Modulating the Fatty Acid Profiles of Hermetia illucens Larvae Fats by Dietary Enrichment with Different Oilseeds: A Sustainable Way for Future Use in Feed and Food
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Biological Material Origin
2.2. Evaluated Parameters
2.3. Experimental Design
2.4. Growing Performances
2.5. Fat and Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
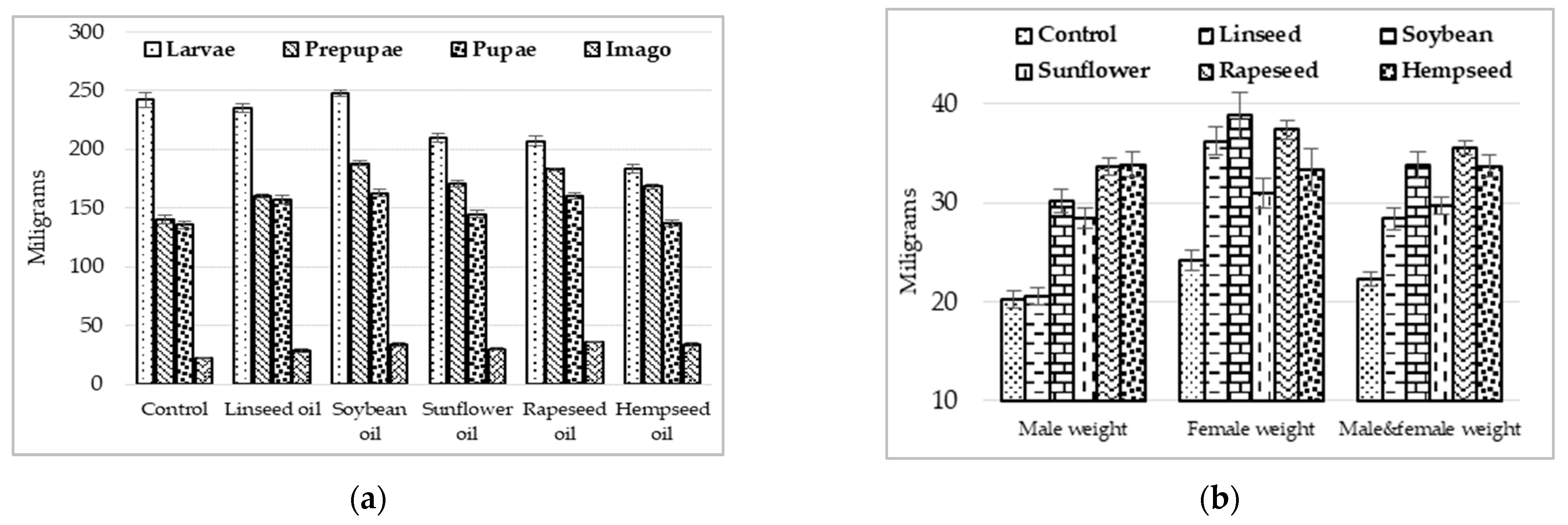
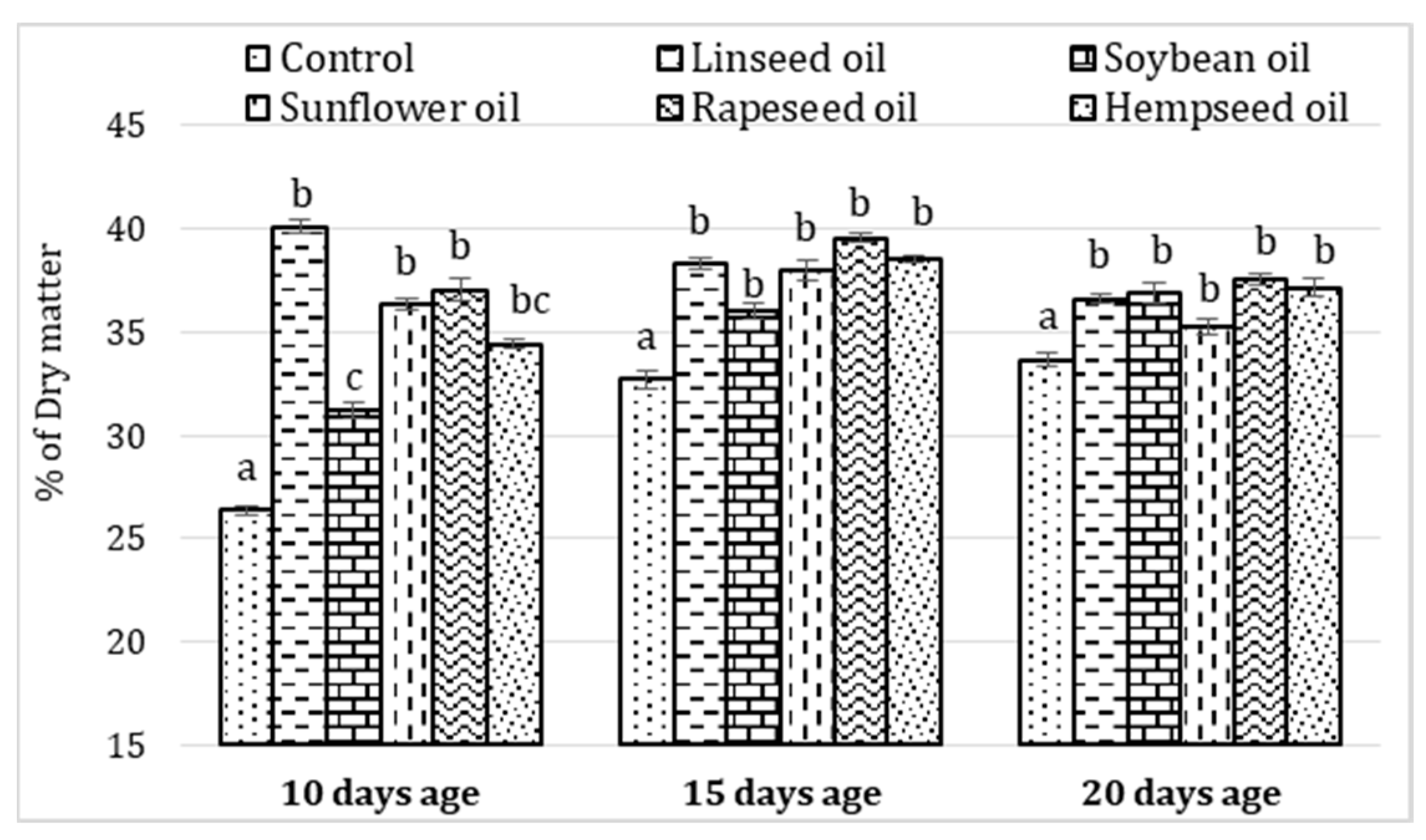
3.1. Growing Performances
3.2. Reproductive Performances of H. illucens Individuals
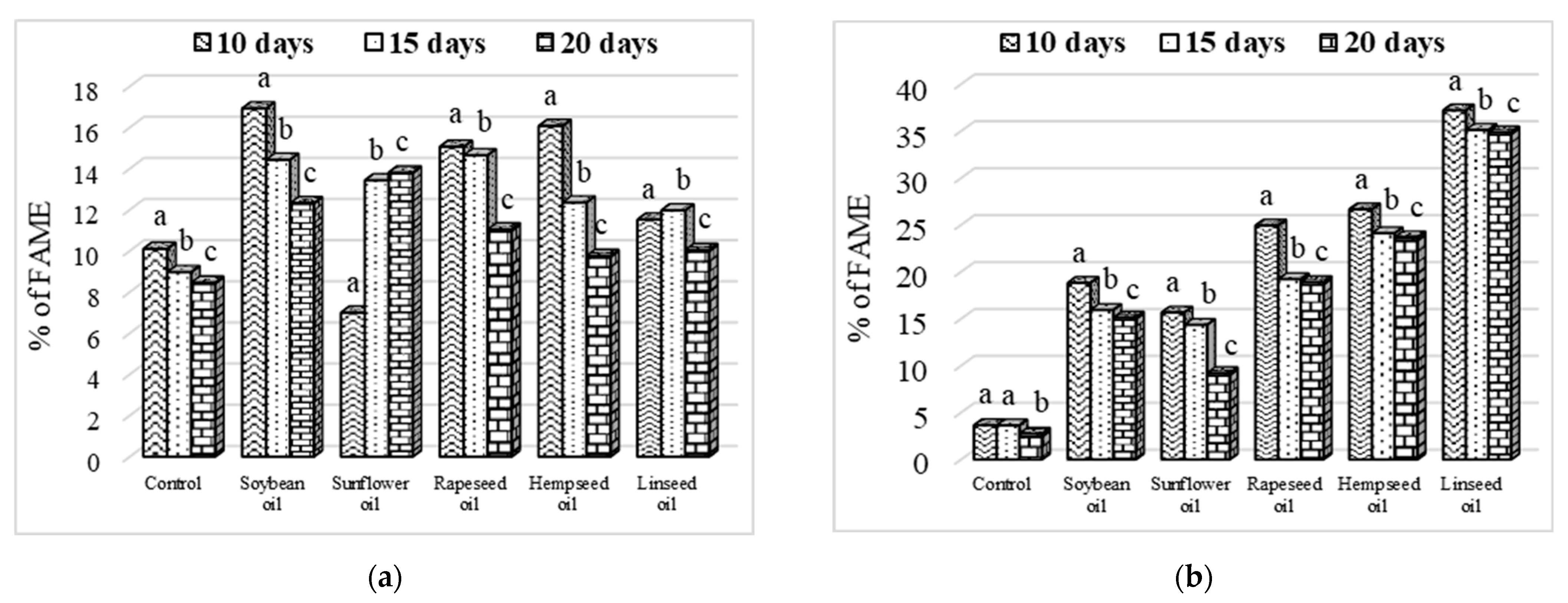
3.3. Larvae Crude Fat Content and Fatty Acid Profile
3.4. Evolution of Fatty Acid Content Depending on Larvae Age
4. Discussion
4.1. Performance Response
4.2. Fats and Fatty Acid Profile of Larvae
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kostadinović, V.S.; Brühl, L.; Mitrev, S.; Mirhosseini, H.; Matthäus, B. Quality evaluation of cold-pressed edible oils from Macedonia. Eur. J. Lipid Sci. Technol. 2015, 117, 2023–2035. [Google Scholar] [CrossRef]
- Sobko, O.; Zikeli, S.; Claupein, W.; Gruber, S. Seed yield, seed protein, oil content, and agronomic characteristics of soybean (Glycine max L. Merrill) depending on different seeding systems and cultivars in Germany. Agronomy 2020, 10, 1020. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Zhang, S.; Azam, M.; Shaibu, A.S.; Feng, Y.; Qi, J.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; et al. Natural variation in fatty acid composition of diverse world soybean germplasms grown in China. Agronomy 2020, 10, 24. [Google Scholar] [CrossRef]
- Czyż, K.; Sokoła-Wysoczańska, E.; Wyrostek, A.; Cholewińska, P. An attempt to enrich pig meat with omega-3 fatty acids using linseed oil ethyl ester diet supplement. Agriculture 2021, 11, 365. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Rolland, Y.; Barreto, P.D.S.; Maltais, M.; Guyonnet, S.; Cantet, C.; Andrieu, S.; Vellas, B. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain lifestyle intervention on muscle strength in older adults: Secondary analysis of the Multidomain Alzheimer Preventive Trial (MAPT). Nutrients 2019, 11, 1931. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Nichols, P.D.; Malau-Aduli, A.E. Enhancing omega-3 long-chain polyunsaturated fatty acid content of dairy-derived foods for human consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; No. 171; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Gahukar, R.T. Edible insects farming: Efficiency and impact on family livelihood, food security, and environment compared with livestock and crops. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 85–111. [Google Scholar]
- Dicke, M. Insects as feed and the sustainable development goals. J. Insects Food Feed 2018, 4, 147–156. [Google Scholar] [CrossRef]
- Govorushko, S. Global status of insects as food and feed source: A review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Yang, K.; Wang, T.; Wang, Y.; Jia, Y.; Yin, Y.; Gu, P.; Miao, H. Toxic effects of industrial flocculants addition on bioconversion of black soldier fly larvae (Hermetia illucens L.). Insects 2022, 13, 683. [Google Scholar] [CrossRef] [PubMed]
- Borkent, S.; Hodge, S. Glasshouse evaluation of the black soldier fly waste product hexafrass™ as an organic fertilizer. Insects 2021, 12, 977. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 2002, 37, 345–352. [Google Scholar] [CrossRef]
- English, G.; Wanger, G.; Colombo, S.M. A review of advancements in black soldier fly (Hermetia illucens) production for dietary inclusion in salmonid feeds. J. Agric. Food Res. 2021, 5, 100164. [Google Scholar] [CrossRef]
- Papuc, T.; Boaru, A.; Ladosi, D.; Struti, D.; Georgescu, B. Potential of black soldier fly (Hermetia illucens) as alternative protein source in salmonid feeds—A review. Indian J. Fish 2020, 67, 160–170. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Brodskii, E.S.; Kovalenko, A.A.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 2016, 468, 209–212. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed–a review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)–Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Hoc, B.; Noël, G.; Carpentier, J.; Francis, F.; Caparros, M.R. Optimization of black soldier fly (Hermetia illucens) artificial reproduction. PLoS ONE 2019, 14, e0216160. [Google Scholar]
- Barroso, F.G.; Sánchez-Muros, M.J.; Rincón, M.Á.; Rodriguez-Rodriguez, M.; Fabrikov, D.; Morote, E.; Guil-Guerrero, J.L. Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 2019, 82, 103237. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2020, 27, 500–509. [Google Scholar] [PubMed]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; Meulenaer, B.D.; Michiels, J.; Eeckhout, M.; Clercq, P.D.; Smet, S.D. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Giannetto, A.; Oliva, S.; Ceccon, L.C.F.; De Araujo, P.F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spano, N.C.; Cappello, T.; et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Biomass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar]
- Truzzi, C.; Giorgini, E.; Annibaldi, A.; Antonucci, M.; Illuminati, S.; Scarponi, G.; Paola, R.; Nunzio, I.; Carla, C.; Matteo, Z.; et al. Fatty acids profile of black soldier fly (Hermetia illucens): Influence of feeding substrate based on coffee-waste silverskin enriched with microalgae. Anim. Feed Sci. Technol. 2020, 259, 114309. [Google Scholar]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life Table of black soldier fly (Hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Broekhoven, S.; Van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar]
- Barroso, F.G.; Sánchez-Muros, M.J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Hoc, B.; Francis, F.; Carpentier, J.; Mostade, L.; Blecker, C.; Purcaro, G.; Megido, R.C. ω3-enrichment of Hermetia illucens (L. 1758) prepupae from oilseed byproducts. J. Saudi Soc. Agric. Sci. 2021, 20, 155–163. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and fatty acid composition of black soldier fly Hermetia illucens (Diptera: Stratiomyidae) larvae are influenced by dietary fat sources and levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.; van Broekhoven, S.; van Huis, A.; van Loon, J.J. Correction: Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2019, 14, e0222043. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Adler, P.H.; Myers, H.M. Development of the black soldier fly (Diptera: Stratiomyidae) in relation to temperature. Environ. Entomol. 2009, 38, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ichiki, R.T.; Shimoda, M.; Morioka, S. Small-scale rearing of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae), in the laboratory: Low-cost and year-round rearing. Appl. Entomol. Zool. 2016, 51, 161–166. [Google Scholar] [CrossRef]
- Hogsette, J.A. New diets for production of house flies and stable flies (Diptera: Muscidae) in the laboratory. J. Econ. Entomol. 1992, 85, 2291–2294. [Google Scholar] [CrossRef]
- Omar, K.A.; Shan, L.; Wang, Y.L.; Wang, X. Stabilizing flaxseed oil with individual antioxidants and their mixtures. Eur. J. Lipid Sci. Technol. 2010, 112, 1003–1011. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, W.T.; Lee, S.B.; Choi, Y.C.; Nho, S.K. Seasonal pupation, adult emergence and mating of black soldier fly, Hermetia illucens (Diptera: Stratiomyidae) in artificial rearing system. Int. J. Indust. Entomol. 2010, 21, 189–191. [Google Scholar]
- Boaru, A.; Vig, A.; Ladoşi, D.; Păpuc, T.; Struţi, D.; Georgescu, B. The use of various oviposition structures for the black soldier fly, Hermetia illucens L. (Diptera: Stratiomydae) in improving the reproductive process in captivity. ABAH Bioflux. 2019, 11, 12–20. [Google Scholar]
- Georgescu, B.; Struți, D.I.; Sima, N.F.; Păpuc, T.A.; Mihaela, B.A. A comprehensive method for the evaluation of Hermetia illucens egg quality parameters: Implications and influence factors. Insects 2022, 13, 17. [Google Scholar]
- AOAO. Official Method of Analysis, 18th ed.; The Association of Official Analytical Chemists (AOAC): Washington, DC, USA, 2005. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; The Association of Official Analytical Chemists (AOAC): Washington DC, USA, 1995. [Google Scholar]
- Timmons, J.S.; Weiss, W.P.; Palmquist, D.L.; Harper, W.J. Relationships among dietary roasted soybeans, milk components, and spontaneous oxidized flavor of milk. Int. J. Dairy Sci. 2001, 84, 2440–2449. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The effects of diet formulation on the yield, proximate composition, and fatty acid profile of the black soldier fly (Hermetia illucens L.) prepupae intended for animal feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, B.; Struţi, D.; Papuc, T.; Cighi, V.; Boaru, A. Effect of the energy content of diets on the development and quality of the fat reserves of larvae and reproduction of adults of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Eur. J. Entomol. 2021, 118, 297–306. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.L.; Lognay, G.; Francis, F.; Caparros, M.R. About lipid metabolism in Hermetia illucens (L. 1758): On the origin of fatty acids in prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- Li, X.; Song, L.; Chen, Y.; Li, Y.; Zuo, T.; Wu, S. Influence of different fatty acids in artificial diets on growth, development and fecundity of Arma chinensis. Sci. Silvae Sin. 2018, 54, 85–93. [Google Scholar]
- Chang, C.L.; Vargas, R.I. Wheat germ oil and its effects on a liquid larval rearing diet for oriental fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 2014, 100, 322–326. [Google Scholar] [CrossRef]
- Senyilmaz, D.; Virtue, S.; Xu, X.; Tan, C.Y.; Griffin, J.L.; Miller, A.K.; Vidal, P.A.; Teleman, A.A. Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature 2015, 525, 124–128. [Google Scholar] [CrossRef]
- Wang, S.; Xu, B.; Wang, H. Appropriate linoleic acid supplemental level in Apis mellifera ligustica worker larvae diet. Chin. J. Clin. Nutr. 2015, 27, 1440–1449. [Google Scholar]
- Bertinetti, C.; Samayoa, A.C.; Hwang, S.Y. Effects of feeding adults of Hermetia illucens (Diptera: Stratiomyidae) on longevity, oviposition, and egg hatchability: Insights into optimizing egg production. J. Insect Sci. 2019, 19, 19. [Google Scholar] [CrossRef] [PubMed]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, B.; Struţi, D.; Păpuc, T.; Ladoşi, D.; Boaru, A. Body weight loss of black soldier fly Hermetia illucens (Diptera: Stratiomyidae) during development in non-feeding stages: Implications for egg clutch parameters. Eur. J. Entomol. 2020, 117, 216–225. [Google Scholar] [CrossRef]
- Zhu, Z.; Ur Rehman, K.; Yu, Y.; Liu, X.; Wang, H.; Tomberlin, J.K.; Sze, S.H.; Cal, M.; Zhang, J.; Yu, Z.; et al. De novo transcriptome sequencing and analysis revealed the molecular basis of rapid fat accumulation by black soldier fly (Hermetia illucens L.) for development of insectival biodiesel. Biotechnol. Biofuels 2019, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical analysis of minor bioactive components and cannabidiolic acid in commercial hemp seed oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- WHO; FAO Expert Consultation. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2003; Volume 916, pp. 1–149. [Google Scholar]
- Thais, S.R.; Sophie, V.D.S.; Catherine, D.; Evert, T.; Ronald, E.V.K.; Nele, J. Endogenous pathway for LC-PUFA synthesis DHA, EPA and ARA by enzymatic desaturation and chain elongation steps. PLoS ONE 2013, 8, e68000. [Google Scholar]

| Specification | Control | Experimental Treatments (Mean ± SE) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Linseed Oil | Soybean Oil | Sunflower Oil | Rapeseed Oil | Hempseed Oil | ||||
| Larvae weight | 10 days age | 142.47 ± 3.18 a | 162.26 ± 2.62 bc | 165.64 ± 2.52 b | 169.45 ± 2.47 b | 152.25 ± 1.73 ac | 183.43 ± 4.17 d | 0.001 |
| 15 days age | 239.74 ± 2.75 a | 282.77 ± 2.90 b | 279.92 ± 2.84 b | 261.32 ± 2.86 c | 262.75 ± 2.89 c | 246.69 ± 2.86 a | 0.001 | |
| 20 days age | 241.80 ± 6.18 a | 234.83 ± 3.91 a | 247.25 ± 2.65 a | 209.27 ± 3.70 b | 206.82 ± 4.50 b | 183.55 ± 3.62 c | 0.001 | |
| Prepupae weight | 140.18 ± 3.56 a | 160.27 ± 1.66 b | 187.76 ± 2.29 d | 170.39 ± 2.54 bc | 183.07 ± 1.07 dc | 168.20 ± 2.34 b | 0.001 | |
| Pupae weight | 135.48 ± 3.52 a | 157.27 ± 3.64 bc | 162.66 ± 3.56 b | 144.72 ± 3.35 ac | 160.17 ± 2.85 b | 136.99 ± 2.82 a | 0.001 | |
| Male weight | 20.28 ± 0.88 a | 20.57 ± 0.83 a | 30.16 ± 1.15 bc | 28.49 ± 1.03 c | 33.70 ± 0.88 b | 33.86 ± 1.30 d | 0.001 | |
| Female weight | 24.22 ± 1.05 a | 36.21 ± 1.43 b | 38.79 ± 2.40 b | 30.93 ± 1.47 c | 37.41 ± 0.96 b | 33.36 ± 2.15 bc | 0.001 | |
| Male and female weight | 22.25 ± 0.71 a | 28.39 ± 1.14 b | 33.86 ± 1.34 c | 29.71 ± 0.90 b | 35.55 ± 0.67 c | 33.69 ± 1.11 c | 0.001 | |
| Parameters | Control | Experimental Treatments (Mean ± SE) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Linseed Oil | Soybean Oil | Sunflower Oil | Rapeseed Oil | Hempseed Oil | |||
| Clutch weight (mg) | 19.97 ± 1.49 a | 17.74 ± 2.25 a | 16.70 ± 0.89 a | 17.97 ± 1.28 a | 15.57 ± 1.60 a | 17.75 ± 1.46 a | 0.361 |
| Eggs number/clutch | 852.30 ± 45.91 a | 771.50 ± 83.48 a | 794.36 ± 47.88 a | 748.00 ± 49.62 a | 850.67 ± 96.14 a | 799.24 ± 76.11 a | 0.672 |
| Egg weight (mg) | 0.0234 ± 0.01 a | 0.0226 ± 0.01 a | 0.0213 ± 0.01 ab | 0.0240 ± 0.01 a | 0.0186 ± 0.01 b | 0.0223 ± 0.01 a | 0.001 |
| Total clutches (n) | 41 | 48 | 42 | 32 | 31 | 28 | - |
| Fatty Acids | Control | Experimental Treatments (Mean ± SE) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Linseed Oil | Soybean Oil | Sunflower Oil | Rapeseed Oil | Hempseed Oil | |||
| Lauric acid (C12:0) | 44.75 ± 0.07 a | 18.67 ± 0.05 b | 21.32 ± 0.08 c | 28.74 ± 0.03 d | 23.95 ± 0.06 e | 25.25 ± 0.06 f | 0.001 |
| Miristic acid (C14:0) | 7.39 ± 0.07 a | 5.64 ± 0.07 b | 7.52 ± 0.09 a | 6.78 ± 0.06 c | 5.87 ± 0.06 d | 7.21 ± 0.05 a | 0.001 |
| Palmitic acid (C16:0) | 23.93 ± 0.03 a | 18.72 ± 0.07 b | 24.10 ± 0.05 c | 21.07 ± 0.03 d | 18.66 ± 0.07 b | 20.16 ± 0.06 e | 0.001 |
| Stearic acid (C18:0) | 7.69 ± 0.07 a | 6.36 ± 0.07 b | 9.34 ± 0.09 c | 8.45 ± 0.07 d | 9.66 ± 0.07 e | 6.41 ± 0.05 b | 0.001 |
| Palmitoleic acid (C16:1) | 4.80 ± 0.06 a | 3.25 ± 0.07 b | 5.53 ± 0.06 c | 4.25 ± 0.07 d | 4.41 ± 0.05 d | 4.45 ± 0.08 d | 0.001 |
| Oleic acid (C18:1n-9) | 5.28 ± 0.07 a | 8.29 ± 0.09 b | 11.45 ± 0.07 c | 12.58 ± 0.03 d | 10.66 ± 0.07 e | 7.92 ± 0.01 f | 0.001 |
| Linoleic acid (C18:2n-6) (LA) | 2.74 ± 0.04 a | 11.74 ± 0.07 b | 9.03 ± 0.02 c | 8.93 ± 0.02 c | 10.25 ± 0.07 d | 12.54 ± 0.09 e | 0.001 |
| γ–linolenic acid (C18:3n-6) | 0.53 ± 0.05 a | 9.89 ± 0.03 b | 4.78 ± 0.06 c | 4.76 ± 0.06 c | 8.04 ± 0.02 d | 8.40 ± 0.07 e | 0.001 |
| α–linolenic acid (C18:3n-3) (ALA) | 0.7 ± 0.06 a | 14.85 ± 0.04 b | 4.55 ± 0.07 c | 1.74 ± 0.07 d | 6.24 ± 0.07 e | 5.24 ± 0.07 f | 0.001 |
| Eicosenoic acid (C20:5n-3) | 0.13 ± 0.02 a | 0.71 ± 0.06 b | 0.45 ± 0.02 c | 0.21 ± 0.02 ac | 0.33 ± 0.03 ac | 0.45 ± 0.02 bc | 0.008 |
| Others fatty acids | 2.06 ± 0.02 a | 1.88 ± 0.04 a | 1.93 ± 0.02 a | 2.49 ± 0.05 a | 1.93 ± 0.03 a | 1.97 ± 0.04 a | 0.078 |
| SFA | 83.76 ± 0.07 a | 49.56 ± 0.11 b | 62.24 ± 0.07 c | 65.13 ± 0.09 d | 58.18 ± 0.06 e | 58.19 ± 0.04 f | 0.001 |
| UFA | 13.76 ± 0.07 a | 48.74 ± 0.06 b | 35.81 ± 0.05 c | 32.50 ± 0.10 d | 39.96 ± 0.02 e | 39.09 ± 0.02 f | 0.001 |
| MUFA | 10.10 ± 0.03 a | 11.52 ± 0.07 b | 16.92 ± 0.05 c | 16.83 ± 0.04 c | 15.06 ± 0.01 d | 12.35 ± 0.07 e | 0.001 |
| PUFA | 3.66 ± 0.06 a | 37.22 ± 0.07 b | 18.84 ± 0.03 c | 15.67 ± 0.07 d | 24.95 ± 0.02 e | 26.67 ± 0.07 f | 0.001 |
| n–3 | 0.38 ± 0.05 a | 15.55 ± 0.07 b | 5.04 ± 0.02 c | 1.91 ± 0.03 d | 6.67 ± 0.07 e | 5.75 ± 0.08 f | 0.001 |
| n–6 | 3.27 ± 0.07 a | 21.66 ± 0.07 b | 13.87 ± 0.05 c | 13.76 ± 0.07 c | 18.24 ± 0.05 d | 20.88 ± 0.09 e | 0.001 |
| UFA/SFA | 0.16 ± 0.04 a | 0.97 ± 0.05 b | 0.56 ± 0.05 c | 0.50 ± 0.03 c | 0.67 ± 0.05 c | 0.67 ± 0.06 c | 0.001 |
| PUFA/SFA | 0.04 ± 0.01 a | 0.77 ± 0.01 b | 0.31 ± 0.02 c | 0.24 ± 0.04 ac | 0.43 ± 0.03 c | 0.44 ± 0.03 c | 0.001 |
| MUFA/SFA | 0.12 ± 0.01 a | 0.25 ± 0.02 b | 0.26 ± 0.04 b | 0.27 ± 0.01 b | 0.26 ± 0.01 b | 0.21 ± 0.02 ab | 0.001 |
| n–6/n–3 | 8.22 ± 0.09 a | 1.39 ± 0.04 b | 2.77 ± 0.07 c | 7.05 ± 0.12 c | 2.77 ± 0.04 d | 3.66 ± 0.08 e | 0.001 |
| Polyunsaturated index (PI) | 3.24 ± 0.04 a | 41.44 ± 0.07 b | 18.15 ± 0.11 c | 12.43 ± 0.07 d | 22.77 ± 0.09 e | 22.95 ± 0.02 e | 0.001 |
| Atherogenic index (AI) | 7.15 ± 0.03 a | 1.22 ± 0.07 b | 2.13 ± 0.04 ce | 2.37 ± 0.06 c | 1.66 ± 0.07 d | 1.91 ± 0.04 e | 0.001 |
| Thrombogenic index (TI) | 4.86 ± 0.02 a | 0.48 ± 0.01 b | 1.34 ± 0.02 c | 1.75 ± 0.02 d | 0.94 ± 0.01 e | 0.98 ± 0.01 e | 0.001 |
| Fatty Acids | Control | Experimental Treatments (Mean ± SE) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Linseed Oil | Soybean Oil | Sunflower Oil | Rapeseed Oil | Hempseed Oil | |||
| Lauric acid (C12:0) | 41.37 ± 0.07 a | 18.86 ± 0.06 b | 30.79 ± 0.08 c | 34.94 ± 0.02 c | 28.73 ± 0.09 e | 23.50 ± 0.07 f | 0.001 |
| Miristic acid (C14:0) | 14.07 ± 0.03 a | 8.34 ± 0.05 b | 8.51 ± 0.06 b | 7.22 ± 0.07 c | 4.77 ± 0.09 d | 10.02 ± 0.04 e | 0.001 |
| Palmitic acid (C16:0) | 24.89 ± 0.04 a | 17.07 ± 0.02 b | 22.34 ± 0.07 c | 22.67 ± 0.08 d | 22.13 ± 0.09 c | 20.33 ± 0.04 e | 0.001 |
| Stearic acid (C18:0) | 4.49 ± 0.07 a | 6.26 ± 0.09 b | 5.24 ± 0.11 c | 4.13 ± 0.05 d | 8.12 ± 0.09 e | 3.84 ± 0.04 f | 0.001 |
| Palmitoleic acid (C16:1) | 4.06 ± 0.03 a | 4.05 ± 0.02 b | 6.12 ± 0.04 c | 5.55 ± 0.07 d | 4.56 ± 0.07 e | 6.65 ± 0.08 f | 0.001 |
| Oleic acid (C18:1n-9) | 4.93 ± 0.03 a | 7.96 ± 0.05 b | 8.24 ± 0.07 c | 7.88 ± 0.03 b | 10.05 ± 0.05 d | 9.43 ± 0.07 e | 0.001 |
| Linoleic acid (C18:2n-6) (LA) | 1.34 ± 0.08 a | 9.43 ± 0.08 b | 7.95 ± 0.06 c | 3.76 ± 0.07 d | 6.16 ± 0.07 e | 8.46 ± 0.08 f | 0.001 |
| γ–linolenic acid (C18:3n-6) | 1.03 ± 0.02 a | 11.04 ± 0.04 b | 6.56 ± 0.07 c | 8.54 ± 0.09 d | 9.91 ± 0.05 e | 9.55 ± 0.08 f | 0.001 |
| α–linolenic acid (C18:3n-3) (ALA) | 1.04 ± 0.03 a | 13.94 ± 0.04 b | 0.77 ± 0.01 c | 1.87 ± 0.03 d | 2.72 ± 0.03 e | 5.65 ± 0.07 f | 0.001 |
| Eicosenoic acid (C20:5n-3) | 0.23 ± 0.02 a | 0.67 ± 0.02 b | 0.66 ± 0.02 c | 0.33 ± 0.02 ad | 0.52 ± 0.02 bcd | 0.47 ± 0.02 d | 0.008 |
| Others fatty acids | 2.55 ± 0.04 a | 2.42 ± 0.05 a | 2.82 ± 0.06 a | 3.11 ± 0.04 b | 2.33 ± 0.03 a | 2.10 ± 0.06 a | 0.048 |
| SFA | 84.82 ± 0.05 a | 50.50 ± 0.15 b | 66.69 ± 0.09 c | 69.04 ± 0.06 d | 63.77 ± 0.07 e | 57.76 ± 0.08 f | 0.001 |
| UFA | 12.65 ± 0.08 a | 47.13 ± 0.03 b | 30.33 ± 0.07 c | 27.78 ± 0.07 d | 33.85 ± 0.04 e | 40.13 ± 0.03 f | 0.001 |
| MUFA | 8.97 ± 0.04 a | 11.97 ± 0.03 b | 14.43 ± 0.07 c | 13.43 ± 0.07 d | 14.65 ± 0.05 c | 16.07 ± 0.04 e | 0.001 |
| PUFA | 3.66 ± 0.07 a | 35.13 ± 0.06 b | 15.94 ± 0.03 c | 14.35 ± 0.05 d | 19.22 ± 0.08 e | 24.12 ± 0.07 f | 0.001 |
| n–3 | 1.25 ± 0.06 a | 14.64 ± 0.07 b | 1.44 ± 0.07 a | 2.15 ± 0.07 c | 3.19 ± 0.08 d | 6.12 ± 0.09 e | 0.001 |
| n–6 | 2.35 ± 0.07 a | 20.45 ± 0.08 b | 14.54 ± 0.04 c | 12.23 ± 0.09 d | 16.03 ± 0.04 e | 17.96 ± 0.08 f | 0.001 |
| UFA/SFA | 0.15 ± 0.01 a | 0.92 ± 0.02 b | 0.45 ± 0.02 c | 0.41 ± 0.02 c | 0.53 ± 0.02 d | 0.69 ± 0.03 e | 0.001 |
| PUFA/SFA | 0.04 ± 0.01 a | 0.69 ± 0.01 b | 0.24 ± 0.02 ac | 0.26 ± 0.02 ac | 0.32 ± 0.02 c | 0.44 ± 0.03 c | 0.001 |
| MUFA/SFA | 0.13 ± 0.03 a | 0.24 ± 0.04 a | 0.22 ± 0.02 a | 0.16 ± 0.03 a | 0.22 ± 0.06 a | 0.24 ± 0.04 a | 0.772 |
| n–6/n–3 | 1.87 ± 0.06 a | 1.43 ± 0.08 b | 10.22 ± 0.06 c | 5.76 ± 0.07 d | 5.06 ± 0.08 e | 2.94 ± 0.03 f | 0.001 |
| Polyunsaturated index (PI) | 3.45 ± 0.07 a | 37.34 ± 0.07 b | 9.5 ± 0.05 c | 7.35 ± 0.09 d | 11.54 ± 0.11 e | 19.76 ± 0.07 f | 0.001 |
| Atherogenic index (AI) | 9.76 ± 0.07 a | 1.45 ± 0.08 b | 2.86 ± 0.06 c | 3.11 ± 0.04 d | 2.06 ± 0.03 e | 2.08 ± 0.03 e | 0.001 |
| Thrombogenic index (TI) | 4.33 ± 0.07 a | 0.55 ± 0.04 b | 1.94 ± 0.07 c | 1.77 ± 0.08 c | 1.35 ± 0.08 d | 0.96 ± 0.02 e | 0.001 |
| Fatty Acids | Control | Experimental Treatments (Mean ± SE) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Linseed Oil | Soybean Oil | Sunflower Oil | Rapeseed Oil | Hempseed Oil | |||
| Lauric acid (C12:0) | 36.33 ± 0.07 a | 18.87 ± 0.06 b | 28.89 ± 0.02 c | 36.76 ± 0.07 d | 27.57 ± 0.06 e | 25.44 ± 0.09 f | 0.001 |
| Miristic acid (C14:0) | 17.24 ± 0.07 a | 8.86 ± 0.06 b | 10.07 ± 0.02 c | 9.44 ± 0.07 d | 11.56 ± 0.06 e | 10.95 ± 0.09 f | 0.001 |
| Palmitic acid (C16:0) | 24.44 ± 0.05 a | 20.44 ± 0.06 b | 26.14 ± 0.08 c | 23.89 ± 0.04 d | 22.22 ± 0.08 e | 22.06 ± 0.04 e | 0.001 |
| Stearic acid (C18:0) | 7.92 ± 0.04 a | 4.82 ± 0.05 b | 4.76 ± 0.07 b | 4.35 ± 0.06 c | 5.91 ± 0.07 d | 5.78 ± 0.04 d | 0.001 |
| Palmitoleic acid (C16:1) | 3.14 ± 0.03 a | 2.92 ± 0.07 b | 4.58 ± 0.05 c | 5.14 ± 0.05 d | 4.21 ± 0.05 e | 4.07 ± 0.09 b e | 0.001 |
| Oleic acid (C18:1n-9) | 5.32 ± 0.07 a | 6.14 ± 0.04 b | 7.77 ± 0.09 c | 8.65 ± 0.09 d | 6.78 ± 0.04 e | 5.65 ± 0.08 f | 0.001 |
| Linoleic acid (C18:2n-6) (LA) | 1.50 ± 0.09 a | 14.22 ± 0.07 b | 7.77 ± 0.05 c | 1.43 ± 0.06 a | 7.86 ± 0.07 c | 9.32 ± 0.09 d | 0.001 |
| γ–linolenic acid (C18:3n-6) | 0.67 ± 0.07 a | 10.05 ± 0.02 b | 4.44 ± 0.07 c | 5.49 ± 0.05 d | 5.94 ± 0.02 e | 7.66 ± 0.07 f | 0.001 |
| α–linolenic acid (C18:3n-3) (ALA) | 0.44 ± 0.07 a | 10.13 ± 0.06 b | 2.77 ± 0.07 c | 2.15 ± 0.04 d | 4.92 ± 0.04 e | 6.45 ± 0.07 f | 0.001 |
| Eicosenoic acid (C20:5n-3) | 0.14 ± 0.06 a | 0.46 ± 0.07 b | 0.23 ± 0.05 a | 0.16 ± 0.08 a | 0.16 ± 0.02 a | 0.26 ± 0.02 ab | 0.008 |
| Others fatty acids | 2.85 ± 0.06 a | 2.13 ± 0.04 a | 2.70 ± 0.07 a | 2.66 ± 0.04 a | 2.69 ± 0.07 a | 2.30 ± 0.04 a | 0.529 |
| SFA | 85.86 ± 0.06 a | 52.90 ± 0.08 b | 69.87 ± 0.03 c | 74.34 ± 0.07 d | 67.24 ± 0.08 e | 64.23 ± 0.09 f | 0.001 |
| UFA | 11.23 ± 0.07 a | 44.92 ± 0.04 b | 27.44 ± 0.07 c | 22.94 ± 0.04 d | 29.94 ± 0.02 e | 33.44 ± 0.07 f | 0.001 |
| MUFA | 8.46 ± 0.06 a | 10.05 ± 0.02 b | 12.32 ± 0.08 c | 13.81 ± 0.12 d | 11.05 ± 0.17 e | 9.76 ± 0.09 f | 0.001 |
| PUFA | 2.76 ± 0.08 a | 34.88 ± 0.05 b | 15.13 ± 0.03 c | 9.22 ± 0.07 d | 18.91 ± 0.04 e | 23.68 ± 0.07 f | 0.001 |
| n–3 | 0.57 ± 0.03 a | 10.65 ± 0.05 b | 3.05 ± 0.02 c | 2.34 ± 0.11 d | 5.13 ± 0.04 e | 6.76 ± 0.09 f | 0.001 |
| n–6 | 2.15 ± 0.04 a | 24.23 ± 0.05 b | 12.14 ± 0.06 c | 6.89 ± 0.07 d | 13.78 ± 0.07 e | 16.95 ± 0.02 f | 0.001 |
| UFA/SFA | 0.13 ± 0.02 a | 0.86 ± 0.03 b | 0.39 ± 0.02 c | 0.32 ± 0.01 d | 0.44 ± 0.03 e | 0.52 ± 0.02 f | 0.001 |
| PUFA/SFA | 0.03 ± 0.01 a | 0.66 ± 0.02 b | 0.22 ± 0.02 c | 0.12 ± 0.03 d | 0.28 ± 0.01 e | 0.37 ± 0.02 f | 0.001 |
| MUFA/SFA | 0.09 ± 0.02 a | 0.19 ± 0.02 b | 0.18 ± 0.03 bc | 0.19 ± 0.02 b | 0.17 ± 0.02 bc | 0.16 ± 0.02 c | 0.772 |
| n–6/n–3 | 3.85 ± 0.06 a | 2.24 ± 0.08 b | 4.1 ± 0.05 c | 2.90 ± 0.05 d | 2.77 ± 0.08 d | 2.51 ± 0.05 e | 0.001 |
| Polyunsaturated index (PI) | 2.34 ± 0.07 a | 34.44 ± 0.09 b | 13.24 ± 0.05 c | 5.77 ± 0.08 d | 17.76 ± 0.03 e | 22.22 ± 0.09 f | 0.001 |
| Atherogenic index (AI) | 11.55 ± 0.07 a | 1.67 ± 0.04 b | 3.45 ± 0.07 c | 4.24 ± 0.09 d | 3.22 ± 0.09 c | 2.80 ± 0.13 e | 0.001 |
| Thrombogenic index (TI) | 6.78 ± 0.09 a | 0.68 ± 0.03 b | 1.91 ± 0.02 c | 2.16 ± 0.04 d | 1.43 ± 0.07 e | 1.16 ± 0.06 f | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu, B.; Boaru, A.M.; Muntean, L.; Sima, N.; Struți, D.I.; Păpuc, T.A.; Georgescu, C. Modulating the Fatty Acid Profiles of Hermetia illucens Larvae Fats by Dietary Enrichment with Different Oilseeds: A Sustainable Way for Future Use in Feed and Food. Insects 2022, 13, 801. https://doi.org/10.3390/insects13090801
Georgescu B, Boaru AM, Muntean L, Sima N, Struți DI, Păpuc TA, Georgescu C. Modulating the Fatty Acid Profiles of Hermetia illucens Larvae Fats by Dietary Enrichment with Different Oilseeds: A Sustainable Way for Future Use in Feed and Food. Insects. 2022; 13(9):801. https://doi.org/10.3390/insects13090801
Chicago/Turabian StyleGeorgescu, Bogdan, Anca Mihaela Boaru, Leon Muntean, Nicușor Sima, Dănuț Ioan Struți, Tudor Andrei Păpuc, and Carmen Georgescu. 2022. "Modulating the Fatty Acid Profiles of Hermetia illucens Larvae Fats by Dietary Enrichment with Different Oilseeds: A Sustainable Way for Future Use in Feed and Food" Insects 13, no. 9: 801. https://doi.org/10.3390/insects13090801
APA StyleGeorgescu, B., Boaru, A. M., Muntean, L., Sima, N., Struți, D. I., Păpuc, T. A., & Georgescu, C. (2022). Modulating the Fatty Acid Profiles of Hermetia illucens Larvae Fats by Dietary Enrichment with Different Oilseeds: A Sustainable Way for Future Use in Feed and Food. Insects, 13(9), 801. https://doi.org/10.3390/insects13090801








