Assessment of Suitable Reference Genes for qRT-PCR Normalization in Eocanthecona furcellata (Wolff)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Conditions
2.2.1. Development Stages
2.2.2. Different Tissues
2.2.3. Temperature Treatment
2.2.4. Starvation Treatment
2.3. Total RNA Extraction and cDNA Synthesis
2.4. Candidate Reference Gene Selection and Primer Design
2.5. qRT-PCR
2.6. Data Analysis
2.7. Validation of Selected Reference Gene
3. Results
3.1. Amplification Efficiency of Primers
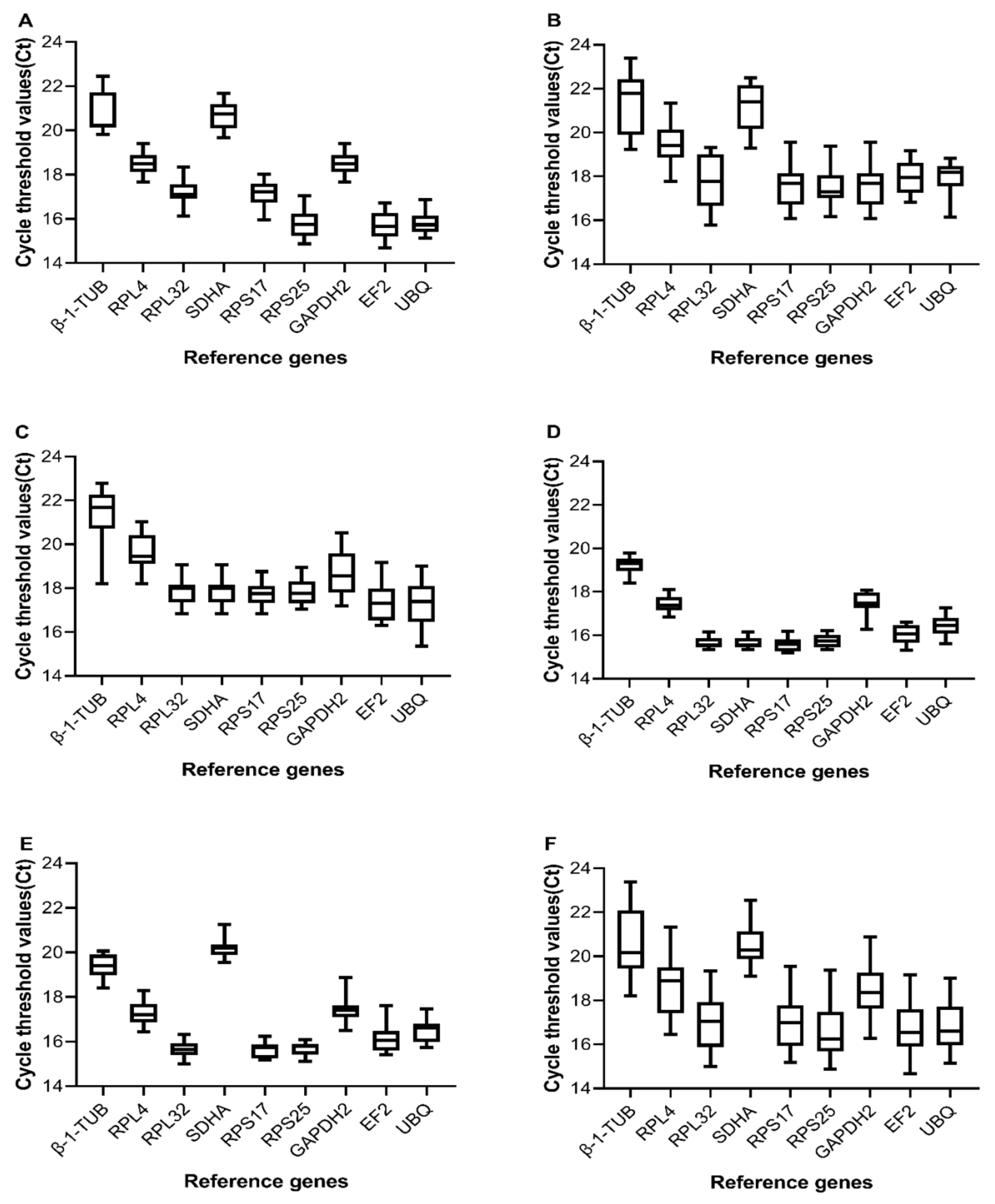
3.2. Expression Profiles of Candidate Reference Genes
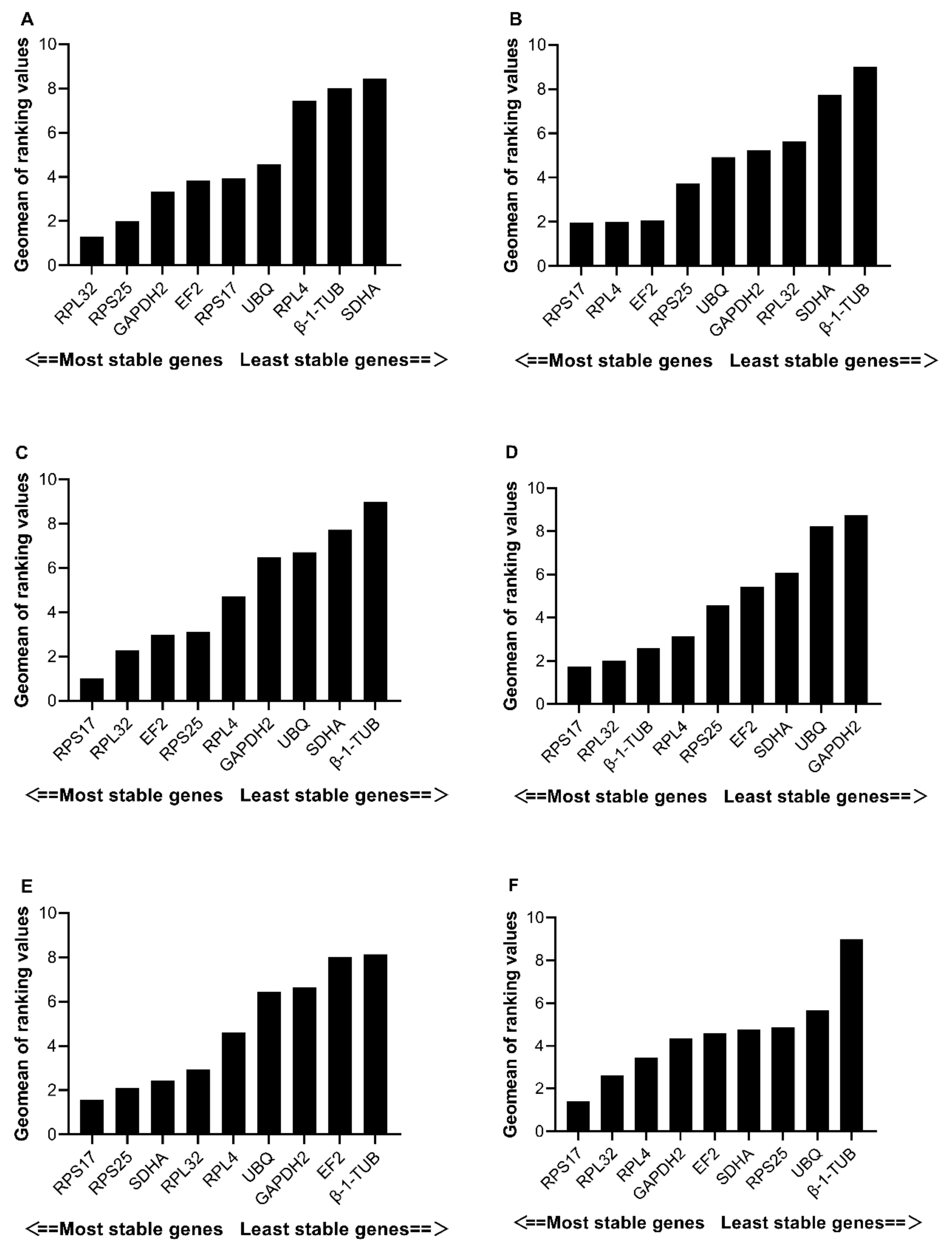
3.3. Expression Stability of Candidate Reference Genes
3.3.1. Developmental Stages
3.3.2. Female Tissues
3.3.3. Male Tissues
3.3.4. Temperature Treatments
3.3.5. Starvation Treatments
3.3.6. All Samples
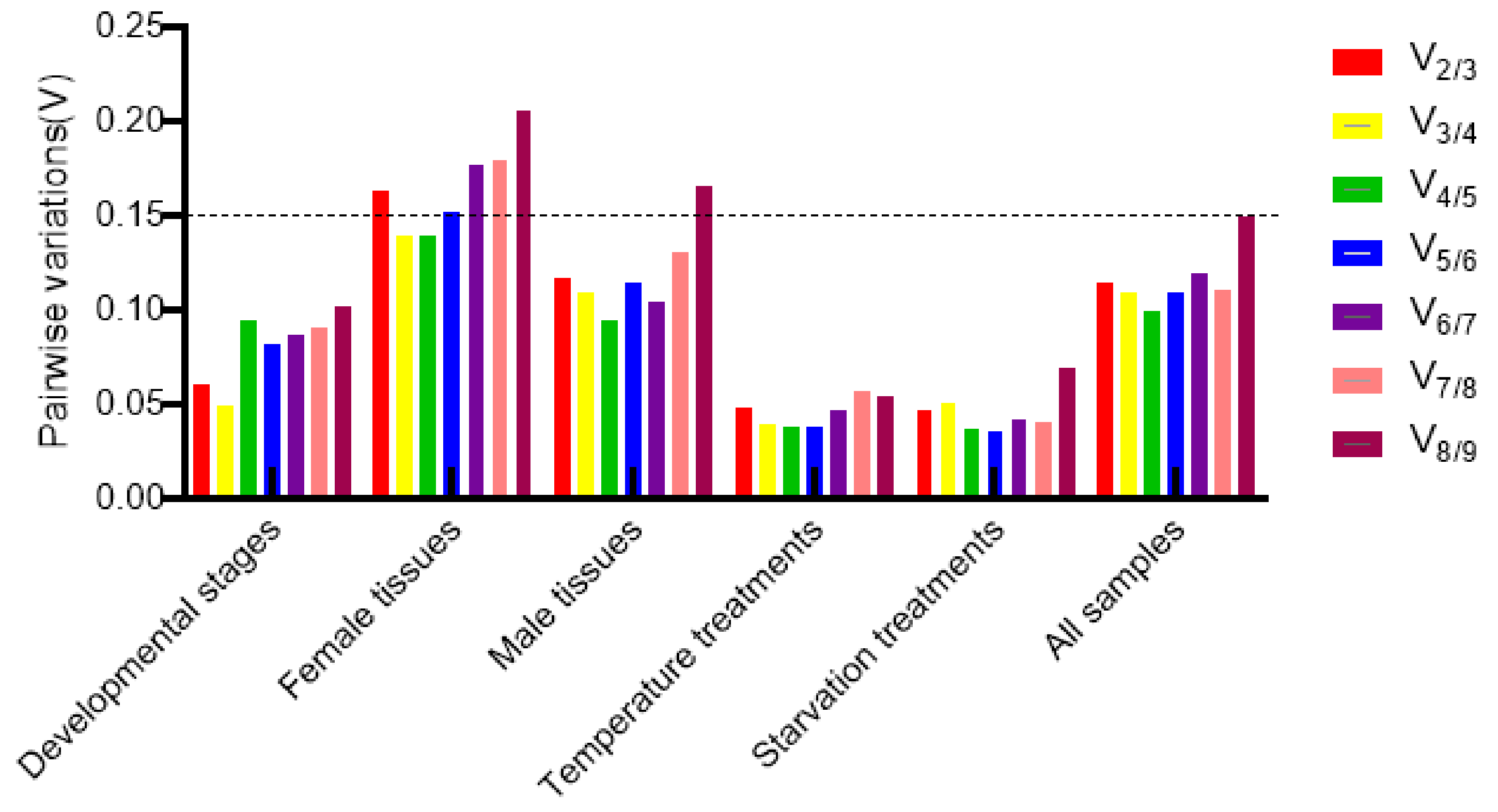
3.4. Optimal Number of Reference Genes Normalized under Different Experimental Conditions
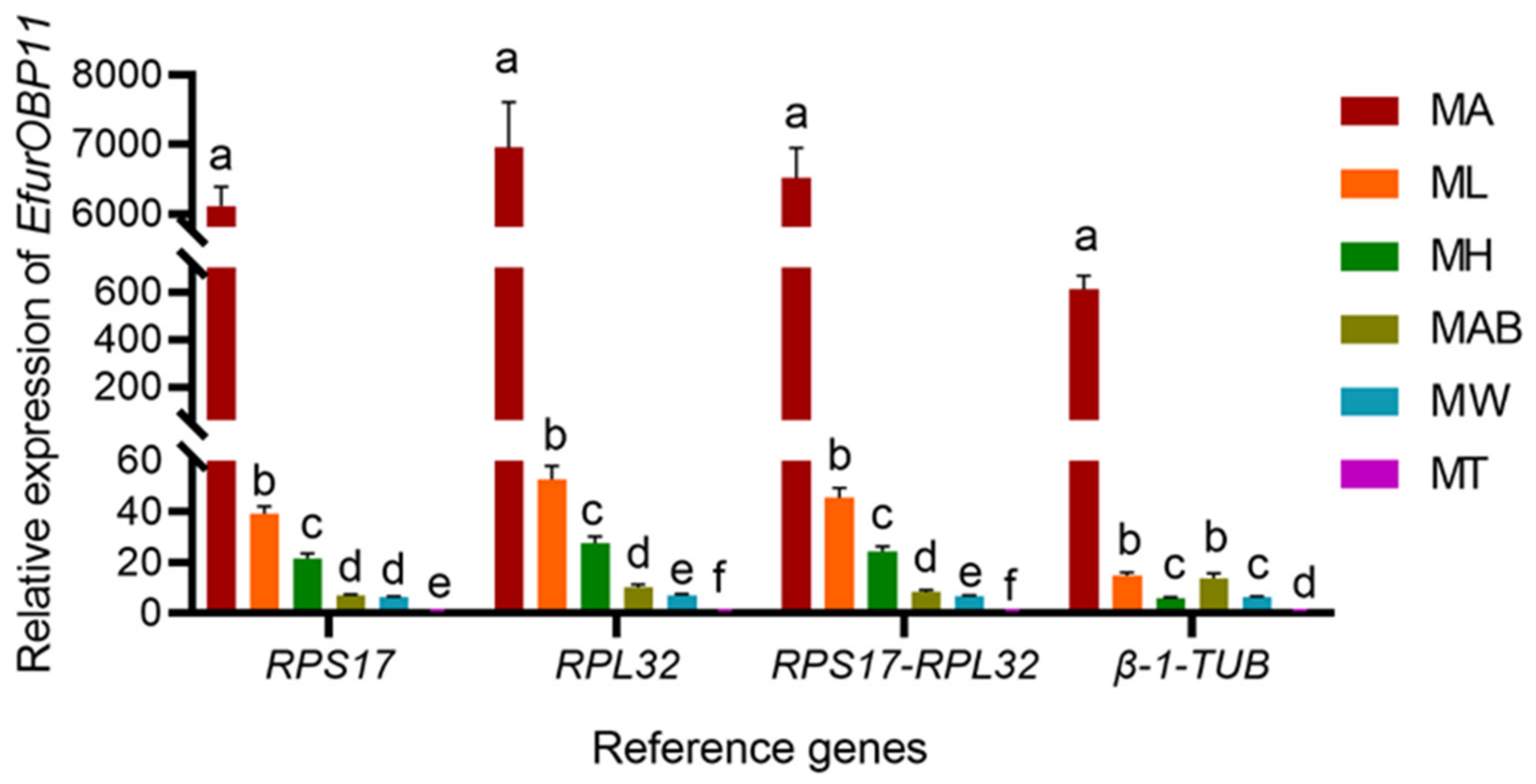
3.5. Validation of Selected Reference Genes in E. furcellata
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.C.; Wang, H.; Ren, H.D.; Hang, C.F. Studies on the biological characteristics of Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae, Asopinae). For. Res. 1998, 92–96. [Google Scholar]
- Pan, Y.N.; Yao, M.Y.; Fu, D.; Guo, F.; Qin, R.; Jiang, L.; Chen, W.L. Predation effects of Eocanthecona furcellata (Wolff) adults against Spodoptera frugiperda larvae. J. Mt. Agric. Biol. 2020, 39, 23–27. [Google Scholar] [CrossRef]
- Lenin, E.A.; Rajan, S.J. Biology of predatory bug Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae) on Corcyra cephalonica Stainton. J. Entomol. Zool. Stud. 2016, 4, 338–340. [Google Scholar]
- Yu, C.; Yao, M.Y.; Zhao, R.N.; Wang, L.; Chen, W.L. Effects of breeding density on growth and development of Eocanthecona furcellata (Wolff). J. Mt. Agric. Biol. 2021, 40, 75–79. [Google Scholar] [CrossRef]
- He, X.N.; Xian, J.D.; Chen, R.; Zhang, Z.Y.; Zeng, R. Effects of four insect feed on development and reproduction of Eocanthecona furcellata (Wolff) (Hemiptera: Asopinae). J. Environ. Entomol. 2013, 35, 799–803. [Google Scholar] [CrossRef]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef]
- Li, R.; Xie, W.; Wang, S.; Wu, Q.; Yang, N.; Yang, X.; Pan, H.; Zhou, X.; Bai, L.; Xu, B.; et al. Reference gene selection for qRT-PCR analysis in the sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2013, 8, e53006. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef]
- Chapuis, M.P.; Tohidi-Esfahani, D.; Dodgson, T.; Blondin, L.; Ponton, F.; Cullen, D.; Simpson, S.J.; Sword, G.A. Assessment and validation of a suite of reverse transcription-quantitative PCR reference genes for analyses of density-dependent behavioural plasticity in the Australian plague locust. BMC Mol. Biol. 2011, 12, 7. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, K.D.; Roy, N.V.; Paepe, A.D. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 12. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Bestkeeper–excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. MiRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Vogt, R.G.; Prestwich, G.D.; Lerner, M.R. Odorant-binding-protein subfamilies associate with distinct classes of olfactory receptor neurons in insects. J. Neurobiol. 1991, 22, 74–84. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Guan, H.; Yang, K. RNA isolation and real-time quantitative. Methods Mol. Biol. 2008, 456, 259–270. [Google Scholar]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Lee, P.D.; Sladek, R.; Greenwood, C.M.T.; Hudson, T.J. Control genes and variability: Absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 2002, 12, 292–297. [Google Scholar] [CrossRef]
- Luo, J.; Ma, C.; Li, Z.; Zhu, B.; Zhang, J.; Lei, C.L.; Jin, S.X.; Joe, J.H.; Chen, L. Assessment of suitable reference genes for qRT-PCR analysis in Adelphocoris suturalis. J. Integr. Agric. 2018, 17, 2745–2757. [Google Scholar] [CrossRef]
- Yuan, M.; Lu, Y.; Zhu, X.; Wan, H.; Shakeel, M.; Zhan, S.; Jin, B.R.; Li, J. Selection and evaluation of potential reference genes for gene expression analysis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) using reverse-transcription quantitative PCR. PLoS ONE 2014, 9, e86503. [Google Scholar] [CrossRef] [Green Version]
- Freitas, F.C.P.; Depintor, T.S.; Agostini, L.T.; Luna-Lucena, D.; Nunes, F.M.F.; Bitondi, M.M.G.; Simões, Z.L.P.; Lourenço, A.P. Evaluation of reference genes for gene expression analysis by real-time quantitative PCR (qPCR) in three stingless bee species (Hymenoptera: Apidae: Meliponini). Sci. Rep. 2019, 9, 17692. [Google Scholar] [CrossRef]
- Sonoda, S.; Ashfaq, M.; Tsumuki, H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (l.) and their expression in relation to developmental stage and temperature. Arch. Insect Biochem. Physiol. 2006, 62, 80–90. [Google Scholar] [CrossRef]
- Shu, B.S.; Hai-Kuo, Y.U.; Dai, J.H.; Xie, Z.G.; Qian, W.Q.; Lin, J.T. Stability evaluation of reference genes for real-time quantitative PCR normalization in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 2471–2482. [Google Scholar] [CrossRef]
- Zhai, Y.; Lin, Q.; Zhou, X.; Zhang, X.; Liu, T.; Yu, Y. Identification and validation of reference genes for quantitative real-time PCR in Drosophila suzukii (Diptera: Drosophilidae). PLoS ONE 2014, 9, e106800. [Google Scholar] [CrossRef]
- Sagri, E.; Koskinioti, P.; Gregoriou, M.E.; Tsoumani, K.T.; Bassiakos, Y.C.; Mathiopoulos, K.D. Housekeeping in tephritid insects: The best gene choice for expression analyses in the medfly and the olive fly. Sci. Rep. 2017, 7, 45634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z. The Rpf84 gene, encoding a ribosomal large subunit protein, RPL22, regulates symbiotic nodulation in Robinia pseudoacacia. Planta 2019, 250, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.J. Reference genes for quantitative real-time PCR analysis in symbiont entomomyces delphacidicola of Nilaparvata lugens (Stål). Sci. Rep. 2017, 7, 42206. [Google Scholar] [CrossRef]
- Jia, B.; Ma, Y.; Pang, B.Q.; Shan, Y.M.; Bao, Q.L.; Han, H.B.; Tan, Y. Screening of reference genes for quantitative real-time PCR in Lygus pratensis (Hemiptera: Miridae). ACTA Entomol. Sin. 2019, 62, 1379–1391. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Yang, Y.; Chai, Y.; Gao, L.L.; Ma, R. Identification and evaluation of reference genes for quantitative PCR normalization in alligator weed flea beetle (Coleoptera: Chrysomelidae). J. Insect Sci. 2021, 21, 7. [Google Scholar] [CrossRef]
- Ehara, H.; Kujirai, T.; Fujino, Y.; Shirouzu, M.; Kurumizaka, H.; Sekine, S. Structural insight into nucleosome transcription by RNA polymerase II with elongation factors. Science 2019, 363, 744–747. [Google Scholar] [CrossRef]
- Lü, J.; Yang, C.; Zhang, Y.; Pan, H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insects: A systematic review. Front. Physiol. 2018, 9, 1560. [Google Scholar] [CrossRef]
- Bansal, R.; Mamidala, P.; Mian, M.A.R.; Mittapalli, O.; Michel, A.P. Validation of reference genes for gene expression studies in Aphis glycines (Hemiptera: Aphididae). J. Econ. Entomol. 2012, 105, 1432–1438. [Google Scholar] [CrossRef]
- Shakeel, M.; Rodriguez, A.; Tahir, U.B.; Jin, F. Gene expression studies of reference genes for quantitative real-time PCR: An overview in insects. Biotechnol. Lett. 2018, 40, 227–236. [Google Scholar] [CrossRef]
- Tan, Q.Q.; Zhu, L.; Li, Y.; Liu, W.; Ma, W.H.; Lei, C.L.; Wang, X.P. A de novo transcriptome and valid reference genes for quantitative real-time PCR in Colaphellus bowringi. PLoS ONE 2015, 10, e0118693. [Google Scholar] [CrossRef]
- Lord, J.C.; Hartzer, K.; Toutges, M.; Oppert, B. Evaluation of quantitative PCR reference genes for gene expression studies in Tribolium castaneum after fungal challenge. J. Microbiol. Methods 2010, 80, 219–221. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Khajuria, C.; Wang, H.; Matz, N.; Cunha Cardoso, D.; Valicente, F.H.; Zhou, X.; Siegfried, B. Validation of reference housekeeping genes for gene expression studies in western corn rootworm (Diabrotica virgifera virgifera). PLoS ONE 2014, 9, e109825. [Google Scholar] [CrossRef]
- Concha, C.; Edman, R.M.; Belikoff, E.J.; Schiemann, A.H.; Carey, B.; Scott, M.J. Organization and expression of the Australian sheep blowfly (Lucilia cuprina) hsp23, hsp24, hsp70 and hsp83 genes. Insect Mol. Biol. 2012, 21, 169–180. [Google Scholar] [CrossRef]
- Ling, D.; Salvaterra, P.M. Robust RT-qPCR data normalization: Validation and selection of internal reference genes during post-experimental data analysis. PLoS ONE 2011, 6, e17762. [Google Scholar] [CrossRef]
- Fu, W.; Xie, W.; Zhang, Z.; Wang, S.; Wu, Q.; Liu, Y.; Zhou, X.; Zhou, X.; Zhang, Y. Exploring valid reference genes for quantitative real-time PCR analysis in Plutella xylostella (Lepidoptera: Plutellidae). Int. J. Biol. Sci. 2013, 9, 792–802. [Google Scholar] [CrossRef] [Green Version]
| Gene | Accession Number | Primer Sequence (5′–3′) | Product Length (bp) | Tm (°C) | Efficiency (%) | R2 |
|---|---|---|---|---|---|---|
| β-1-TUB | ON505066 | F: ACTGACACATTCTCTTGGAGGT R: GGTGGCGTTATAAGGTTCTACA | 157 | 55.0 | 101.1 | 0.996 |
| RPL4 | ON505068 | F: TTCCCGAAATCCCTCTTGTTG R: GTCTCCTATTACGCATCTTACCT | 162 | 55.0 | 97.5 | 0.996 |
| RPL32 | ON505067 | F: AGGAGGAACTGGCGTAAGC R: GGAACTAACAGCATGAGCGATT | 213 | 55.0 | 103.3 | 0.996 |
| SDHA | ON505070 | F: GCTCCAGAACTTAATGTTGTGT R: TACGCCAATGCTCCTCAATAG | 170 | 55.0 | 102.6 | 0.995 |
| RPS17 | ON505073 | F: CGCTATCATTCCTACCAAACCT R: CTCCAACATCTTCAACATTCCA | 217 | 55.0 | 95.0 | 0.997 |
| RPS25 | ON505074 | F: GTCTCCTATTACGCATCTTACCT R: CTGCTTTAGTCGCCCTGGTA | 115 | 55.0 | 93.7 | 0.995 |
| GAPDH2 | ON505069 | F: TCTGTGGTGTCAACTTGGATG R: CGTCTTCTGAGTAGCGGTAAC | 167 | 55.0 | 99.7 | 0.996 |
| EF2 | ON505072 | F: TGGAGGTATCTATGGTGTACTGA R: AATGGCTTGGTGTTGGTGTC | 228 | 55.0 | 104.8 | 0.995 |
| UBQ | ON505071 | F: CGGCAAGACTATCACACTAGAAG R: ATACCTCCTCTGAGACGAAGTAC | 204 | 55.0 | 100.5 | 0.990 |
| Experimental Conditions | Reference Gene | ∆Ct | BestKeeper | NormFinder | geNorm | ||||
|---|---|---|---|---|---|---|---|---|---|
| Stability | Rank | Stability | Rank | Stability | Rank | Stability | Rank | ||
| Developmental stages | β-1-TUB | 0.904 | 8 | 0.789 | 8 | 0.785 | 8 | 0.622 | 8 |
| RPL4 | 0.802 | 7 | 0.888 | 9 | 0.662 | 7 | 0.549 | 7 | |
| RPL32 | 0.542 | 1 | 0.422 | 3 | 0.153 | 1 | 0.306 | 1 | |
| SDHA | 0.992 | 9 | 0.544 | 7 | 0.901 | 9 | 0.705 | 9 | |
| RPS17 | 0.574 | 4 | 0.48 | 5 | 0.248 | 3 | 0.332 | 4 | |
| RPS25 | 0.556 | 2 | 0.477 | 4 | 0.217 | 2 | 0.306 | 1 | |
| GAPDH2 | 0.674 | 5 | 0.377 | 1 | 0.417 | 5 | 0.427 | 5 | |
| EF2 | 0.567 | 3 | 0.525 | 6 | 0.256 | 4 | 0.313 | 3 | |
| UBQ | 0.728 | 6 | 0.388 | 2 | 0.53 | 6 | 0.486 | 6 | |
| Female tissues | β-1-TUB | 1.915 | 9 | 1.207 | 9 | 1.774 | 9 | 1.267 | 9 |
| RPL4 | 1.055 | 2 | 0.739 | 4 | 0.614 | 2 | 0.434 | 1 | |
| RPL32 | 1.211 | 6 | 1.04 | 8 | 0.933 | 7 | 0.543 | 3 | |
| SDHA | 1.497 | 8 | 0.961 | 7 | 1.176 | 8 | 1.082 | 8 | |
| RPS17 | 1.03 | 1 | 0.788 | 5 | 0.633 | 3 | 0.434 | 1 | |
| RPS25 | 1.109 | 4 | 0.704 | 3 | 0.713 | 4 | 0.618 | 4 | |
| GAPDH2 | 1.174 | 5 | 0.931 | 6 | 0.780 | 5 | 0.698 | 5 | |
| EF2 | 1.102 | 3 | 0.631 | 1 | 0.552 | 1 | 0.791 | 6 | |
| UBQ | 1.309 | 7 | 0.647 | 2 | 0.906 | 6 | 0.944 | 7 | |
| Male tissues | β-1-TUB | 1.525 | 9 | 1.03 | 9 | 1.413 | 9 | 0.966 | 9 |
| RPL4 | 0.924 | 5 | 0.613 | 4 | 0.657 | 5 | 0.529 | 5 | |
| RPL32 | 0.796 | 3 | 0.471 | 3 | 0.468 | 3 | 0.264 | 1 | |
| SDHA | 1.146 | 8 | 0.825 | 7 | 0.905 | 8 | 0.806 | 8 | |
| RPS17 | 0.737 | 1 | 0.423 | 1 | 0.326 | 1 | 0.264 | 1 | |
| RPS25 | 0.824 | 4 | 0.444 | 2 | 0.508 | 4 | 0.358 | 3 | |
| GAPDH2 | 0.976 | 7 | 0.813 | 6 | 0.714 | 7 | 0.623 | 6 | |
| EF2 | 0.794 | 2 | 0.671 | 5 | 0.361 | 2 | 0.468 | 4 | |
| UBQ | 0.97 | 6 | 0.846 | 8 | 0.661 | 6 | 0.693 | 7 | |
| Temperature treatments | β-1-TUB | 0.407 | 3 | 0.302 | 5 | 0.247 | 3 | 0.24 | 1 |
| RPL4 | 0.418 | 4 | 0.316 | 6 | 0.276 | 4 | 0.24 | 1 | |
| RPL32 | 0.398 | 2 | 0.21 | 1 | 0.22 | 2 | 0.285 | 4 | |
| SDHA | 0.484 | 7 | 0.288 | 4 | 0.359 | 7 | 0.384 | 7 | |
| RPS17 | 0.376 | 1 | 0.26 | 3 | 0.173 | 1 | 0.253 | 3 | |
| RPS25 | 0.477 | 6 | 0.241 | 2 | 0.349 | 6 | 0.351 | 6 | |
| GAPDH2 | 0.598 | 9 | 0.374 | 8 | 0.517 | 9 | 0.461 | 9 | |
| EF2 | 0.460 | 5 | 0.327 | 7 | 0.336 | 5 | 0.316 | 5 | |
| UBQ | 0.529 | 8 | 0.410 | 9 | 0.42 | 8 | 0.422 | 8 | |
| Starvation treatments | β-1-TUB | 0.653 | 9 | 0.407 | 6 | 0.575 | 9 | 0.509 | 9 |
| RPL4 | 0.465 | 4 | 0.416 | 7 | 0.286 | 4 | 0.351 | 4 | |
| RPL32 | 0.479 | 5 | 0.292 | 3 | 0.313 | 5 | 0.301 | 1 | |
| SDHA | 0.458 | 3 | 0.365 | 4 | 0.282 | 3 | 0.301 | 1 | |
| RPS17 | 0.404 | 1 | 0.284 | 2 | 0.114 | 1 | 0.333 | 3 | |
| RPS25 | 0.437 | 2 | 0.244 | 1 | 0.221 | 2 | 0.375 | 5 | |
| GAPDH2 | 0.516 | 6 | 0.439 | 9 | 0.381 | 6 | 0.399 | 6 | |
| EF2 | 0.636 | 8 | 0.426 | 8 | 0.553 | 8 | 0.468 | 8 | |
| UBQ | 0.535 | 7 | 0.406 | 5 | 0.409 | 7 | 0.425 | 7 | |
| All samples | β-1-TUB | 1.374 | 9 | 1.182 | 9 | 1.180 | 9 | 1.050 | 9 |
| RPL4 | 0.946 | 3 | 1.079 | 8 | 0.604 | 2 | 0.510 | 3 | |
| RPL32 | 0.942 | 2 | 0.973 | 6 | 0.622 | 4 | 0.355 | 1 | |
| SDHA | 1.266 | 8 | 0.729 | 1 | 1.042 | 8 | 0.958 | 8 | |
| RPS17 | 0.879 | 1 | 0.913 | 4 | 0.488 | 1 | 0.355 | 1 | |
| RPS25 | 0.946 | 4 | 1.002 | 7 | 0.626 | 5 | 0.631 | 4 | |
| GAPDH2 | 0.962 | 6 | 0.859 | 2 | 0.628 | 6 | 0.707 | 5 | |
| EF2 | 0.972 | 5 | 0.913 | 4 | 0.621 | 3 | 0.770 | 6 | |
| UBQ | 1.127 | 7 | 0.909 | 3 | 0.860 | 7 | 0.857 | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.-N.; Zhao, R.-N.; Fu, D.; Yu, C.; Pan, C.-N.; Zhou, W.; Chen, W.-L. Assessment of Suitable Reference Genes for qRT-PCR Normalization in Eocanthecona furcellata (Wolff). Insects 2022, 13, 773. https://doi.org/10.3390/insects13090773
Pan Y-N, Zhao R-N, Fu D, Yu C, Pan C-N, Zhou W, Chen W-L. Assessment of Suitable Reference Genes for qRT-PCR Normalization in Eocanthecona furcellata (Wolff). Insects. 2022; 13(9):773. https://doi.org/10.3390/insects13090773
Chicago/Turabian StylePan, Ying-Na, Ru-Na Zhao, Di Fu, Chun Yu, Chun-Ni Pan, Wei Zhou, and Wen-Long Chen. 2022. "Assessment of Suitable Reference Genes for qRT-PCR Normalization in Eocanthecona furcellata (Wolff)" Insects 13, no. 9: 773. https://doi.org/10.3390/insects13090773
APA StylePan, Y.-N., Zhao, R.-N., Fu, D., Yu, C., Pan, C.-N., Zhou, W., & Chen, W.-L. (2022). Assessment of Suitable Reference Genes for qRT-PCR Normalization in Eocanthecona furcellata (Wolff). Insects, 13(9), 773. https://doi.org/10.3390/insects13090773






