (Table 1).

Table 1.
The developmental times of Paracoccus marginatus individuals reared on four different host plants (F0–F2).
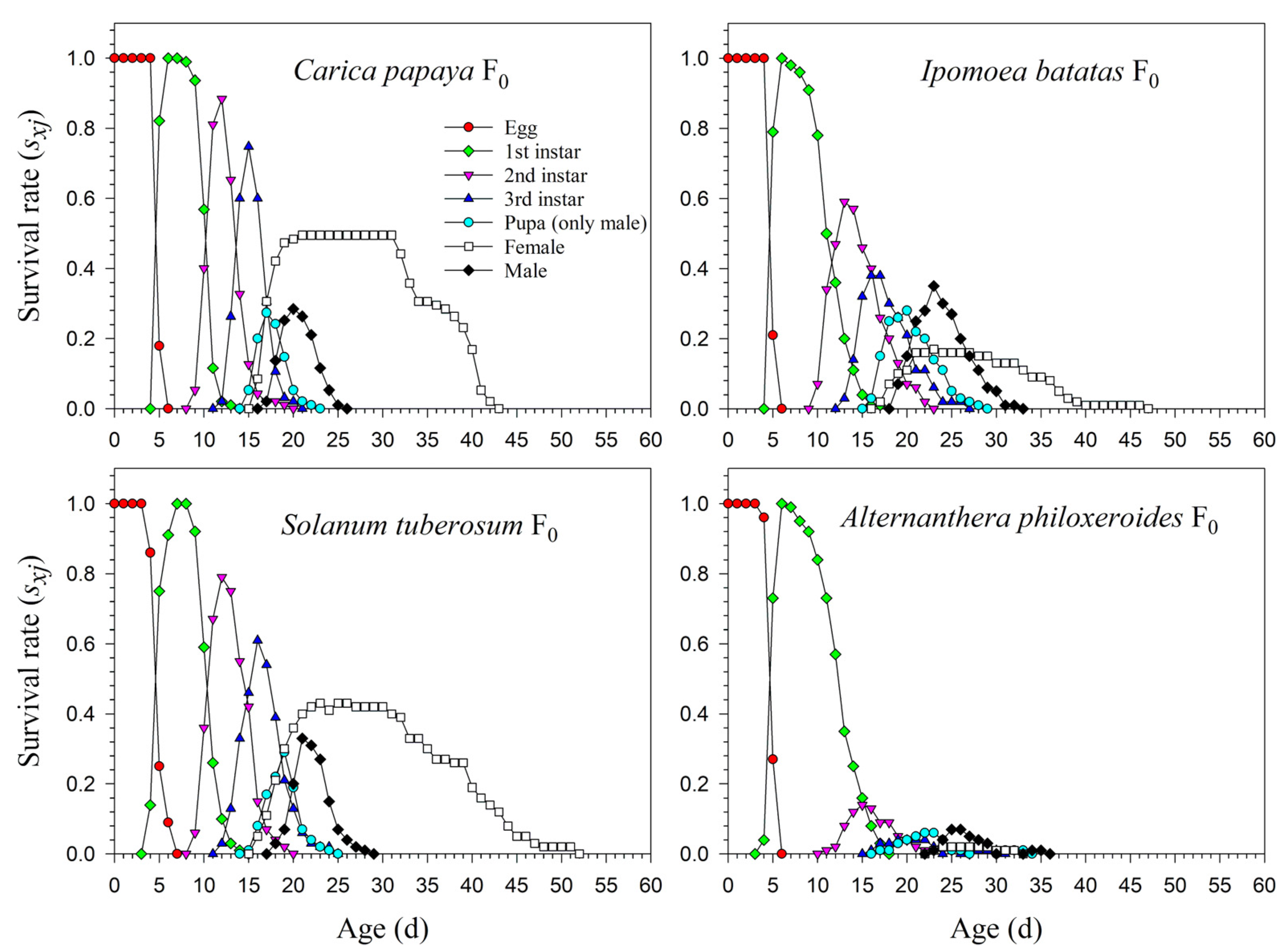
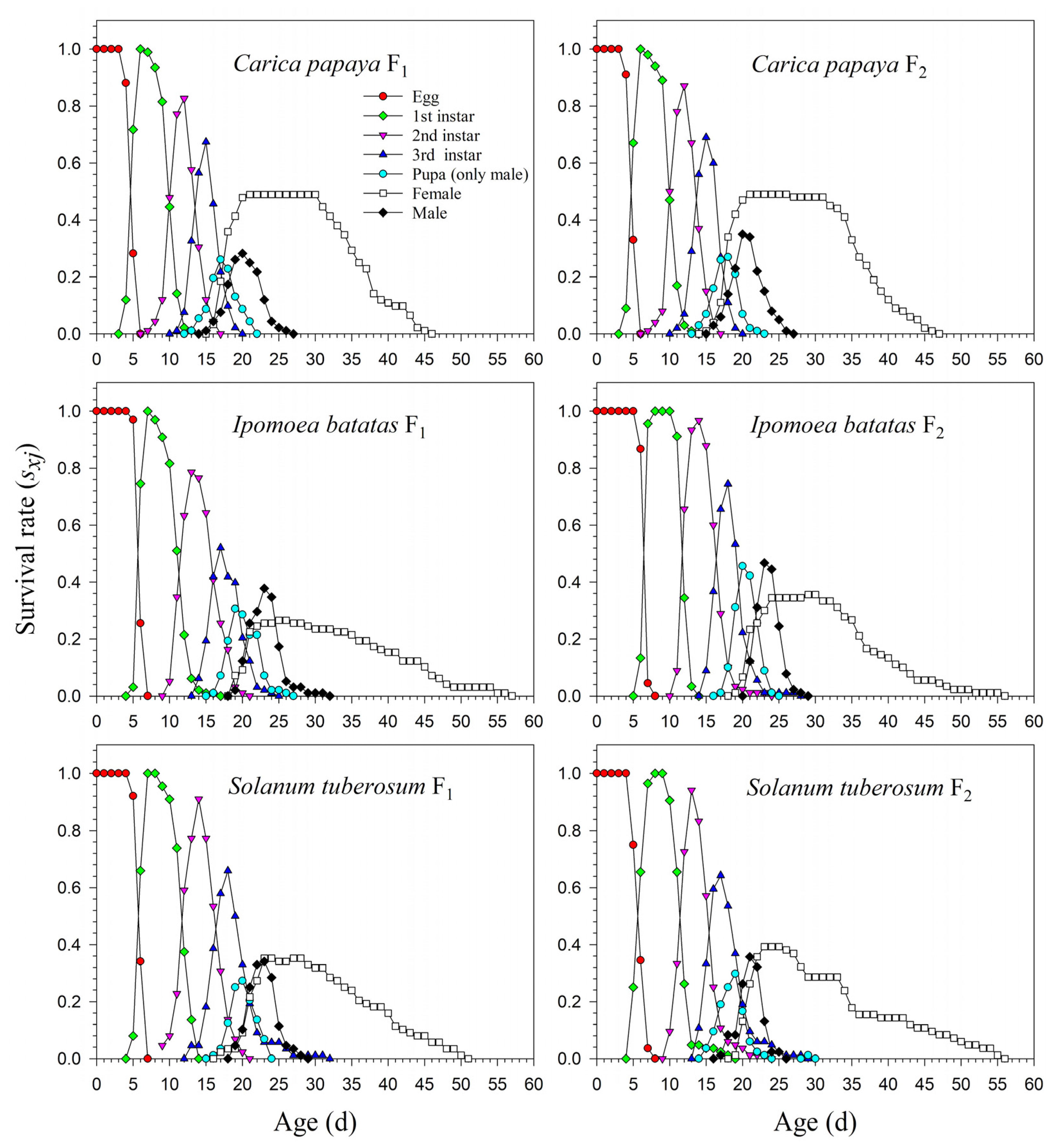
The age-stage life table is capable of describing the stage differentiation; therefore, obvious stage overlapping can be observed. When P. marginatus individuals were reared on A. philoxeroides in F0, the probability of an egg surviving to the 2nd instar was extremely low (i.e., 0.150, 11 individuals), and significantly lower than on other host plants. Only two eggs successfully developed into female adults (Figure 1). In contrast, the survival rates to the 2nd instar when reared on C. papaya were as high as 0.924 and 0.930 in F1 and F2, respectively; higher survival rates to female adulthood (0.489 and 0.490, respectively) were also observed in F1 and F2. Similar high survival rates occurred in the male adults (0.435 in F1 and 0.440 in F2). Lower survival rates were observed when reared on I. batatas and S. tuberosum (Figure 2). The narrow distribution of male adult survival curves (sxj) showed that all male adults had shorter lifespans than the females.
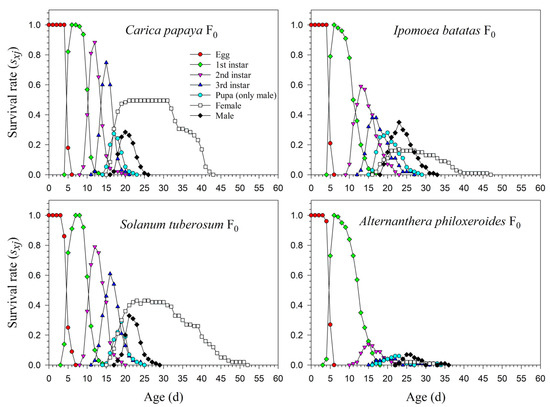
Figure 1.
Age-stage-specific survival rates (sxj) of Paracoccus marginatus individuals reared on four different host plants (F0).
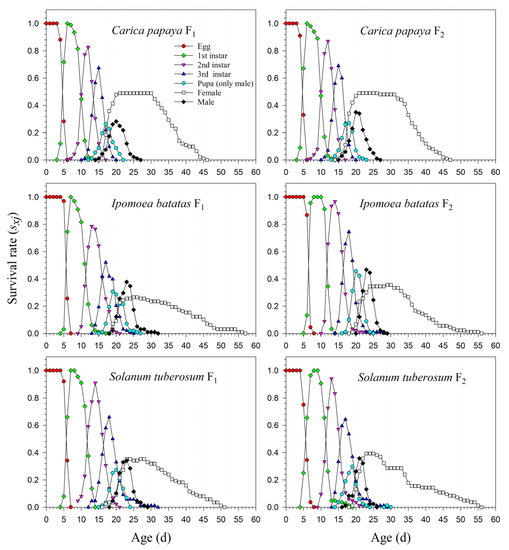
Figure 2.
Age-stage-specific survival rates (sxj) of Paracoccus marginatus individuals reared on three different host plants (F1–F2).
The preadult survival rate of P. marginatus reared on A. philoxeroides in F0 was extremely low (sa = 0.110), while no significant differences occurred among C. papaya, I. batatas, and S. tuberosum. The preadult survival rate of P. marginatus reared on I. batatas increased to 0.933 in F2. Higher proportions of female adults of P. marginatus were observed in F0 on C. papaya (Nf/N = 0.495) and S. tuberosum (Nf/N = 0.450). The Nf/N value on I. batatas was 0.170. An extremely low Nf/N value (0.023) was observed on A. philoxeroides. In the F2 generation, the Nf/N values remained constant on C. papaya and S. tuberosum, but increased to 0.367 on I. batatas. In F0, a significantly high proportion of male adults (Nm/N) of P. marginatus was observed on I. batatas (0.650). The Nm/N values on C. papaya and S. tuberosum were 0.411 and 0.440, respectively. An extremely low Nm/N ratio (0.090) was observed on A. philoxeroides. The Nm/N ratio did not change from F1 to F2 (Table 2).

Table 2.
Preadult survival rates (sa), proportions of female adults (Nf/N), proportions of male adults (Nm/N), and fecundity rates (F) of Paracoccus marginatus individuals reared on four different host plants (F0–F2).
In the F0 generation, the highest fecundity (F) of P. marginatus occurred on C. papaya (202.70 hatched eggs/female), which was significantly higher than in the other three plants. Paracoccus marginatus produced, on average, 6.50 eggs/female when reared on A. philoxeroides. None of the eggs produced on this host were viable, so the mean fecundity was zero (Table 2). Lower fecundity rates were observed on I. batatas and S. tuberosum, with 98.83 hatched eggs/female and 127.46 hatched eggs/female, respectively. On I. batatas, the fecundity increased in F1 (229.50 hatched eggs/female) and F2 (203.76 hatched eggs/female) (Table 2).
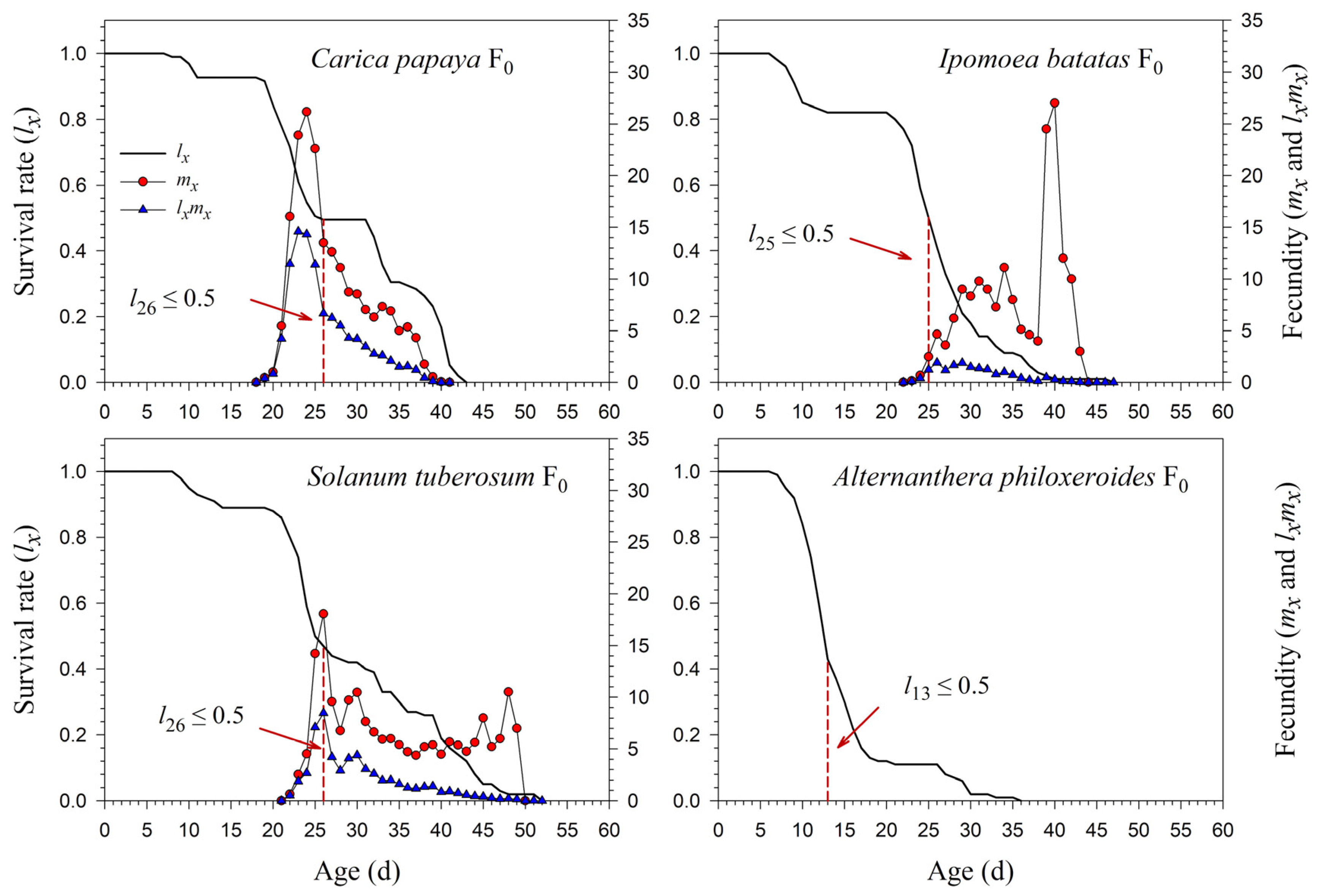
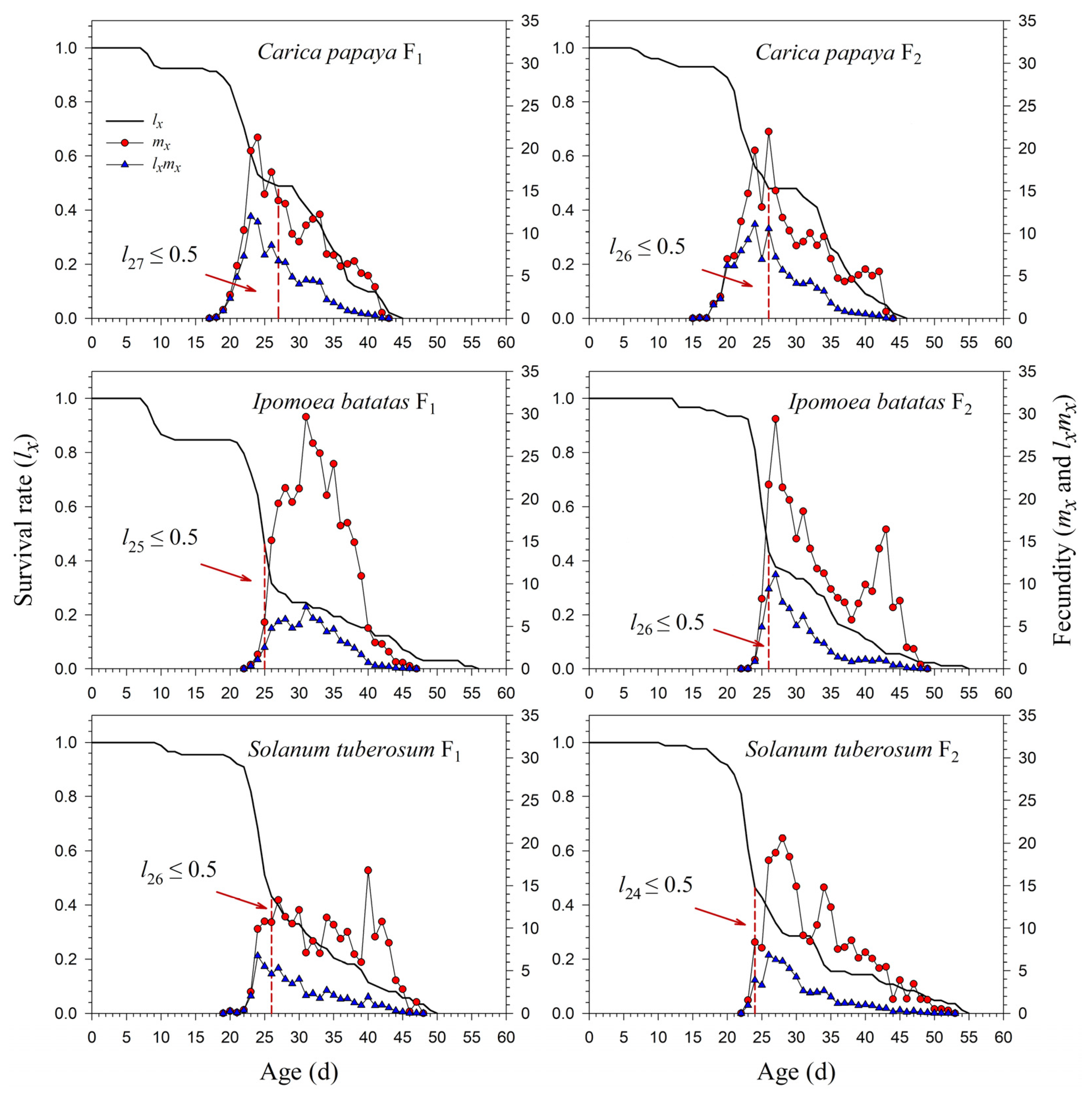
The age-specific survival rate (lx) curve is the simplified version of sxj; thus, the stage differentiation is not observable. The 50% survival rates of P. marginatus in F0 occurred at 26, 25, 26, and 13 d on C. papaya, I. batatas, S. tuberosum, and A. philoxeroides, respectively (Figure 3). In F2, the 50% survival rates of P. marginatus on I. batatas and S. tuberosum changed at 26 and 24 d, respectively (Figure 4). Higher curves of the age-specific fecundity (mx) and net maternity (lxmx) were observed on C. papaya in F0. Although there was a relatively high peak of 27 eggs at 40 d on I. batatas, the low survival rate (lx) caused the net maternity rates (lxmx) to be very low. When reared on S. tuberosum, the high peak of mx (18.4 eggs) occurred at 26 d, and the remaining mx values were, for the most part, greater than 5 eggs (Figure 3). The mx and lxmx values on C. papaya and S. tuberosum did not change significantly; they did, however, increase on I. batatas during the F1 and F2 generations (Figure 4).
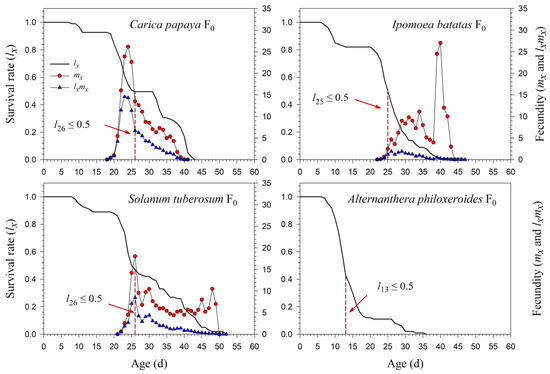
Figure 3.
Age-specific survival (lx), fecundity (mx), and net maternity (lxmx) rates of Paracoccus marginatus individuals reared on four different host plants (F0). The red vertical dashed lines in the figure denote the age at which the survival rate lx ≤ 0.5.
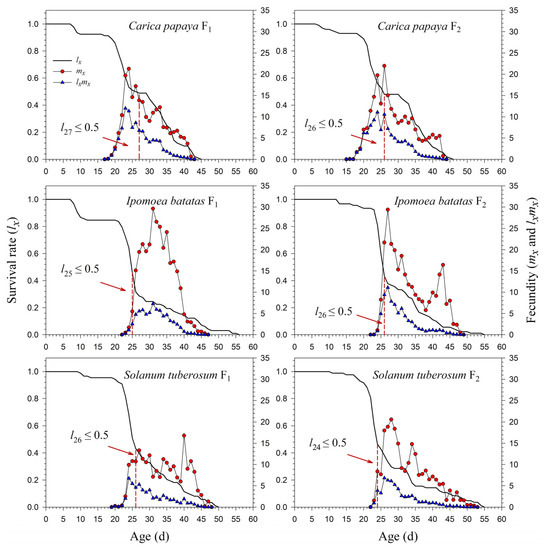
Figure 4.
Age-specific survival (lx), fecundity (mx), and net maternity (lxmx) rates of Paracoccus marginatus individuals reared on three different host plants (F1–F2). The red vertical dashed lines in the figure denote the age at which the survival rate lx ≤ 0.5.
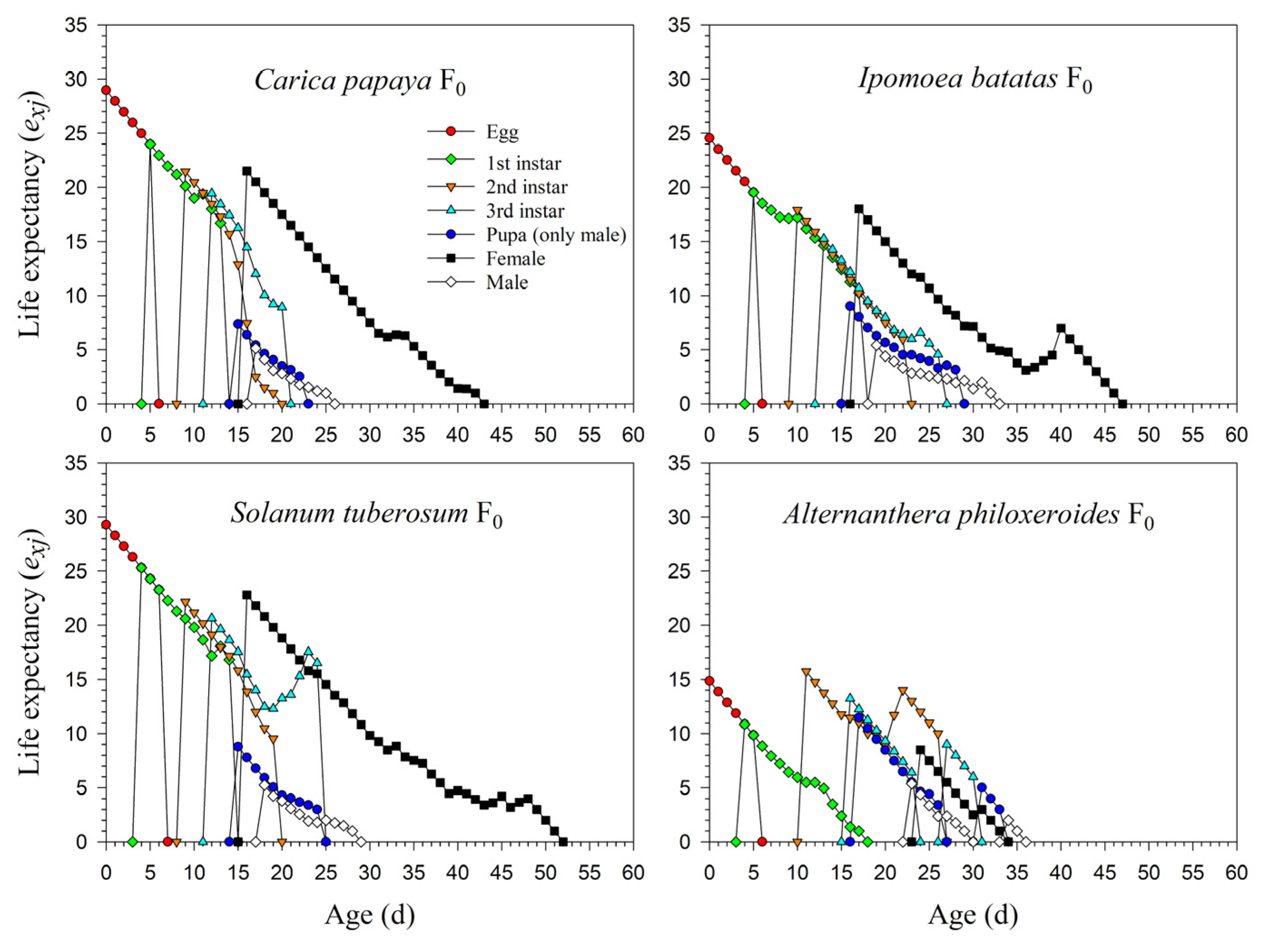
The life expectancy rates of newly laid eggs of P. marginatus were 29.0, 24.5, 29.3, and 14.9 d in F0. The survival rate from the 1st instar to the 2nd instar on A. philoxeroides was extremely low (0.15), and individuals surviving to the 2nd instar could, for the most part, complete their development to adults; hence, the exj curve of the 2nd instar was significantly higher than in the 1st instar (Figure 5). The detailed exj curves on C. papaya, I. batatas, and S. tuberosum during F1 and F2 are shown in Supplementary Figure S1.
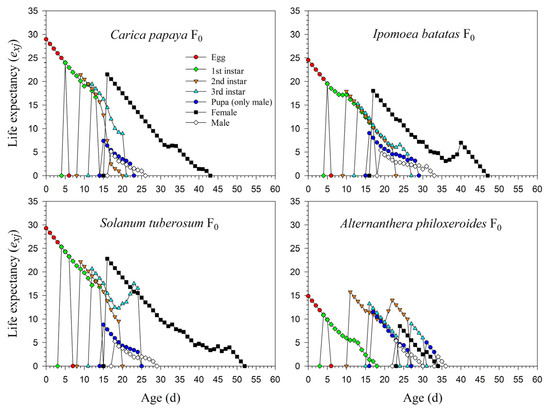
Figure 5.
Age-stage-specific life expectancy (exj) rates of Paracoccus marginatus individuals reared on four different host plants (F0).
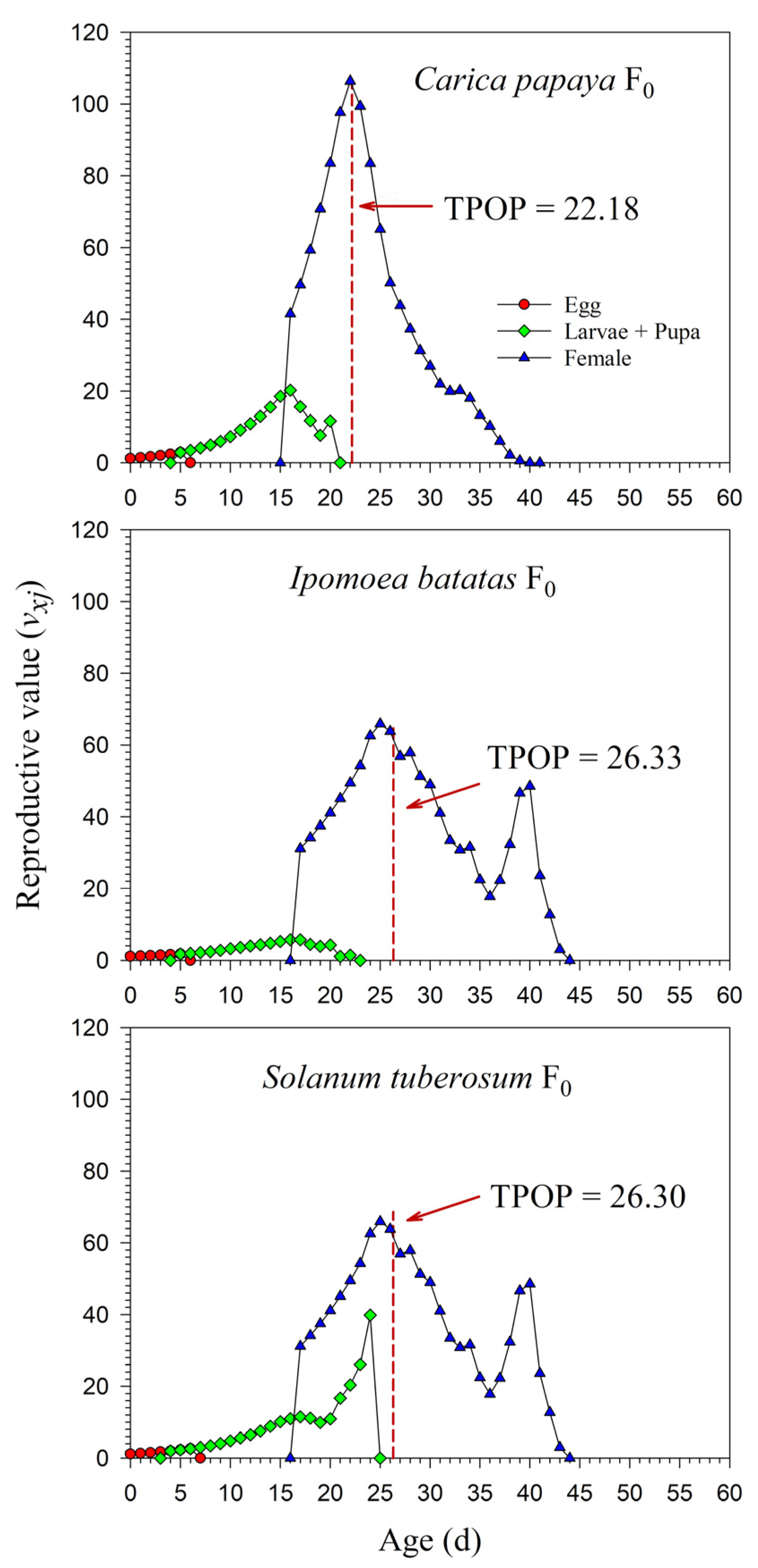
The age-stage-specific reproductive values (vxj) at age zero were exactly equal to the finite rates of increase (λ), i.e., 1.1945, 1.0970, and 1.1475. The vxj increased with age. When reared on I. batatas in F0, the vxj curve significantly increased when female adults emerged. Similar increases in the vxj curves were observed on C. papaya and S. tuberosum; due to the high percentage of female adults, however, this increase was not obvious. The peak dates of vxj were close to the total preoviposition period (TPOP) (Figure 6). The detailed vxj curves on C. papaya, I. batatas, and S. tuberosum for F1 and F2 are shown in Supplementary Figure S2.
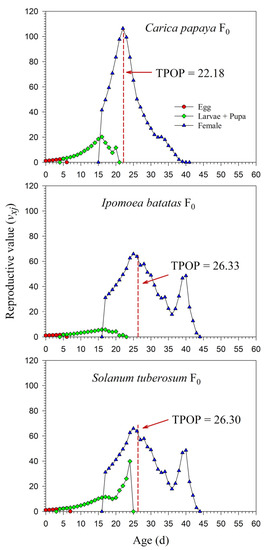
Figure 6.
Age-stage-specific reproductive value (vxj) rates of Paracoccus marginatus individuals reared on three different host plants (F0). The red vertical dashed line in each figure denotes the total preoviposition period (TPOP).
3.2. Population Parameters of P. marginatus after Host Plant Shifting
There were significant differences in the population parameters in the F0 generation of P. marginatus after host plant shifting. The highest values of the net reproductive rate (R0), intrinsic rate of increase (r), and finite rate of increase (λ) for P. marginatus occurred on C. papaya (i.e., 100.26 offspring, 0.1778 d−1 and 1.1945 d−1). Significantly lower R0, r, and λ values were observed when reared on I. batatas and S. tuberosum. Only inviable eggs were produced on A. philoxeroides; thus, the population parameters could not be estimated on this host. The mean generation time (T) of P. marginatus reared on C. papaya was significantly shorter than on I. batatas and S. tuberosum. Although the R0, r, and λ values were not significantly changed in the F1 and F2 individuals when reared on C. papaya and S. tuberosum, higher values did occur in the F1 and F2 generations when reared on I. batatas (Table 3).

Table 3.
Population parameters of Paracoccus marginatus individuals reared on three different host plants (F0–F2).
3.3. Population Projection of P. marginatus
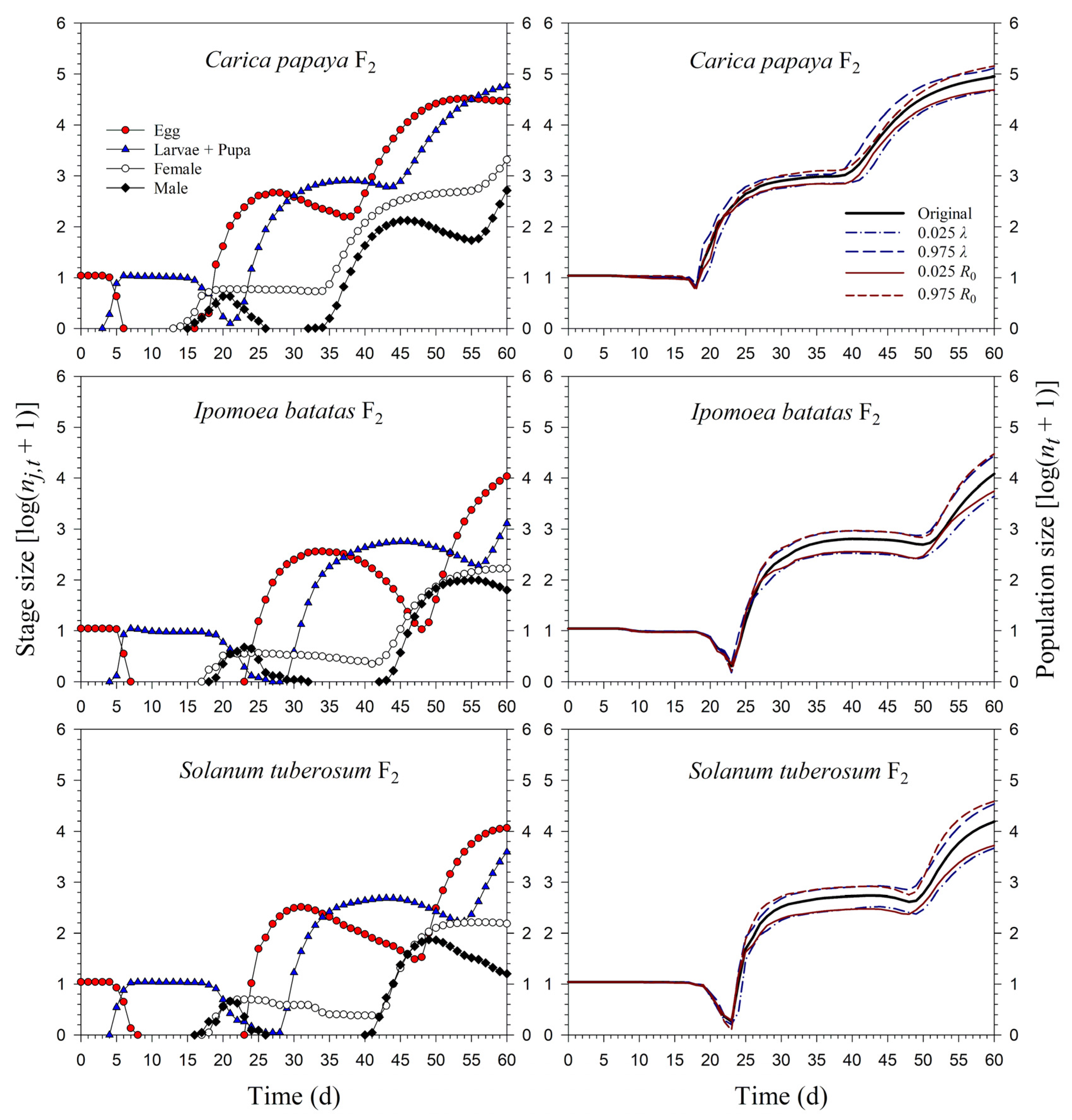
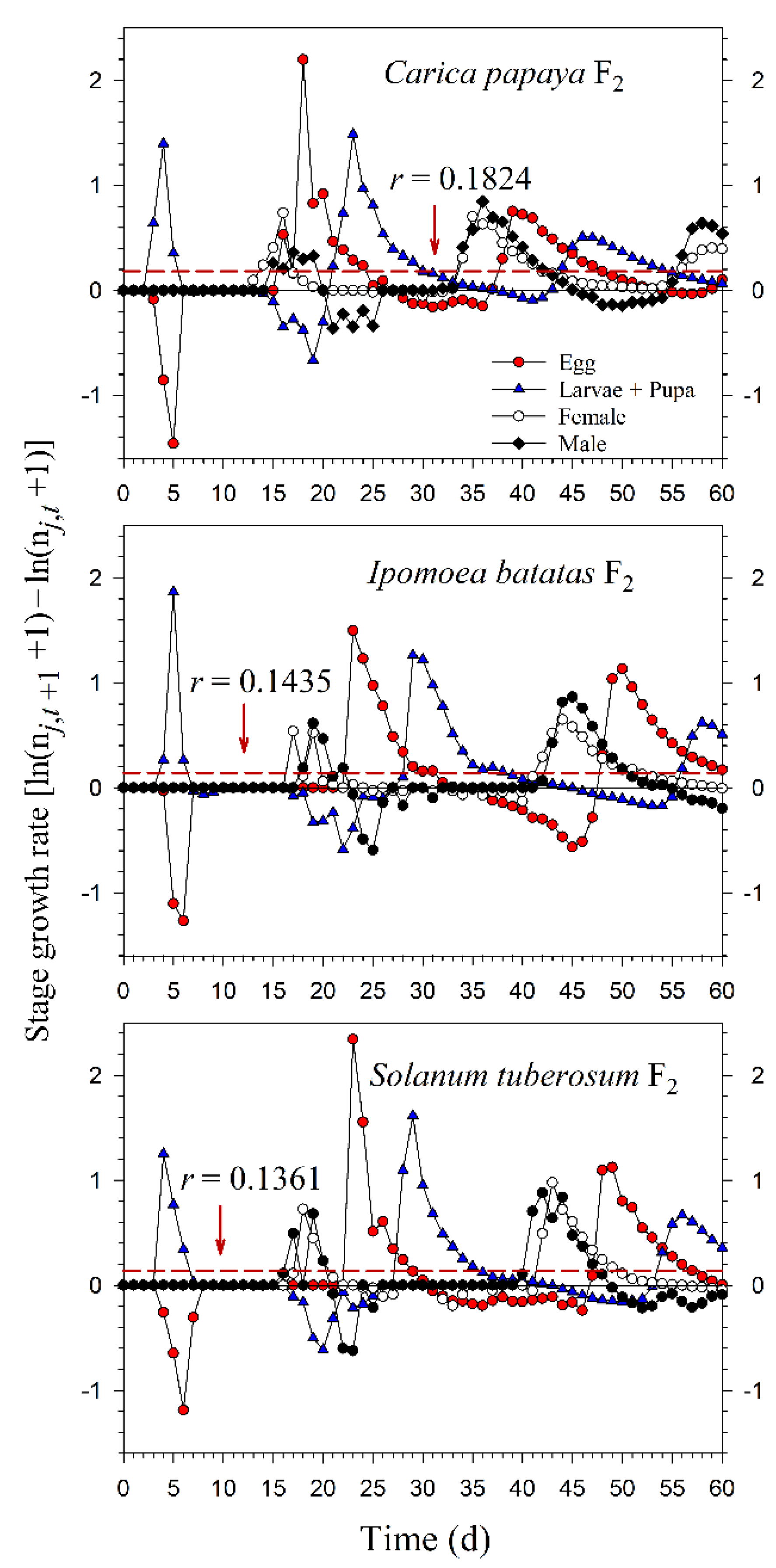
Starting with an initial population of 10 newly enclosed 1st-instar nymphs, P. marginatus could develop to the third generation on C. papaya within 60 d, with a population size reaching as many as 89,552 individuals. However, only two intact generations were observed on I. batatas and S. tuberosum, where the population sizes at 60 d were 12,067 and 15,555 individuals, respectively. When the life tables of the 2.5th and 97.5th percentiles of the net reproductive rate (R0) were used to project the variability of the population growth, the population sizes on C. papaya ranged from 49,033 to 144,038. However, when the life tables of the 2.5th and 97.5th percentiles of the finite rate of increase (λ) were used to project the variability of population growth, the population sizes of P. marginatus ranged from 47,452 to 131,289 (Figure 7). The growth rate curves of all stages fluctuated around the intrinsic rate of increase (r) (Figure 8).
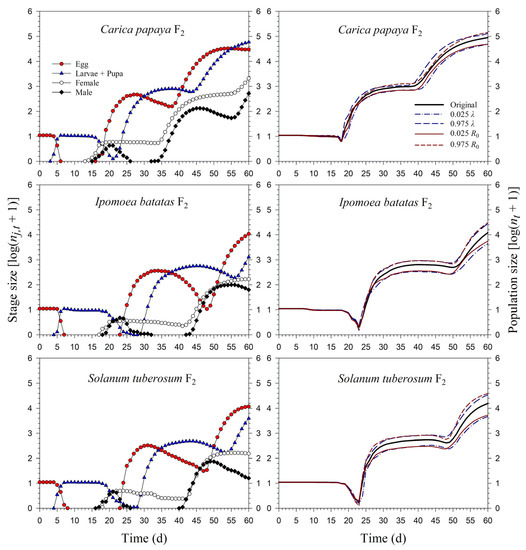
Figure 7.
Stage size (log (nj,t + 1)) and population size (log (nt + 1)) rates of Paracoccus marginatus individuals reared on three different host plants (F2).
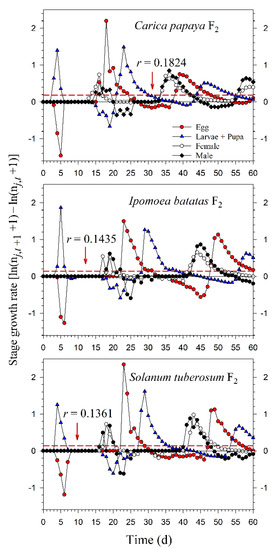
Figure 8.
Fluctuations in the growth rates of each life stage of Paracoccus marginatus individuals reared on three different host plants (F2). The red vertical dashed lines in each figure denote the intrinsic rate of increase (r).
4. Discussion
With the intention to improve our understanding in the fitness changes of P. marginatus after host plant shifting, we investigated the development, fecundity, and population parameters in P. marginatus within three consecutive generations after being transferred from S. tuberosum to C. papaya, I. batatas, and A. philoxeroides. In addition, the population growth rates of the insects on C. papaya, I. batatas, and S. tuberosum were projected. The study showed that P. marginatus transferred to C. papaya had a higher fitness level. When transferred to I. batatas, the fitness decreased initially and then recovered after two generations. Paracoccus marginatus individuals could rapidly expand their populations on the above host plants. Alternanthera philoxeroides was not suitable for the development of P. marginatus.
Multiple factors such as the population growth and total egg production should be adequately considered when evaluating the fitness of an insect population. The construction and comparison of life tables is the most comprehensive method for describing the population growth, development, survival, and reproduction of a species. Insects of different sexes and stages will usually demonstrate different responses when exposed to variations in their host plants, the numbers and composition of their biological enemies, extreme climate conditions, and pesticides, and consequently it is necessary to take all of these into consideration prior to formulating an effective pest management strategy [30,31,32,33]. In order to accomplish this, life tables are fundamental to achieving a comprehensive assessment of a population’s fitness on a given host plant. Thus, it was important to use the age-stage, two-sex life table method to assessed the fitness changes that occurred after host plant shifting in P. marginatus.
The age-stage, two-sex life table not only includes the male component of a population, but is also capable of describing the overlapping and differentiation of each stage [29]. Although the males and females of P. marginatus have different numbers of developmental stages, the stage differentiation can still be precisely described.
The hatch rates of eggs vary with the age of the female adults; hence, using only hatched eggs will enable a more accurate estimate of the population parameters being studied [14,34]. The highest fecundity of P. marginatus was observed on C. papaya (F = 215.27 hatched eggs/female). Seni et al. (2015) reported the fecundity of P. marginatus on C. papaya as 291 total eggs/female (greater than 215.27 hatched eggs/female) [35]; however, the hatch rate was omitted in their study.
By using the age-stage, two-sex life table, He et al. (2021) reported a longer developmental duration and lower intrinsic rate of increase for Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) when reared on soybean, while a shorter developmental duration and higher intrinsic rate occurred on sunflower [36]. Karimi-Pormehr et al. (2018) reported a shorter developmental time, higher survival rate, and greater fecundity in Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) on a more suitable cultivar (‘19A1′) of barley, while noting a longer developmental time and lower fecundity when reared on a less suitable cultivar (‘Fajr30′) [37]. In this study, when P. marginatus was reared on C. papaya, the developmental durations of the 1st, 2nd, and 3rd instar (female) individuals were significantly reduced (Supplementary Table S1); however, the reverse occurred when reared on I. batatas and S. tuberosum. While the fecundity of P. marginatus was significantly higher on C. papaya overall in this study, it was significantly lower on I. batatas (F0). The insects have trade-offs between development and reproduction. When the basic ‘development’ need of insects are met by suitable host plants, insects tend to allocate more energy to reproduction.
Our results showed that P. marginatus reared on C. papaya had a significantly higher proportion of female adults (Nf/N), while a lower Nf/N occurred on I. batatas in the F0 generation. Lewontin (1965) demonstrated that the first age of reproduction plays an important role in the values of r and λ [38]. When P. marginatus was reared on C. papaya, reproduction in the F0 generation started at 18 d, but advanced to 15 d in F2. The three-day change resulted in the value of r increasing from 0.1778 d−1 (F0) to 0.1824 d−1 (F2), while λ increased from 1.1945 d−1 (F0) to 1.2000 d−1 (F2) (Figure 4 and Figure 5). Consequently, this change resulted in P. marginatus reared on C. papaya having higher values for their population parameters (r and λ) due to their higher survival and fecundity rates on this host. The opposite was true when reared on I. batatas and S. tuberosum.
By using the age-stage, two-sex life table, the stage structure and fluctuations in growth rate in different stages can be observed using population projection. In addition, the life tables constructed based on the 2.5th and 97.5th percentiles of R0 and λ can be used to disclose the variabilities that occur during population growth [29].
When host plant shifting happens, the fitness of the insect population to the new host plant may recover after a few generations. Quezada et al. (2015) showed that Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) consecutively reared on less nutritional host plants for three generations would show an adaptive response [39]. Meihls et al. (2008) demonstrated that after three generations of being reared on Bt corn, the survival rate of Diabrotica v. virgifera (LeConte) (Coleoptera: Chrysomelidae) was comparable to beetles reared on normal corn [40]. In this study, when P. marginatus transferred from S. tuberosum to C. papaya, all population parameters were significantly higher than on other plants during three generations. This demonstrated that even though P. marginatus initially survived on S. tuberosum for multiple generations, the insects transferred to C. papaya still had a higher fitness level. However, after transferal to I. batatas, the fitness of P. marginatus initially decreased in F0 and then rebounded in F1 and F2. Paracoccus marginatus showed a higher ability to recover fitness on I. batata. Based on the observation that females were unable to produce viable eggs on A. philoxeroides, we concluded that this host was unsuitable for P. marginatus. This differences in the fitness of P. marginatus to host plants may be due to the volatiles, nutrients of host plants, and so on (unpublished data from the authors).
The age-stage, two-sex life table has been used in a number of studies involving the adaptation of insects on different host plants. Guo et al. (2021) reported that compared with being reared on potato and tobacco, Spodoptera frugiperda reared on maize exhibited a shorter developmental time in the larval period, more female individuals, and a higher reproductive rate [41]. Nemati-Kalkhoran et al. (2018) reported the life table characteristics of Rhyzopertha dominica (Coleoptera: Bostrichidae) on different barley cultivars, demonstrating that a higher net reproductive rate and intrinsic rate of increase occurred on the cultivar ‘Mahoor’ [42]. Jaleel et al. (2018) reported that Bactrocera dorsalis (Diptera: Tephritidae) females produced more eggs on guava than banana [43].
Cipollini and Peterson (2018) pointed out the potential effects of host shifting, including the importance of phytophagous insects being able to find and utilize their ancestral hosts, potentially leading to host range expansions [44]. The present study reports the fitness of P. marginatus after transferal from S. tuberosum to C. papaya, I. batatas, and A. philoxeroides. Ipomoea batatas and S. tuberosum are important food crops and C. papaya is an important fruit [45,46,47]. Our results demonstrate the potential damage of P. marginatus to I. batatas and S. tuberosum, and again verify the severe damage of P. marginatus to C. papaya, even if the insects transfer from suboptimal host plants. These results indicate that outbreaks of P. marginatus are possible in the future, and should they occur may result in serious economic damage. This study provides new insights into the host plant fitness, prevalence, and potential pest control of P. marginatus.
Supplementary Materials
The following supporting information are available at: https://www.mdpi.com/article/10.3390/insects13090804/s1. Table S1: Developmental times of Paracoccus marginatus individuals reared on four different host plants (F0–F2). Figure S1: Age-stage-specific life expectancy (exj) rates of Paracoccus marginatus individuals reared on three different host plants (F1–F2). Figure S2: Age-stage-specific reproductive value (vxj) rates of Paracoccus marginatus individuals reared on three different host plants (F1–F2).
Author Contributions
Conceptualization, H.-Y.C. and J.-W.F.; methodology, H.-Y.C., M.-Z.S. and J.-Y.L.; formal analysis, H.-Y.C., M.-Z.S. and J.-Y.L.; investigation, H.-Y.C., L.-Z.Z. and J.-W.F.; data curation, H.-Y.C., M.-Z.S. and J.-W.F.; writing—original draft preparation, H.-Y.C.; writing—review and editing, H.-Y.C., M.-Z.S., J.-Y.L., L.-Z.Z. and J.-W.F.; visualization, H.-Y.C. and M.-Z.S.; project administration, M.-Z.S., J.-Y.L., L.-Z.Z. and J.-W.F.; funding acquisition, J.-W.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Research Special Foundation of Public Research Institutes of Fujian Province (2020R1022007, 2020R1024005, 2019R1024-1), Open Project of Fujian Key Laboratory of Agro-Products Quality and Safety (APQSKF201901), Agricultural Product Quality Safety and Nutrition Health Innovation Team Project of Fujian Academy of Agricultural Sciences (CXTD2021002), and the “5511” Collaborative innovation Project (XTCXGC2021020).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
We would like to extend our gratitude to the Hsin Chi of the Theoretical and Applied Ecology Department of Entomology National Chung Hsing University Taiwan, and thank Cecil L. Smith (University of Georgia, USA) for the language editing of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, F.Z.; Liu, Z.H.; Shen, H.; Yu, F.; Ma, J.; Hu, X.N.; Zeng, L. Morphological and molecular identification of Paracoccus marginatus (Hemiptera: Pseudococcidae) in Yunnan, China. Fla. Entomol. 2014, 97, 1469–1473. [Google Scholar] [CrossRef]
- Miller, D.R.; Williams, D.J. Notes on a new mealybug (Hemiptera: Coccoidea: Pseudococcidae) pest in Florida and the Caribbean: The papaya mealybug, Paracoccus marginatus Williams and Granara de Willink. Insecta Mundi. 1999, 13, 179–181. [Google Scholar]
- Finch, E.A.; Beale, T.; Chellappan, M.; Goergen, G.; Gadratagi, B.G.; Khan, M.A.; Rehman, M.A.; Rwomushana, I.; Sarma, A.K.; Wyckhuys, K.A.; et al. The potential global distribution of the papaya mealybug, Paracoccus marginatus, a polyphagous pest. Pest Manag. Sci. 2021, 77, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, J.U.; George, M.; Ajesh, G.; Jithine, J.R.; Lekshmi, N.R.; Deepasree, M.I. A review on Paracoccus marginatus Williams, papaya mealybug (Hemiptera: Pseudococcidae). J. Entomol. Zool. Stud. 2016, 4, 528–533. [Google Scholar]
- Huang, F.; Zhang, Z.J.; Li, W.D.; Lin, W.C.; Zhang, P.J.; Zhang, J.M.; Bei, Y.W.; He, Y.P.; Lu, Y.B. Host plant probing analysis reveals quick settlement of the solenopsis mealybug during host shift. J. Econ. Entomol. 2014, 107, 1419–1425. [Google Scholar] [CrossRef]
- Lu, H.; Yang, P.C.; Xu, Y.Y.; Luo, L.; Zhu, J.J.; Cui, N.; Kang, L.; Cui, F. Performances of survival, feeding behavior, and gene expression in aphids reveal their different fitness to host alteration. Sci. Rep. 2016, 6, 19344. [Google Scholar] [CrossRef]
- Ning, S.Y.; Zhang, W.C.; Sun, Y.; Feng, J.N. Development of insect life tables: Comparison of two demographic methods of Delia antiqua (Diptera: Anthomyiidae) on different hosts. Sci. Rep. 2017, 7, 4821. [Google Scholar] [CrossRef]
- Milanović, S.; Janković-Tomanić, M.; Kostić, I.; Kostić, M.; Morina, F.; Živanović, B.; Lazarević, J. Behavioural and physiological plasticity of gypsy moth larvae to host plant switching. Entomol. Exp. Et Appl. 2016, 158, 152–162. [Google Scholar] [CrossRef]
- Mody, K.; Unsicker, S.B.; Linsenmair, K.E. Fitness related diet-mixing by intraspecific host-plant-switching of specialist insect herbivores. Ecology 2007, 88, 1012–1020. [Google Scholar] [CrossRef]
- Fan, Y.J.; Fen, L.; Abd, A.A.H.M.; Yi, X.Q.; Zhang, M.; Nicolas, D.; Gao, X.W. The damage risk evaluation of Aphis gossypii on wheat by host shift and fitness comparison in wheat and cotton. J. Integr. Agric. 2018, 17, 631–639. [Google Scholar] [CrossRef]
- Amarasekare, K.G.; Mannion, C.M.; Osborne, L.S.; Epsky, N.D. Life history of Paracoccus marginatus (Hemiptera: Pseudococcidae) on four host plant species under laboratory conditions. Environ. Entomol. 2008, 37, 630–635. [Google Scholar] [CrossRef]
- Nisha, R.; Kennedy, J.S. Life cycle of papaya mealybug Paracoccus marginatus Williams and Granara de Willink on different host plants vis-à-vis divergent natural selection. J. Entomol. Zool. Stud. 2017, 5, 91–102. [Google Scholar]
- Huang, Y.B.; Chi, H. Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: A case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J. Agric. For. 2012, 61, 37–45. [Google Scholar]
- Mou, D.F.; Lee, C.C.; Smith, C.L.; Chi, H. Using viable eggs to accurately determine the demographic and predation potential of Harmonia dimidiata (Coleoptera: Coccinellidae). J. Appl. Entomol. 2015, 139, 579–591. [Google Scholar] [CrossRef]
- Chi, H.; Liu, S. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2022. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Chi, H.; You, M.S.; Atlıhan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 102–123. [Google Scholar] [CrossRef]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Euler, L. De aptissima figura rotarum dentibus tribuenda. Novi Comment. Acad. Sci. Petropolitanae 1760, 17, 299–316. [Google Scholar]
- Lotka, A.J. Studies on the mode of growth of material aggregates. Am. J. Sci. 1907, 24, 199–216. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Fisher, R.A. The Genetical Theory of Natural Selection; Clarendon Press: Oxford, UK, 1930. [Google Scholar]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Chi, H. Timing-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2022. [Google Scholar]
- Huang, H.W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum: With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Fu, J.W.; Shi, M.Z.; Wang, T.; Li, J.Y.; Zheng, L.Z.; Wu, G. Demography and population projection of flea beetle, Agasicles hygrophila (Coleoptera: Chrysomelidae), fed on alligator weed under elevated CO2. J. Econ. Entomol. 2016, 109, 1116–1124. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.M.; Chi, H.; Wang, R.C.; Wang, Y.P.; Xu, Y.Y.; Li, X.D.; Yin, P.; Zheng, F.Q. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J. Econ. Entomol. 2018, 111, 2143–2152. [Google Scholar] [CrossRef]
- Mastoi, M.I.; Adam, N.A.; Muhamad, R.; Ghani, I.A.; Gilal, A.A.; Khan, J.; Bhatti, A.R.; Zia, A.; Sahito, J.G.M. Efficiency of Acerophagus papayae on different host stage combinations of papaya mealybug, Paracoccus marginatus. Pak. J. Agric. Sci. 2018, 55, 375–379. [Google Scholar]
- Shi, M.Z.; Li, J.Y.; Ding, B.; Fu, J.W.; Zheng, L.Z.; Chi, H. Indirect effect of elevated CO2 on population parameters and growth of Agasicles hygrophila (Coleoptera: Chrysomelidae), a biocontrol agent of alligatorweed (Amaranthaceae). J. Econ. Entomol. 2019, 112, 1120–1129. [Google Scholar] [CrossRef]
- Jha, R.K.; Chi, H.; Tang, L.C. Effects of survival rate and fecundity on the intrinsic rate of increase of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Formos. Entomol. 2012, 32, 223–235. [Google Scholar]
- Seni, A.; Sahoo, A.K. Biology of Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Pseudococcidae) on papaya, parthenium and brinjal plants. Res. Crop. 2015, 16, 722–727. [Google Scholar] [CrossRef]
- He, L.M.; Wu, Q.L.; Gao, X.W.; Wu, K.M. Population life tables for the invasive fall armyworm, Spodoptera frugiperda fed on major oil crops planted in China. J. Integr. Agric. 2021, 20, 745–754. [Google Scholar] [CrossRef]
- Karimi-Pormehr, M.S.; Borzoui, E.; Naseri, B.; Dastjerdi, H.R.; Mansouri, S.M. Two-sex life table analysis and digestive physiology of Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) on different barley cultivars. J. Stored Prod. Res. 2018, 75, 64–71. [Google Scholar] [CrossRef]
- Lewontin, R.C. Selection for Colonizing Ability; Baker, H.G., Stebbins, G.L., Eds.; The Genetics of Colonizing Species; Academic Press: San Diego, CA, USA, 1965; pp. 77–94. [Google Scholar]
- Quezada, G.R.; Seehausen, M.L.; Bauce, É. Adaptation of an outbreaking insect defoliator to chronic nutritional stress. J. Evolution. Biol. 2015, 28, 347–355. [Google Scholar] [CrossRef]
- Meihls, L.N.; Higdon, M.L.; Siegfried, B.D.; Miller, N.J.; Sappington, T.W.; Ellersieck, M.R.; Spencerc, T.A.; Hibbard, B.E. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc. Natl. Acad. Sci. USA 2008, 105, 19177–19182. [Google Scholar] [CrossRef]
- Guo, J.F.; Zhang, M.D.; Gao, Z.P.; Wang, D.J.; He, K.L.; Wang, Z.Y. Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: Potential risks to potato and tobacco crops. Insect Sci. 2021, 28, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Nemati-Kalkhoran, M.; Razmjou, J.; Borzoui, E.; Naseri, B. Comparison of life table parameters and digestive physiology of Rhyzopertha dominica (Coleoptera: Bostrichidae) fed on various barley cultivars. J. Insect Sci. 2018, 18, 31. [Google Scholar] [CrossRef]
- Jaleel, W.; Tao, X.B.; Wang, D.S.; Lu, L.H.; He, Y.R. Using two-sex life table traits to assess the fruit preference and fitness of Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2018, 111, 2936–2945. [Google Scholar] [CrossRef]
- Cipollini, D.; Peterson, D.L. The potential for host switching via ecological fitting in the emerald ash borer-host plant system. Oecologia 2018, 187, 507–519. [Google Scholar] [CrossRef]
- Tan, S.L. Sweet potato-Ipomoea batatas-a great health food. Agric. Sci. 2015, 3, 15–28. [Google Scholar]
- Singh, A.; Yemmireddy, V. Fate of Salmonella spp. in fresh-cut papaya (Carica papaya L.) at different storage temperature and relative humidity. LWT Food Sci. Technol. 2021, 148, 111810. [Google Scholar] [CrossRef]
- Tian, W.J.; He, G.D.; Qin, L.J.; Li, D.D.; Meng, L.L.; Huang, Y.; He, T.B. Genome-wide analysis of the NRAMP gene family in potato (Solanum tuberosum): Identification, expression analysis and response to five heavy metals stress. Ecotoxicol. Environ. Saf. 2021, 208, 111661. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).