Aedes Mosquito Surveillance Using Ovitraps, Sweep Nets, and Biogent Traps in the City of Yaoundé, Cameroon
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
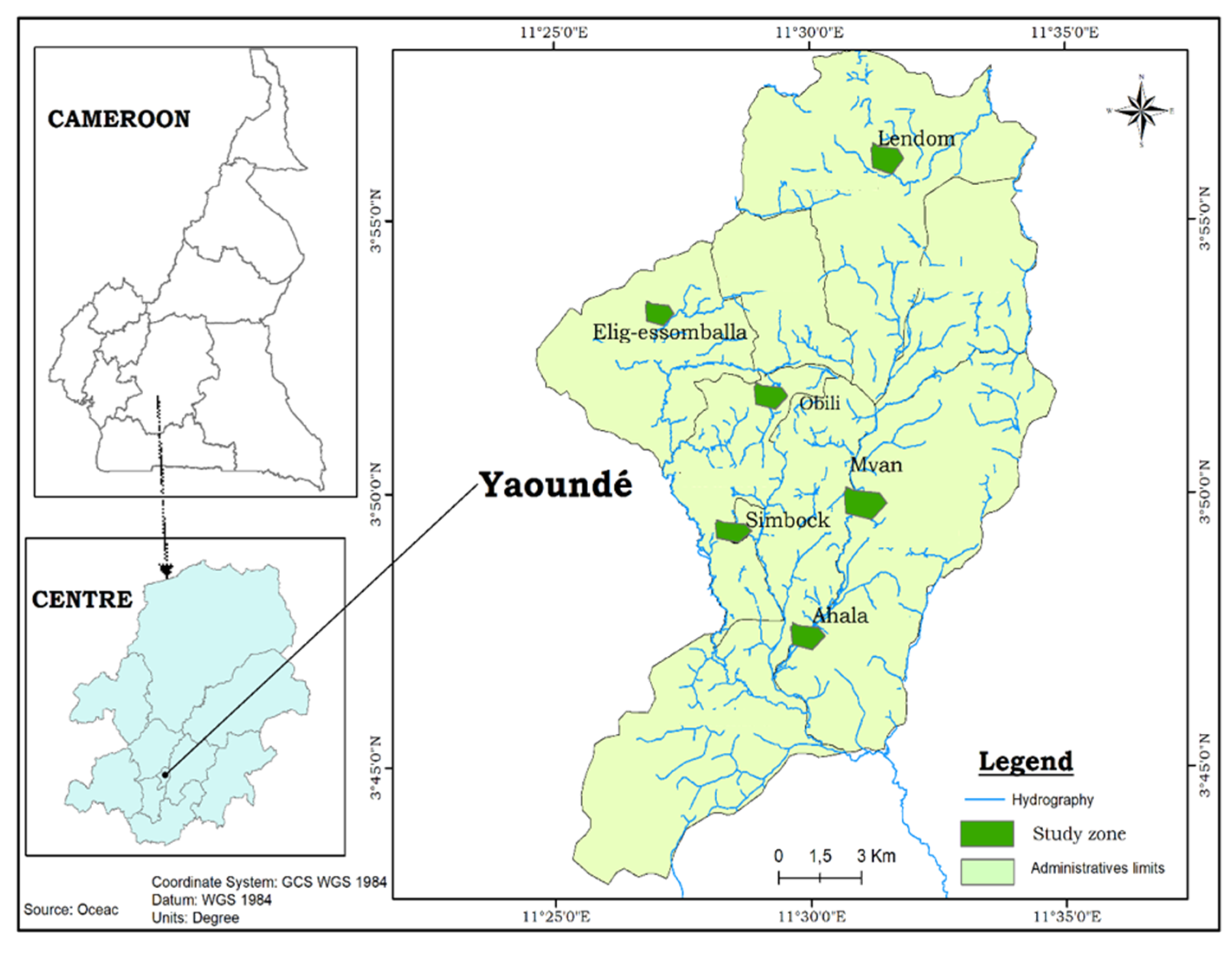
2.1. Study Sites
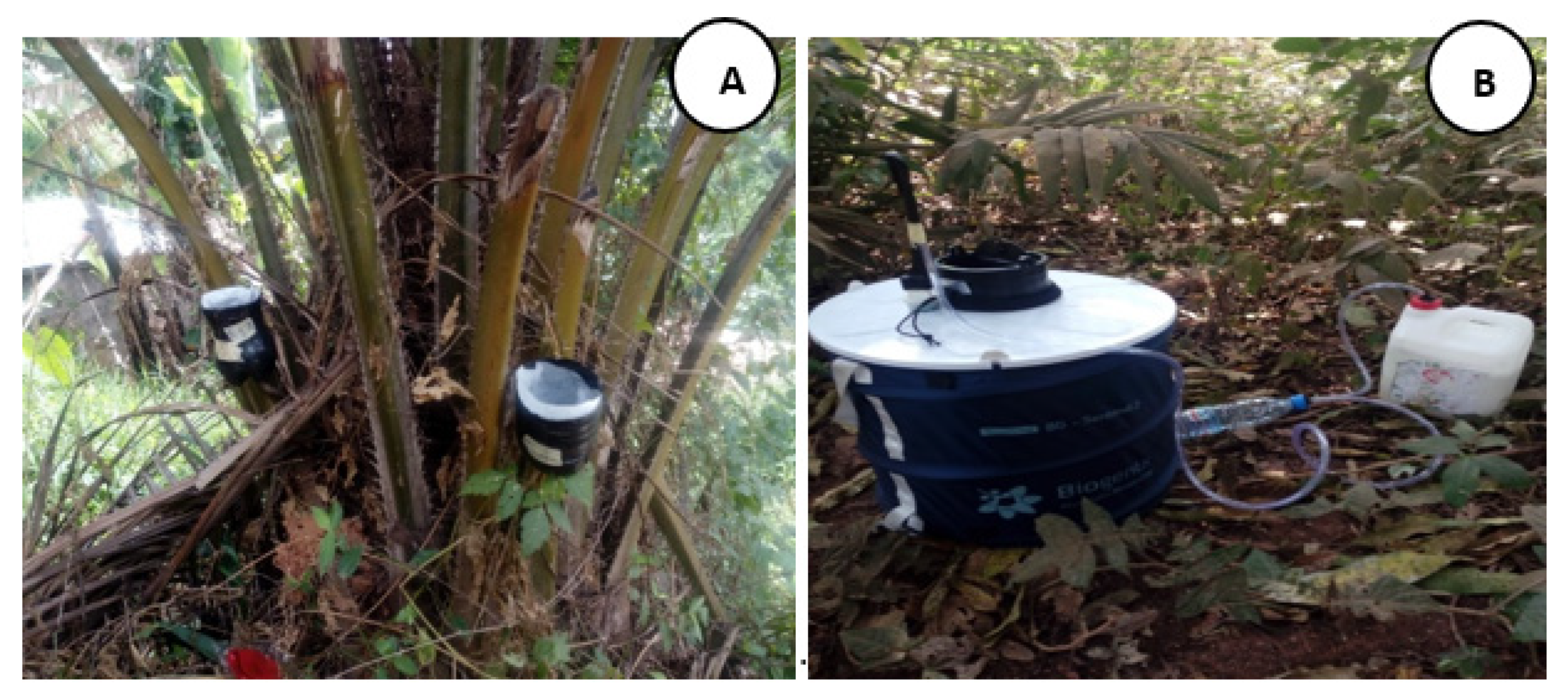
2.2. Study Design
2.3. Data Analysis
3. Results
3.1. Aedes Mosquito Distribution following Each Sampling Method across Study Sites
3.2. Ovitraps Positivity Indices in Each Ecological Zone
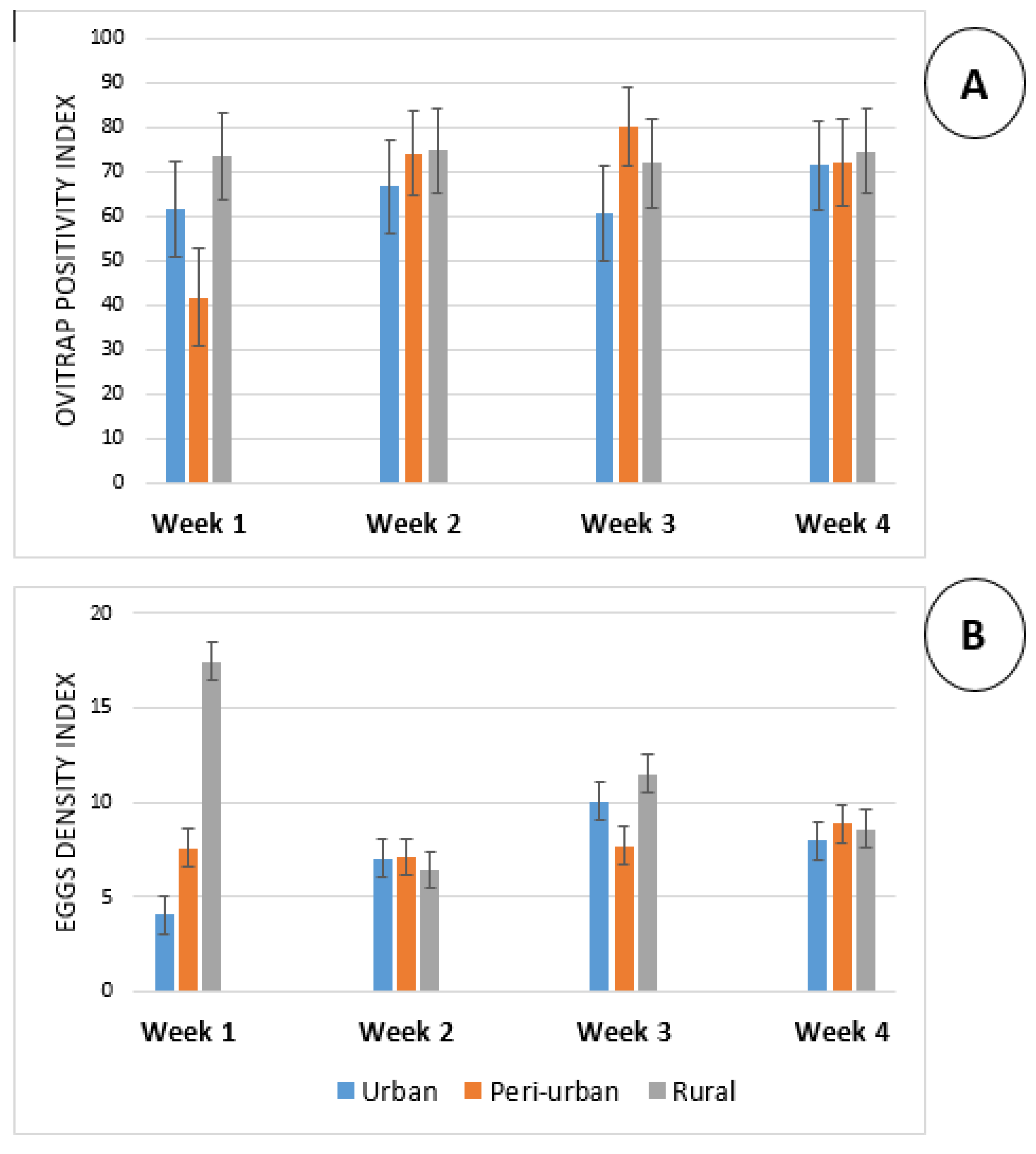
3.3. Weekly Variation of the OPI and EDI
3.4. Abundance of Adults Aedes Mosquito across Study Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Balasubramanian, R.; Ouedraogo, M.; Wandji Nana, L.R.; Mogeni, O.D.; Jeon, H.J.; van Pomeren, T.; Haselbeck, A.; Lim, J.K.; Prifti, K.; et al. The Epidemiology of Dengue Outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon 2020, 6, e04389. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.; Barrera, R.; Makio, A.; Mutisya, J.; Koka, H.; Owaka, S.; Koskei, E.; Nyunja, A.; Eyase, F.; Coldren, R.; et al. Dengue Outbreak in Mombasa City, Kenya, 2013–2014: Entomologic Investigations. PLoS Negl. Trop. Dis. 2016, 10, e0004981. [Google Scholar] [CrossRef] [PubMed]
- Simo, F.B.N.; Bigna, J.J.; Well, E.A.; Kenmoe, S.; Sado, F.B.Y.; Weaver, S.C.; Moundipa, P.F.; Demanou, M. Chikungunya Virus Infection Prevalence in Africa: A Contemporaneous Systematic Review and Meta-Analysis. Public Health 2019, 166, 79–88. [Google Scholar] [CrossRef] [PubMed]
- WHO. Weekly Bulletin on Outbreaks and Other Emergencies; World Health Organization: Geneva, Switzerland, 2021; 21p. [Google Scholar]
- Galani, B.; Mapouokam, D.; Simo, F.; Mohamadou, P.; Njintang, N.; Moundipa, P. Investigation of Dengue-Malaria Coinfection among Febrile Patients Consulting at Ngaoundere Regional Hospital, Cameroon. J. Med. Virol. 2021, 93, 3350–3361. [Google Scholar] [CrossRef]
- Nana-Ndjangwo, S.M.; Djiappi-Tchamen, B. Laboratory of Parasitology and Ecology, Department of Animal Physiology and Biology, Faculty of Science, University of Yaoundé I, Cameroon; Institut de Recherche de Yaoundé (IRY), Organisation de Coordination pour la lutte Contre les Endémies en Afrique Centrale (OCEAC), Yaoundé, Cameroon. 2022; manuscript in preparation. [Google Scholar]
- Simo, N.; Yousseu, F.; Mbarga, E.; Bigna, J.; Melong, A.; Ntoude, A.; Kamgang, B.; Bouyne, R.; Fewou, M.; Demanou, M. Investigation of an Outbreak of Dengue Virus Serotype 1 in a Rural Area of Kribi, South Cameroon: A Cross-Sectional Study. Intervirology 2018, 61, 265–271. [Google Scholar]
- Tchuandom, S.B.; Tchadji, J.C.; Tchouangueu, T.F.; Biloa, M.Z.; Atabonkeng, E.P.; Fumba, M.I.M.; Massom, E.S.; Nchinda, G.; Kuiate, J.-R. A Cross-Sectional Study of Acute Dengue Infection in Paediatric Clinics in Cameroon. BMC Public Health 2019, 19, 958. [Google Scholar] [CrossRef]
- Yousseu, F.B.S.; Nemg, F.B.S.; Ngouanet, S.A.; Mekanda, F.M.O.; Demanou, M. Detection and Serotyping of Dengue Viruses in Febrile Patients Consulting at the New-Bell District Hospital in Douala, Cameroon. PLoS ONE 2018, 13, e0204143. [Google Scholar] [CrossRef]
- Demanou, M.; Pouillot, R.; Grandadam, M.; Boisier, P.; Kamgang, B.; Hervé, J.P.; Rogier, C.; Rousset, D.; Paupy, C. Evidence of Dengue Virus Transmission and Factors Associated with the Presence of Anti-Dengue Virus Antibodies in Humans in Three Major Towns in Cameroon. PLoS Negl. Trop. Dis. 2014, 8, e2950. [Google Scholar] [CrossRef]
- Monamele, G.C.; Demanou, M. First Documented Evidence of Dengue and Malaria Co-Infection in Children Attending Two Health Centers in Yaoundé, Cameroon. Pan Afr. Med. J. 2018, 29, 227. [Google Scholar] [CrossRef] [PubMed]
- Peyrefitte, C.N.; Rousset, D.; Pastorino, B.A.; Pouillot, R.; Bessaud, M.; Tock, F.; Mansaray, H.; Merle, O.L.; Pascual, A.M.; Paupy, C. Chikungunya Virus, Cameroon, 2006. Emerg. Infect. Dis. 2007, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Maurice, D.; Alain, S.-M.S.; Christophe, V.; Rene, N.; Irene, K.T.; Marthe, I.N.; Richard, N. Molecular Characterization of Chikungunya Virus from Three Regions of Cameroon. Virol. Sin. 2015, 30, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Bamou, R.; Mayi, M.P.A.; Djiappi-Tchamen, B.; Nana-Ndjangwo, S.M.; Nchoutpouen, E.; Cornel, A.J.; Awono-Ambene, P.; Parola, P.; Tchuinkam, T.; Antonio-Nkondjio, C. An Update on the Mosquito Fauna and Mosquito-Borne Diseases Distribution in Cameroon. Parasit. Vectors 2021, 14, 527. [Google Scholar] [CrossRef]
- Djiappi-Tchamen, B.; Nana-Ndjangwo, M.S.; Tchuinkam, T.; Makoudjou, I.; Nchoutpouen, E.; Kopya, E.; Talipouo, A.; Bamou, R.; Mayi, M.P.A.; Awono-Ambene, P.; et al. Aedes Mosquito Distribution along a Transect from Rural to Urban Settings in Yaoundé, Cameroon. Insects 2021, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Tedjou, A.N.; Kamgang, B.; Yougang, A.P.; Njiokou, F.; Wondji, C.S. Update on the Geographical Distribution and Prevalence of Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae), Two Major Arbovirus Vectors in Cameroon. PLoS Negl. Trop. Dis. 2019, 13, e0007137. [Google Scholar] [CrossRef]
- Kamgang, B.; Vazeille, M.; Tedjou, A.N.; Wilson-Bahun, T.A.; Yougang, A.P.; Mousson, L.; Wondji, C.S.; Failloux, A.-B. Risk of Dengue in Central Africa: Vector Competence Studies with Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) Populations and Dengue 2 Virus. PLoS Negl. Trop. Dis. 2019, 13, e0007985. [Google Scholar] [CrossRef]
- da Costa, C.F.; Dos Passos, R.A.; Pereira Lima, J.B.; Roque, R.A.; Vanderson de Souza, S.; Campolina, T.B.; Costa Secundino, N.F.; Paolucci Pimenta, P.F. Transovarial Transmission of DENV in Aedes Aegypti in the Amazon Basin: A Local Model of Xenomonitoring. Parasit. Vectors 2017, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-de-Lima, V.H.; Santos Andrade, P.D.; Matsumiya Thomazelli, L.; Toledo Marrelli, M.; Roberto Urbinatti, P.; Marques de Sa Almeida, R.M.; Lima-Camara, T.N. Silent Circulation of Dengue Virus in Aedes Albopictus (Diptera:Culicidae) Resulting from Natural Vertical Transmisssion. Sci. Rep. 2020, 10, 3855. [Google Scholar] [CrossRef]
- Focks, D. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. In Special Programme for Research and Training in Tropical Diseases (TDR); Dengue Bull; WHO: Gainesville, FL, USA, 2003. [Google Scholar]
- Garjito, T.A.; Hidajat, M.C.; Kinansi, R.R.; Setyaningsih, R.; Anggraeni, Y.M.; Mujiyanto; Trapsilowati, W.; Jastal; Ristiyanto; Satoto, T.B.T.; et al. Stegomyia Indices and Risk of Dengue Transmission: A Lack of Correlation. Front. Public Health 2020, 8, 328. [Google Scholar] [CrossRef]
- Wijayanti, S.P.M.; Sunaryo, S.; Suprihatin, S.; McFarlane, M.; Rainey, S.M.; Dietrich, I.; Schnettler, E.; Biek, R.; Kohl, A. Dengue in Java, Indonesia: Relevance of Mosquito Indices as Risk Predictors. PLoS Negl. Trop. Dis. 2016, 10, e0004500. [Google Scholar] [CrossRef] [Green Version]
- Hemme, R.R.; Smith, E.A.; Felix, G.; White, B.J.; Diaz-Garcia, M.I.; Rodriguez, D.; Ruiz-Valcarcel, J.; Acevedo, V.; Amador, M.; Barrera, R. Multi-Year Mass-Trapping with Autocidal Gravid Ovitraps Has Limited Influence on Insecticide Susceptibility in Aedes Aegypti (Diptera: Culicidae) From Puerto Rico. J. Med. Entomol. 2022, 59, 314–319. [Google Scholar] [CrossRef] [PubMed]
- James, L.D.; Winter, N.; Stewart, A.T.M.; Feng, R.S.; Nandram, N.; Mohammed, A.; Duman-Scheel, M.; Romero-Severson, E.; Severson, D.W. Field Trials Reveal the Complexities of Deploying and Evaluating the Impacts of Yeast-Baited Ovitraps on Aedes Mosquito Densities in Trinidad, West Indies. Sci. Rep. 2022, 12, 4047. [Google Scholar] [CrossRef]
- Kpan, M.D.S.; Adja, A.M.; Guindo-Coulibaly, N.; Assouho, K.F.; Kouadio, A.M.N.; Azongnibo, K.R.M.; Zoh, D.D.; Zahouli, B.Z.J.; Remoue, F.; Fournet, F. Spatial Heterogeneity and Seasonal Distribution of Aedes (Stegomyia) Aegypti (L) in Abidjan, Côte d’Ivoire. Vector-Borne Zoonotic Dis. 2021, 21, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, H.; Leng, P.; Zhu, J.; Yao, S.; Zhu, Y.; Wu, H. Analysis of the Spatial Distribution of Aedes Albopictus in an Urban Area of Shanghai, China. Parasit. Vectors 2021, 14, 501. [Google Scholar] [CrossRef]
- Ortega-Morales, A.I.; Moreno-García, M.; González-Acosta, C.; Correa-Morales, F. Mosquito Surveillance in Mexico: The Use of Ovitraps for Aedes Aegypti, Ae. Albopictus, and Non-Target Species. Fla. Entomol. 2018, 101, 623–626. [Google Scholar] [CrossRef]
- PAHO. Dengue and Dengue Haemorragic Fever in the Americas: Guidelines for Prevention and Control; Scientific Publication N° 548; PAHO: Washington, DC, USA, 1994. [Google Scholar]
- Barrera, R.; Mackay, A.J.; Amador, M. A Novel Autocidal Ovitrap for the Surveillance and Control of Aedes Aegypti. J. Am. Mosq. Control Assoc. 2013, 29, 293–296. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Eiras, Á.E.; Lourenço-de-Oliveira, R. Field Evaluation of Effectiveness of the BG-Sentinel, a New Trap for Capturing Adult Aedes Aegypti (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 2006, 101, 321–325. [Google Scholar] [CrossRef]
- Service, M.W. (Ed.) Mark-Recapture Techniques and Adult Dispersal. In Mosquito Ecology: Field Sampling Methods; Springer: Dordrecht, The Netherlands, 1993; pp. 652–751. ISBN 978-94-011-1868-2. [Google Scholar]
- Mayi, M.P.A.; Bamou, R.; Djiappi-Tchamen, B.; Fontaine, A.; Jeffries, C.L.; Walker, T.; Antonio-Nkondjio, C.; Cornel, A.J.; Tchuinkam, T. Habitat and Seasonality Affect Mosquito Community Composition in the West Region of Cameroon. Insects 2020, 11, 312. [Google Scholar] [CrossRef]
- Dibo, M.R.; Chiaravalloti-Neto, F.; Battigaglia, M.; Mondini, A.; Favaro, E.A.; Barbosa, A.A.; Glasser, C.M. Identification of the Best Ovitrap Installation Sites for Gravid Aedes (Stegomyia) Aegypti in Residences in Mirassol, State of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz 2005, 100, 339–343. [Google Scholar] [CrossRef]
- Morato, V.C.G.; Teixeira, M.D.G.; Gomes, A.C.; Bergamaschi, D.P.; Barreto, M.L. Infestation of Aedes Aegypti Estimated by Oviposition Traps in Brazil. Rev. Saúde Pública 2005, 39, 553–558. [Google Scholar] [CrossRef]
- Suchel Les Climats Du Cameroun, Tome III; Thèse Université de Université Bordeaux III: Bordeaux, France, 1987; Volume 4, p. 1186.
- Edwards, F.W. Mosquitoes of the Ethiopian Region. HI—Culicine Adults and Pupae. In Mosquitoes Ethiop. Reg. HI—Culicine Adults Pupae; Trustees of the British Museum: London, UK, 1941. [Google Scholar]
- Jupp, P. Mosquitoes of Southern Africa: Culicinae and Toxorhynchitinae Hartebees Spoort (South Africa); Ekogilde Publishers: Hartebeespoort, South Africa, 1996. [Google Scholar]
- Gomes, A.C. Medidas Dos Níveis de Infestação Urbana Para Aedes (Stegomyia) Aegypti e Aedes (Stegomyia) Albopictus Em Programa de Vigilancia Entomológica. Inf. Epidemiológico SUS Braz. 1998, 7, 49–57. [Google Scholar] [CrossRef]
- Honório, N.A.; Codeço, C.T.; Alves, F.D.C.; Magalhães, M.D.A.; Lourenço-De-Oliveira, R. Temporal Distribubtion of Aedes Aegypti in Different Districts of Rio de Janeiro, Brazil, Measured by Two Types of Traps. J. Med. Entomol. 2009, 46, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Wijegunawardana, N.D.A.D.; Gunawardene, Y.I.N.S.; Chandrasena, T.G.A.N.; Dassanayake, R.S.; Udayanga, N.W.B.A.L.; Abeyewickreme, W. Evaluation of the Effects of Aedes Vector Indices and Climatic Factors on Dengue Incidence in Gampaha District, Sri Lanka. BioMed Res. Int. 2019, 2019, e2950216. [Google Scholar] [CrossRef] [PubMed]
- De Las Liagas, L.; Tyagi, B.; Bersales, L. Aedes Dengue Vector Ovitrap Surveillance System: A Framework for Mosquito Density Prediction. Southeast Asian J. Trop. Med. Public Health 2016, 47, 712–718. [Google Scholar]
- Hii, Y.L.; Zhu, H.; Ng, N.; Ng, L.C.; Rocklöv, J. Forecast of Dengue Incidence Using Temperature and Rainfall. PLoS Negl. Trop. Dis. 2012, 6, e1908. [Google Scholar] [CrossRef]
- Martini, M.; Prihatnolo, A.; Hestiningsih, R. Modification Ovitrap to Control of Aedes Sp Population in Central Java. In Proceedings of the ASEAN/Asian Academic Society International Conference Proceeding Series, Bandar Seri Begawan, Brunei, 9 October 2013. [Google Scholar]
- Wibowo, A. The Difference Existence of Aedes Sp Larvae Based on Ovitrap Locating in Samarinda City Indonesia. Indian J. Public Health Res. Dev. 2018, 9, 1405–1409. [Google Scholar]
- Tantowijoyo, W.; Arguni, E.; Johnson, P.; Budiwati, N.; Nurhayati, P.I.; Fitriana, I.; Wardana, S.; Ardiansyah, H.; Turley, A.P.; Ryan, P.; et al. Spatial and Temporal Variation in Aedes Aegypti and Aedes Albopictus (Diptera: Culicidae) Numbers in the Yogyakarta Area of Java, Indonesia, With Implications for Wolbachia Releases. J. Med. Entomol. 2016, 53, 188–198. [Google Scholar] [CrossRef]
- Bagny, L.; Delatte, H.; Elissa, N.; Quilici, S.; Fontenille, D. Aedes (Diptera: Culicidae) Vectors of Arboviruses in Mayotte (Indian Ocean): Distribution Area and Larval Habitats. J. Med. Entomol. 2009, 46, 198–207. [Google Scholar] [CrossRef]
- Wat’senga Tezzo, F.; Fasine, S.; Manzambi Zola, E.; Marquetti, M.D.C.; Binene Mbuka, G.; Ilombe, G.; Mundeke Takasongo, R.; Smitz, N.; Bisset, J.A.; Van Bortel, W.; et al. High Aedes Spp. Larval Indices in Kinshasa, Democratic Republic of Congo. Parasit. Vectors 2021, 14, 92. [Google Scholar] [CrossRef]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public. Health 2018, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Malaysia. Guidelines of the Use of Ovitrap Surveillance; Ministry of Health Malaysia: Putrajaya, Malaysia, 1997; pp. 1–7. [Google Scholar]
- McGregor, B.L.; Connelly, C.R. A Review of the Control of Aedes Aegypti (Diptera: Culicidae) in the Continental United States. J. Med. Entomol. 2021, 58, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Reinbold-Wasson, D.D.; Reiskind, M.H. Comparative Skip-Oviposition Behavior Among Container Breeding Aedes Spp. Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2021, 58, 2091–2100. [Google Scholar] [CrossRef]
- Rey, J.R.; O’Connell, S.M. Oviposition by Aedes Aegypti and Aedes Albopictus: Influence of Congeners and of Oviposition Site Characteristics. J. Vector Ecol. 2014, 39, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, M.; Bacchi, M.; Bellini, R.; Maini, S. On the Competition Occurring Between Aedes Albopictus and Culex Pipiens (Diptera: Culicidae) in Italy. Environ. Entomol. 2003, 32, 1313–1321. [Google Scholar] [CrossRef]
- Devi, N.; Jauhari, R.; Mondal, R. Ovitrap Surveillance of Aedes Mosquitoes (Diptera: Culicidae) in Selected Areas of Dehradun District, Uttarakhand, India. Glob. J. Med. Res. Dis. 2013, 13, 53–57. [Google Scholar]
- Dalpadado, R.; Amarasinghe, D.; Gunathilaka, N.; Ariyarathna, N. Bionomic Aspects of Dengue Vectors Aedes Aegypti and Aedes Albopictus at Domestic Settings in Urban, Suburban and Rural Areas in Gampaha District, Western Province of Sri Lanka. Parasit. Vectors 2022, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Simo Tchetgna, H.; Yousseu, F.; Kamgang, B.; Tedjou, A.; McCall, P.; Wondji, C. Concurent Circulation of Dengue Serotype 1, 2 and 3 among Acute Febrile Patients in Cameroon. MedRxiv 2021, 15, e0009860. [Google Scholar]
- Kamgang, B.; Nchoutpouen, E.; Simard, F.; Paupy, C. Notes on the Blood-Feeding Behavior of Aedes Albopictus (Diptera: Culicidae) in Cameroon. Parasit. Vectors 2012, 5, 57. [Google Scholar] [CrossRef]
- Li, Y.; Su, X.; Zhou, G.; Zhang, H.; Puthiyakunnon, S.; Shuai, S.; Cai, S.; Gu, J.; Zhou, X.; Yan, G.; et al. Comparative Evaluation of the Efficiency of the BG-Sentinel Trap, CDC Light Trap and Mosquito-Oviposition Trap for the Surveillance of Vector Mosquitoes. Parasit. Vectors 2016, 9, 446. [Google Scholar] [CrossRef] [Green Version]
| Ecological Zones | |||||||
|---|---|---|---|---|---|---|---|
| Urban | Peri-Urban | Rural | Total | ||||
| Obili | Mvan | Simbock | Ahala | Lendom | Elig-Essomballa | ||
| Ovitraps | 1660 | 1834 | 3033 | 1560 | 1618 | 5618 | 15,323 |
| Sweep nets | 320 | 328 | 169 | 427 | 99 | 41 | 1384 |
| Biogent-Sentinel trap | 11 | 13 | 1 | 24 | 5 | 0 | 54 |
| Total | 1991 | 2175 | 3203 | 2011 | 1722 | 5659 | 16,761 |
| Sampling Methods | ||||
|---|---|---|---|---|
| Species | Ovitraps | Sweep Net | Biogent Sentinel Trap | Total |
| Aedes albopictus | 8315 | 1348 | 54 | 9717 |
| Aedes aegypti | 2033 | 3 | 0 | 2036 |
| Aedes contigus | 1047 | 0 | 0 | 1047 |
| Aedes simpsoni | 2042 | 3 | 0 | 2045 |
| Aedes spp. | 1418 | 0 | 0 | 1418 |
| Aedes (neomelaniconion) palpalis | 0 | 1 | 0 | 1 |
| Culex culiciomayia group | 371 | 4 | 0 | 375 |
| Toxorhynchites | 43 | 1 | 0 | 44 |
| Culex moucheti | 13 | 12 | 0 | 25 |
| Culex lutzia tigripes | 7 | 1 | 0 | 8 |
| Culex duttoni | 0 | 1 | 0 | 1 |
| Culex quinquefasciatus | 2 | 2 | 0 | 4 |
| Culex spp. | 26 | 6 | 0 | 32 |
| Eretmapodites | 6 | 2 | 0 | 8 |
| Total | 15,323 | 1384 | 54 | 16,761 |
| Area Ovitraps Index (Species Index) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Areas | N | n | OPI (%) | Number of Eggs | EDI | Ae. albopictus | Ae. aegypti | Ae. contigus | Ae. simpsoni |
| Obili | 331 | 193 | 58.3 ± 10.80 b | 1660 | 8.60 | 1658 (0.80) | 2 (0.01) | 0 | 0 |
| Mvan | 391 | 277 | 70.84 ± 9.95 a,b | 1780 | 6.42 | 1656 (0.81) | 26 (0.02) | 98 (0.04) | 0 |
| Simbock | 345 | 252 | 73.04 ± 9.72 a,b | 2266 | 8.99 | 1129 (0.67) | 418 (0.31) | 390 (0.23) | 329 (0.15) |
| Ahala | 381 | 236 | 61.94 ± 10.63 a,b | 1557 | 6.59 | 1361 (0.74) | 1 (0.004) | 176 (0.11) | 19 (0.01) |
| Lendom | 388 | 334 | 86.08 ± 7.58 a | 4576 | 13.70 | 1457 (0.54) | 1395 (0.36) | 192 (0.09) | 1532 (0.41) |
| Elig-essomballa | 391 | 240 | 61.38 ± 10.66 a,b | 1598 | 6.65 | 1054 (0.60) | 191 (0.19) | 191 (0.21) | 162 (0.14) |
| Total | 2227 | 1532 | 13,437 | 8315 (61.88%) | 2033 (15.12%) | 1047 (7.79%) | 2042 (15.19%) | ||
| Species | Urban | Peri-Urban | Rural | |||
|---|---|---|---|---|---|---|
| Obili | Mvan | Simbock | Ahala | Lendom | Elig-Essomballa | |
| Ae. albopictus + Ae. aegypti | 1.03 (2/193) | 2.52% (7/277) | 22.22% (56/252) | 0.42% (1/236) | 14.16% (34/240) | 21.25% (71/334) |
| Ae. albopictus + Ae. contigus | 0 | 3.97% (11/277) | 17.06% (43/252) | 9.32% (22/236) | 14.16% (34/240) | 8.08% (27/334) |
| Ae. albopictus + Ae. simpsoni | 0 | 0 | 12.69% (32/252) | 1.27% (3/236) | 11.25% (27/240) | 22.75% (76/334) |
| Ae. aegypti + Ae. contigus | 0 | 0.72% (2/277) | 12.69% (32/252) | 0.42% (1/236) | 5.41% (13/240) | 06.88% (23/334) |
| Ae. aegypti + Ae. simpsoni | 0 | 0 | 9.12% (23/252) | 0 | 5.41% (13/240) | 19.46% (65/334) |
| Ae. albopictus + Ae. aegypti + Ae. simpsoni | 0 | 0 | 9.12% (23/252) | 0 | 4.58% (11/240) | 18.56% (62/334) |
| Ae. albopictus + Ae. aegypti + Ae. contigus | 0 | 0 | 16.66% (42/252) | 0.42% (1/236) | 5% (12/240) | 6.58% (22/334) |
| Ae. albopictus + Ae. contigus + Ae. simpsoni | 0 | 0 | 7.93% (20/252) | 0.84% (2/236) | 4.58% (11/240) | 7.18% (24/334) |
| Ae. aegypti + Ae. contigus + Ae. simpsoni | 0 | 0 | 6.34% (16/252) | 0 | 5% (5/240) | 6.58% (22/334) |
| Ecological Zones | ||||||||
|---|---|---|---|---|---|---|---|---|
| Urban | Peri-Urban | Rural | Total | |||||
| Sampling Methods | Species | Obili | Mvan | Simbock | Ahala | Elig-Essomballa | Lendom | |
| Sweep net | Ae. albopictus | 320 | 328 | 166 | 425 | 10 | 99 | 1348 |
| Ae. aegypti | 0 | 0 | 0 | 0 | 3 | 0 | 3 | |
| Ae. simpsoni | 0 | 0 | 0 | 0 | 3 | 0 | 3 | |
| Aedes palpalis | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Biogent Sentinel trap | Ae. albopictus | 11 | 13 | 1 | 24 | 0 | 5 | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djiappi-Tchamen, B.; Nana-Ndjangwo, M.S.; Nchoutpouen, E.; Makoudjou, I.; Ngangue-Siewe, I.N.; Talipouo, A.; Mayi, M.P.A.; Awono-Ambene, P.; Wondji, C.; Tchuinkam, T.; et al. Aedes Mosquito Surveillance Using Ovitraps, Sweep Nets, and Biogent Traps in the City of Yaoundé, Cameroon. Insects 2022, 13, 793. https://doi.org/10.3390/insects13090793
Djiappi-Tchamen B, Nana-Ndjangwo MS, Nchoutpouen E, Makoudjou I, Ngangue-Siewe IN, Talipouo A, Mayi MPA, Awono-Ambene P, Wondji C, Tchuinkam T, et al. Aedes Mosquito Surveillance Using Ovitraps, Sweep Nets, and Biogent Traps in the City of Yaoundé, Cameroon. Insects. 2022; 13(9):793. https://doi.org/10.3390/insects13090793
Chicago/Turabian StyleDjiappi-Tchamen, Borel, Mariette Stella Nana-Ndjangwo, Elysée Nchoutpouen, Idene Makoudjou, Idriss Nasser Ngangue-Siewe, Abdou Talipouo, Marie Paul Audrey Mayi, Parfait Awono-Ambene, Charles Wondji, Timoléon Tchuinkam, and et al. 2022. "Aedes Mosquito Surveillance Using Ovitraps, Sweep Nets, and Biogent Traps in the City of Yaoundé, Cameroon" Insects 13, no. 9: 793. https://doi.org/10.3390/insects13090793
APA StyleDjiappi-Tchamen, B., Nana-Ndjangwo, M. S., Nchoutpouen, E., Makoudjou, I., Ngangue-Siewe, I. N., Talipouo, A., Mayi, M. P. A., Awono-Ambene, P., Wondji, C., Tchuinkam, T., & Antonio-Nkondjio, C. (2022). Aedes Mosquito Surveillance Using Ovitraps, Sweep Nets, and Biogent Traps in the City of Yaoundé, Cameroon. Insects, 13(9), 793. https://doi.org/10.3390/insects13090793








