Changes in the Host Gut Microbiota during Parasitization by Parasitic Wasp Cotesia vestalis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
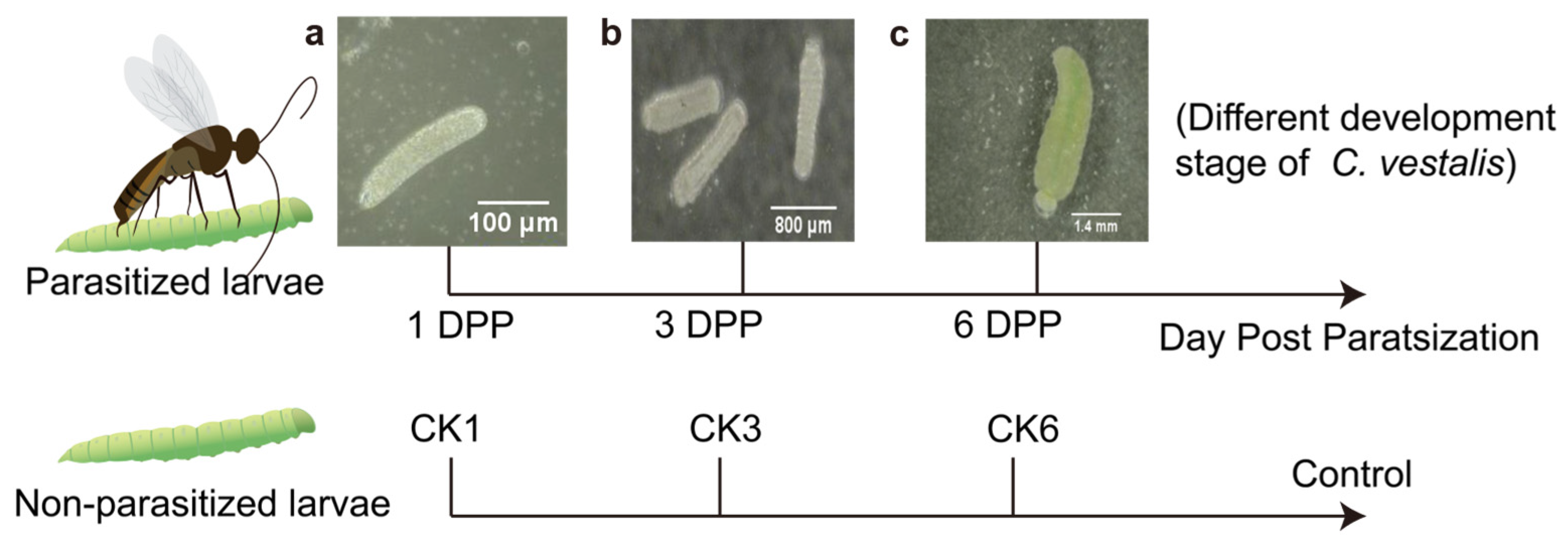
2.1. Insect Rearing and Sample Collection
2.2. DNA Extraction and PCR Amplification of 16S rDNA Sequencing
2.3. Sequence Data Analysis
2.4. Isolation of Host Gut Bacteria
3. Results
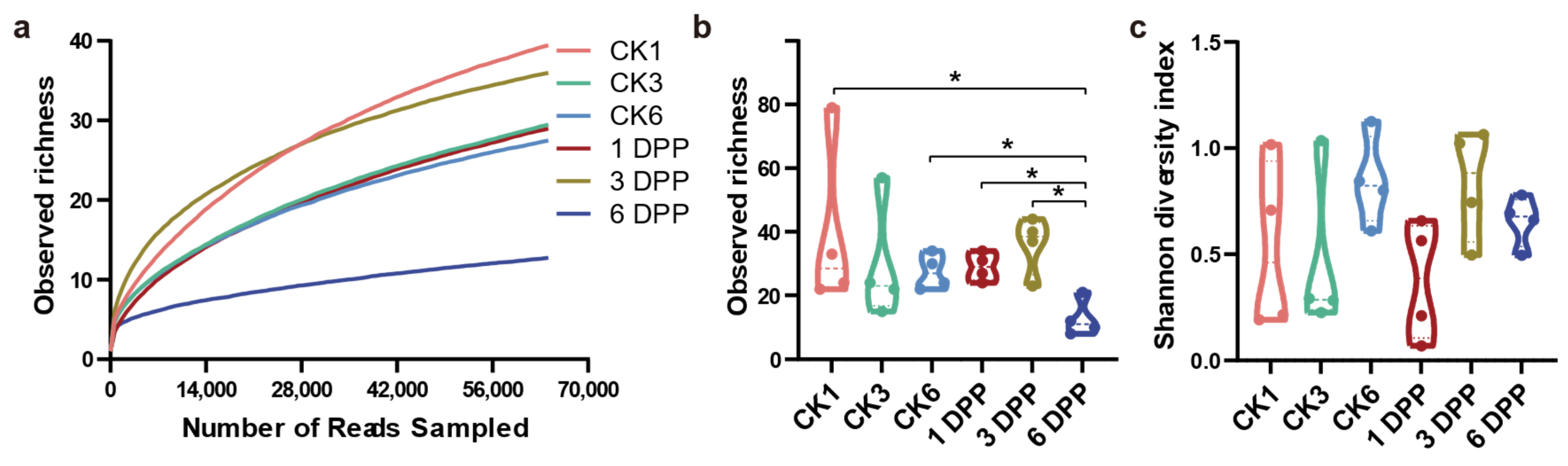
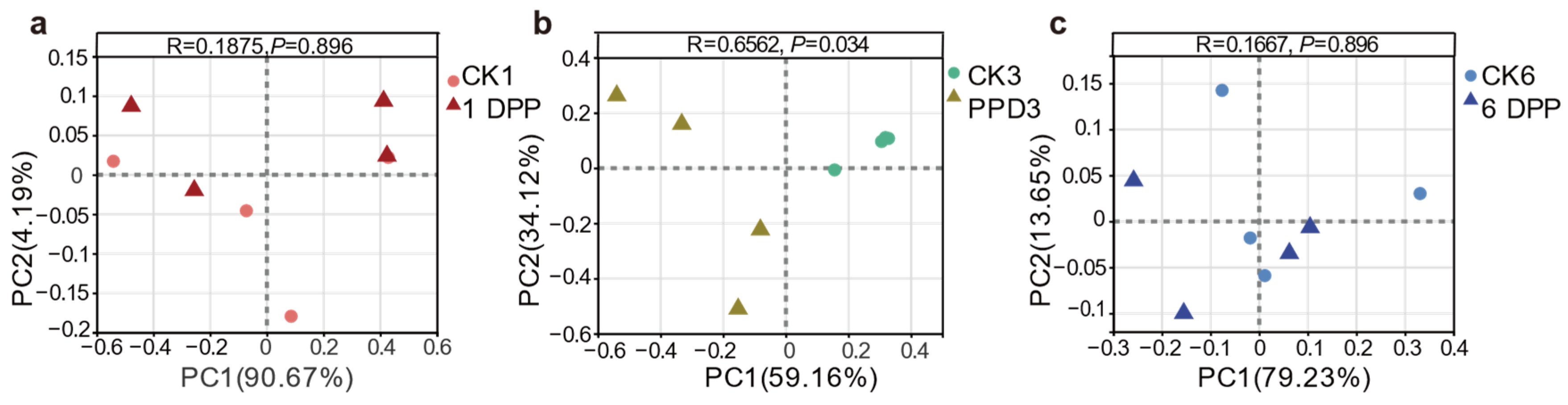
3.1. Effects of Parasitization on Host Gut Microbial Community Diversity and Structure by C. Vestalis
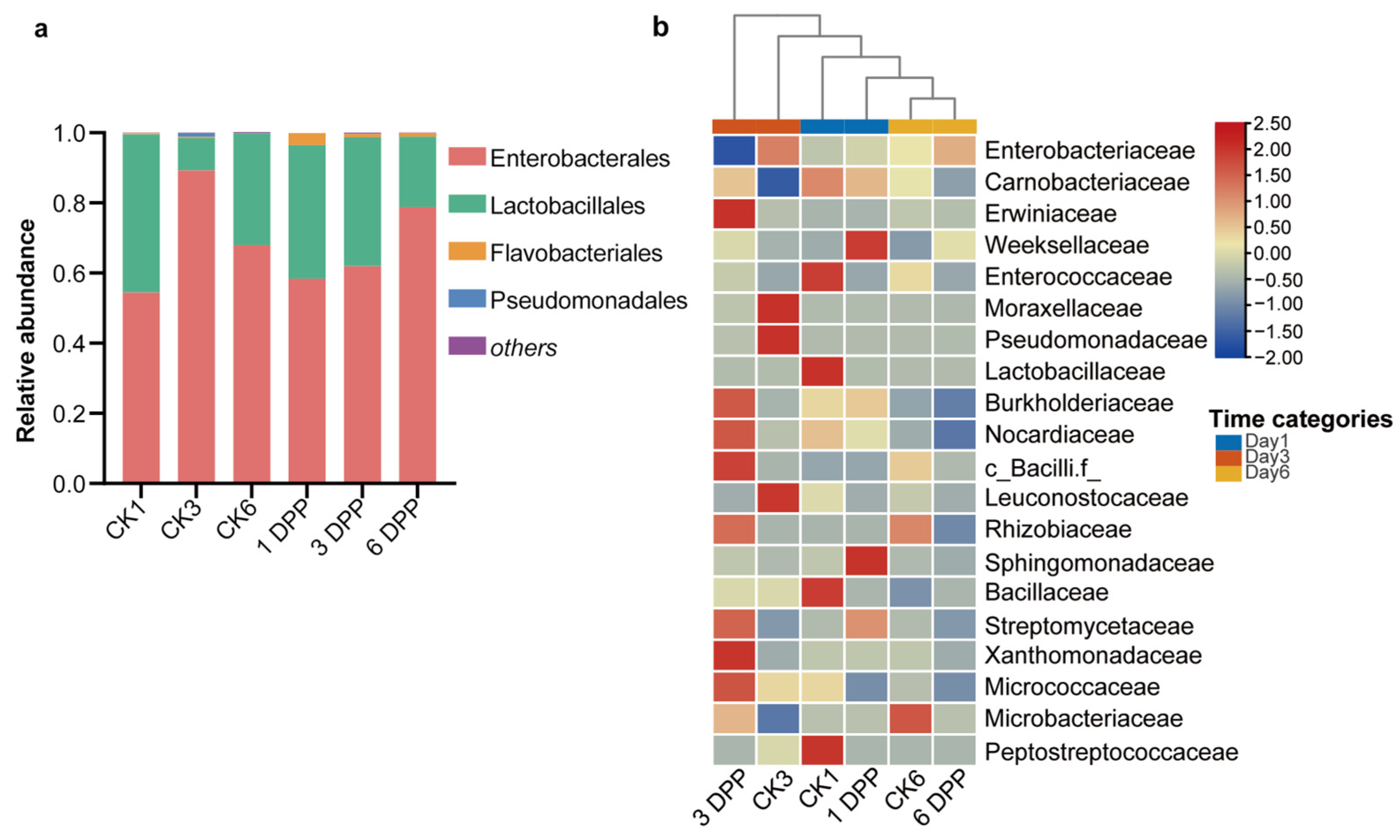
3.2. Impact of Parasitization on the Composition of Host-Gut Microbiota
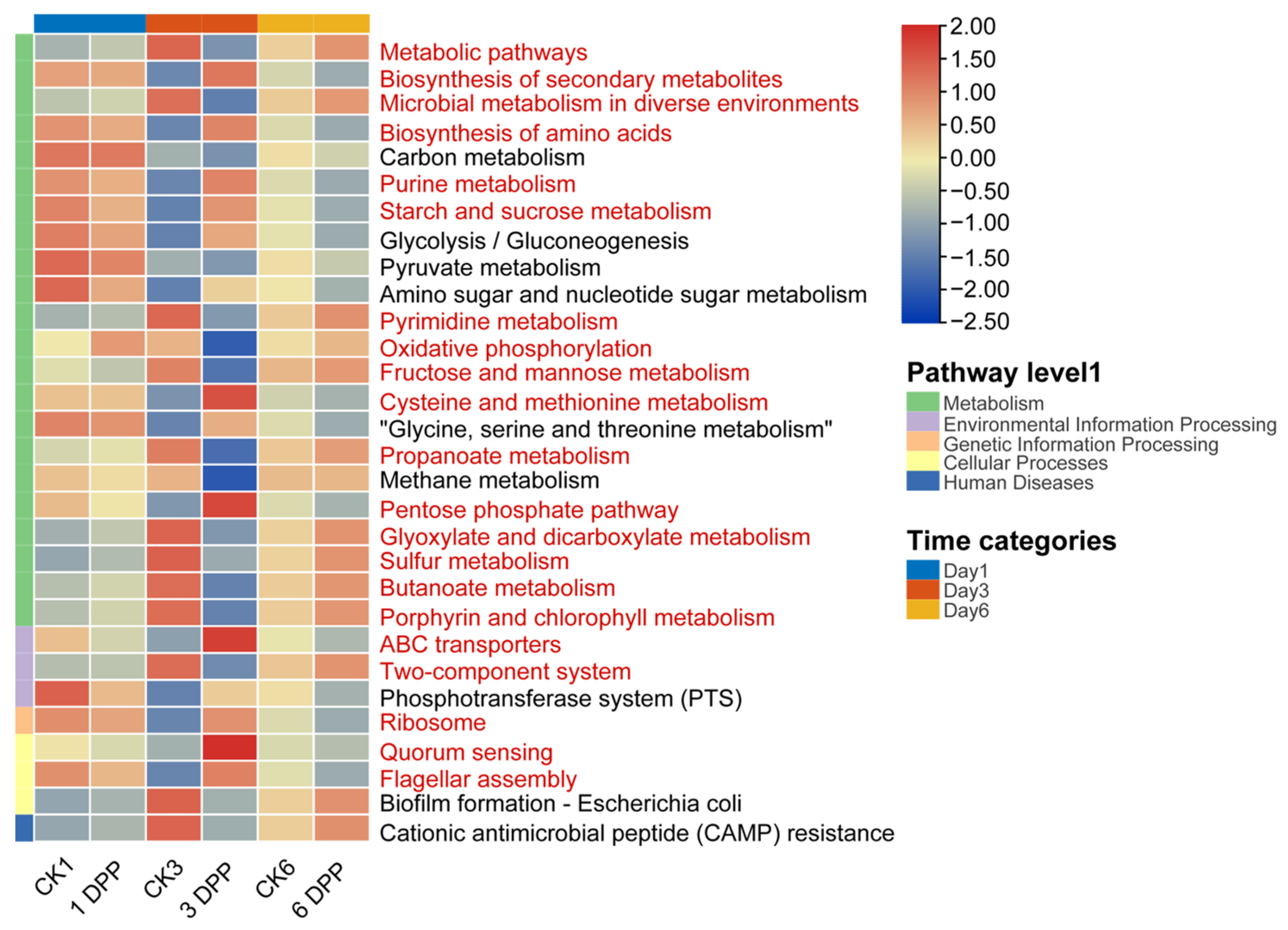
3.3. Effects of Parasitization on Host-Gut Microbial Function by C. vestalis
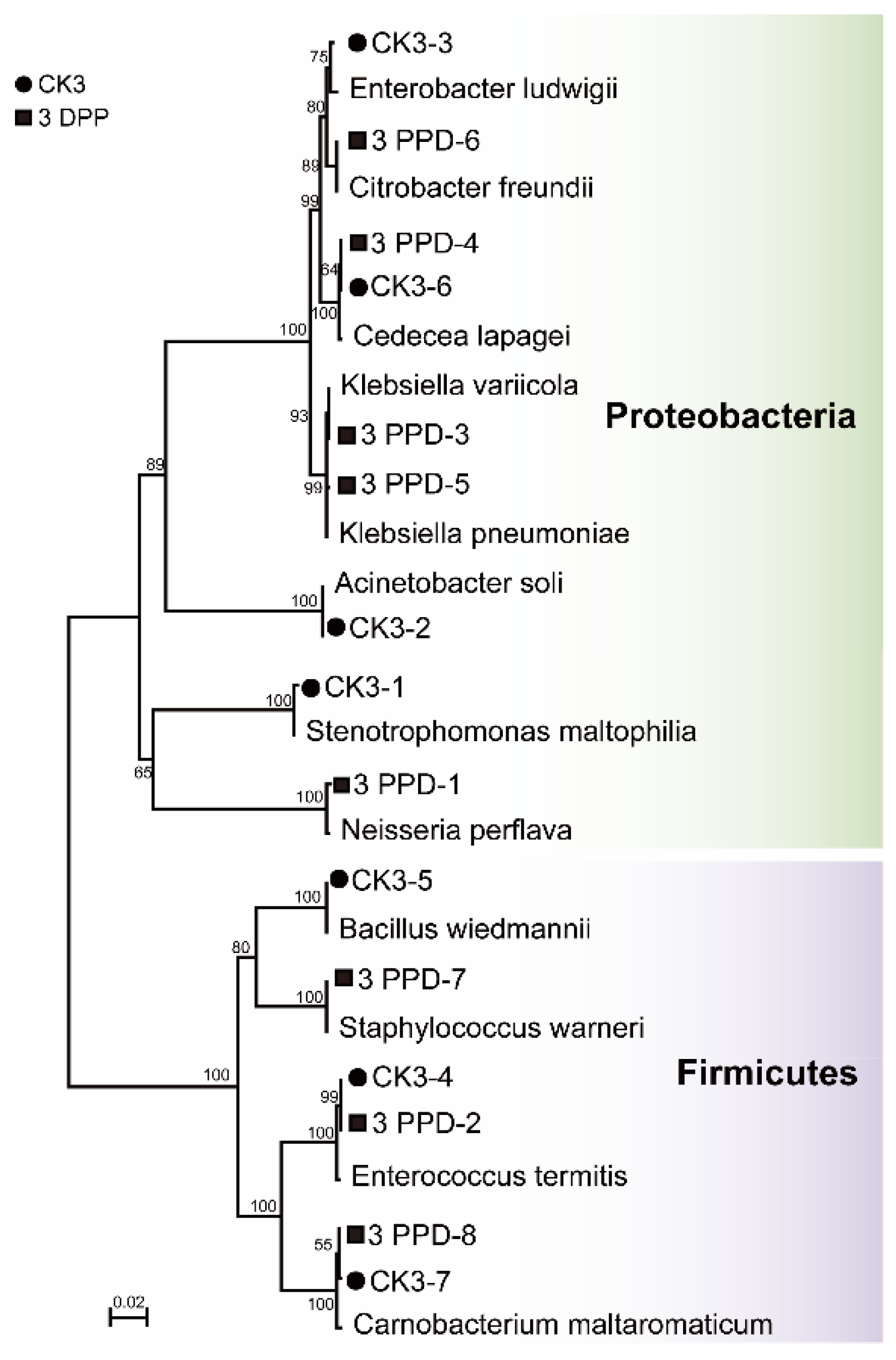
3.4. Isolation and Culture of Bacteria from Parasitized and Non-Parasitized Host Gut
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Tokuda, G. Cellulolytic Systems in Insects. Annu. Rev. Entomol. 2009, 55, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Powell, J.E.; Steele, M.I.; Dietrich, C.; Moran, N.A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eterovic, M.; Kirfel, P.; Grotmann, J.; Vilcinskas, A. Fitness costs of infection with Serratia symbiotica are associated with greater susceptibility to insecticides in the pea aphid Acyrthosiphon pisum: Serratia symbiotica correlates with susceptibility to insecticides in the pea aphid. Pest Manag. Sci. 2018, 74, 1829–1836. [Google Scholar]
- Jia, Y.; Jin, S.; Hu, K.; Geng, L.; Han, C.; Kang, R.; Pang, Y.; Ling, E.; Tan, E.; Pan, Y.; et al. Gut microbiome modulates Drosophila aggression through octopamine signaling. Nat. Commun. 2021, 12, 2698. [Google Scholar] [CrossRef]
- Raza, M.; Wang, Y.; Cai, Z.; Bai, S.; Awan, U.; Zhang, Z.-Y.; Zheng, W.; Zhang, H. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Bai, L.; Jiang, Y.; Huang, W.; Wang, L.; Li, S.; Zhu, G.; Wang, D.; Huang, Z.; Li, X.; et al. A natural symbiotic bacterium drives mosquito refractoriness to Plasmodium infection via secretion of an antimalarial lipase. Nat. Microbiol. 2021, 6, 806–817. [Google Scholar] [CrossRef]
- Charroux, B.; Royet, J. Gut-Microbiota interactions in non-mammals: What can we learn from Drosophila? Semin. Immunol. 2012, 24, 17–24. [Google Scholar] [CrossRef]
- Buchon, N.; Broderick, N.; Lemaitre, B. Gut homeostasis in a microbial world: Insights from Drosophila melanogaster, Nature reviews. Microbiology 2013, 11, 615–626. [Google Scholar]
- Pernice, M.; Simpson, S.J.; Ponton, F. Towards an integrated understanding of gut microbiota using insects as model systems. J. Insect Physiol. 2014, 69, 12–18. [Google Scholar] [CrossRef]
- Xu, L.; Deng, J.; Zhou, F.; Cheng, C.; Lu, M. Gut microbiota in an invasive bark beetle infected by a pathogenic fungus accelerates beetle mortality. J. Pest Sci. 2019, 92, 343–351. [Google Scholar] [CrossRef]
- Fredensborg, B.L.; Kálvalí, I.; Johannesen, T.B.; Stensvold, C.R.; Kapel, C. Parasites modulate the gut-microbiome in insects: A proof-of-concept study. PLoS ONE 2020, 15, e0227561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavichiolli de Oliveira, N.; Cônsoli, F. Beyond host regulation: Changes in gut microbiome of permissive and nonpermissive hosts following parasitization by the wasp Cotesia flavipes. FEMS Microbiol. Ecol. 2019, 96, fiz206. [Google Scholar] [CrossRef] [PubMed]
- Gloder, G.; Bourne, M.E.; Verreth, C.; Wilberts, L.; Bossaert, S.; Crauwels, S.; Dicke, M.; Poelman, E.H.; Jacquemyn, H.; Lievens, B. Parasitism by endoparasitoid wasps alters the internal but not the external microbiome in host caterpillars. Anim. Microbiome 2021, 3, 73. [Google Scholar] [CrossRef]
- Gao, X.; Niu, R.; Xiangzhen, Z.; Wang, L.; Ji, J.; Niu, L.; Wu, C.; Zhang, S.; Luo, J.; Cui, J. Characterization and comparison of the bacterial microbiota of Lysiphlebia japonica parasitioid wasps and their aphid host Aphis gosypii. Pest Manag. Sci. 2021, 77, 2710–2718. [Google Scholar] [CrossRef]
- Polenogova, O.; Kabilov, M.; Maksim, T.; Rotskaya, U.N.; Krivopalov, A.; Morozova, V.; Mozhaitseva, K.; Kryukova, N.; Alikina, T.Y.; Kryukov, V.; et al. Parasitoid envenomation alters the Galleria mellonella midgut microbiota and immunity, thereby promoting fungal infection. Sci. Rep. 2019, 9, 4012. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Lei, J.; Darby, A.C.; Kadowaki, T. Trypanosomatid parasite dynamically changes the transcriptome during infection and modifies honey bee physiology. Commun. Biol. 2020, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Beckage, N.; Gelman, D. Wasp parasitoid disruption of host development: Implications for new biologically based strategies for insect control. Annu. Rev. Entomol. 2004, 49, 299–330. [Google Scholar] [CrossRef] [Green Version]
- Schafellner, C.; Marktl, R.C.; Nussbaumer, C.; Schopf, A. Parasitism-Induced effects of Glyptapanteles liparidis (Hym., Braconidae) on the juvenile hormone titer of its host, Lymantria dispar: The role of the parasitoid larvae. J. Insect Physiol. 2004, 50, 1181–1189. [Google Scholar] [CrossRef]
- Oliver, K.; Russell, J.; Moran, N.; Hunter, M. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [Green Version]
- Christoph, V.; Lukas, G.; Paula, R. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 2010, 6, 109–111. [Google Scholar]
- Chaplinska, M.; Gerritsma, S.; Dini-Andreote, F.; Salles, J.; Wertheim, B. Bacterial communities differ among Drosophila melanogaster populations and affect host resistance against parasitoids. PLoS ONE 2016, 11, e0167726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark Jervis, P.F. Towards a general perspective on life-history evolution and diversification in parasitoid wasps. Biol. J. Linn. Soc. 2011, 104, 443–461. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, A.; Caccia, S.; Congiu, T.; Ferrarese, R.; Eguileor, M.D. Structure and function of the extraembryonic membrane persisting around the larvae of the parasitold Toxoneuron nigriceps. J. Insect Physiol. 2006, 52, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.S. Host nutrition determines blood nutrient composition and mediates parasite developmental success: Manduca sexta L. parasitized by Cotesia congregata (Say). J. Exp. Biol. 2005, 208, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Ji, X.; Yin, Y.; Wan, N. The effect of nucleopolyhedrovirus infection and/or parasitism by Microplitis pallidipes on hemolymph proteins, sugars and lipids in Spodoptera exigua larvae. BioControl 2013, 58, 777–788. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Wang, Z.; Chen, T.; Zhou, S.; Chen, J.; Pang, L.; Ye, X.; Shi, M.; Huang, J.; et al. Symbiotic bracovirus of a parasite manipulates host lipid metabolism via tachykinin signaling. PLoS Pathog. 2021, 17, e1009365. [Google Scholar] [CrossRef]
- Siebert, A.; Doucette, L.; Simpson-Haidaris, P.J.; Werren, J. Parasitoid wasp venom elevates sorbitol and alters expression of metabolic genes in human kidney cells. Toxicon 2019, 161, 57–64. [Google Scholar] [CrossRef]
- Thoetkiattikul, H.; Beck, M.; Strand, M. Inhibitor B-like proteins from a polydnavirus inhibit NF- B activation and suppress the insect immune response. Proc. Natl. Acad. Sci. USA 2005, 102, 11426–11431. [Google Scholar] [CrossRef] [Green Version]
- Strand, M.R. Polydnaviruses: Abrogation of invertebrate immune systems. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 250–256. [Google Scholar]
- Lu, Z. A metalloprotease homolog venom protein from a parasitoid wasp suppresses the toll pathway in host hemocytes. Front. Immunol. 2018, 9, 2301. [Google Scholar]
- Ha, E.-M.; Oh, C.-T.; Ryu, J.-H.; Bae, Y.-S.; Kang, S.-W.; Jang, I.-H.; Brey, P.; Lee, W.-J. An antioxidant system required for host protection against gut infection in drosophila. Dev. Cell 2005, 8, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponton, F.; Morimoto, J.; Robinson, K.; Kumar, S.; Cotter, S.; Wilson, K.; Simpson, S. Macronutrients modulate survival to infection and immunity in Drosophila. J. Anim. Ecol. 2019, 89, 460–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Yao, Z.; Raza, M.F.; Cai, Z.; Zhang, H. Regulatory mechanisms of microbial homeostasis in insect gut. Insect Sci. 2020, 28, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Furlong, M.; Wright, D.; Dosdall, L. Diamondback moth ecology and management: Problems, progress and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
- Talekar, N.S.; Shelton, A.M. Biology, ecology and management of the Diamondback Moth. Annu. Rev. Entomol. 2003, 38, 275–301. [Google Scholar] [CrossRef]
- Li, Z.; Xia, F.; Liu, S.S.; You, M.; Furlong, M.J. Biology, ecology and management of the Diamondback Moth in China. Annu. Rev. Entomol. 2015, 61, 277–296. [Google Scholar] [CrossRef]
- Yang, F.; Saqib, H.; Chen, J.; Ruan, Q.; Vasseur, L.; He, W.; You, M. Differential profiles of gut microbiota and metabolites associated with host shift of Plutella xylostella. Int. J. Mol. Sci. 2020, 21, 6283. [Google Scholar] [CrossRef]
- Li, S.; Xu, X.; De Mandal, S.; Shakeel, M.; Hua, Y.; Shoukat, R.; Fu, D.; Jin, F. Gut microbiota mediate Plutella xylostella susceptibility to Bt Cry1Ac protoxin is associated with host immune response. Environ. Pollut. 2021, 271, 116271. [Google Scholar] [CrossRef]
- Xia, X.; Zheng, D.; Zhong, H.; Qin, B.; Gurr, G.; Vasseur, L.; Lin, H.; Bai, J.; He, W.; You, M. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS ONE 2013, 8, e68852. [Google Scholar]
- Lin, X.L.; Kang, Z.W.; Pan, Q.J.; Liu, T.X. Evaluation of five antibiotics on larval gut bacterial diversity of Plutella xylostella (Lepidoptera: Plutellidae). Insect Sci. 2015, 22, 619–628. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rassoulian, G.; Karimzadeh, J.; Hosseini-Naveh, V.; Farazmand, H. Biological study of Plutella xylostella (L.) (Lep: Plutellidae) and it’s solitary endoparasitoid, Cotesia vestalis (Haliday) (Hym. Braconidae) under laboratory conditions. Pak. J. Biol. Sci. PJBS 2011, 14, 1090–1099. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes. DNA Res. 2014, 21, 217–227. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Glckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.; Tiedje, J.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5264–5267. [Google Scholar] [CrossRef] [Green Version]
- Claesson, M.; O’Sullivan, O.; Wang, Q.; Nikkilä, J.; Marchesi, J.; Smidt, H.; de Vos, W.; Ross, R.; O’Toole, P. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS ONE 2009, 4, e6669. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-Source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537. [Google Scholar] [CrossRef] [Green Version]
- Douglas, G.; Maffei, V.; Zaneveld, J.; Yurgel, S.; Brown, J.; Taylor, C.; Huttenhower, C.; Langille, M. PICRUSt2: An improved and extensible approach for metagenome inference. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Douglas, G.; Maffei, V.; Zaneveld, J.; Yurgel, S.; Brown, J.; Taylor, C.; Huttenhower, C.; Langille, M. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Chen, J.; Wang, B.; Li, Y.; Ye, Y.; Ma, W.; Ma, L. Effects on community composition and function Pinus massoniana infected by Bursaphelenchus xylophilus. BMC Microbiol. 2022, 22, 157. [Google Scholar] [CrossRef]
- Bletz, M.; Goedbloed, D.; Sanchez, E.; Reinhardt, T.; Tebbe, C.; Bhuju, S.; Geffers, R.; Jarek, M.; Vences, M.; Steinfartz, S. Amphibian gut microbiota shifts differentially in community structure but converges on habitat-specific predicted functions. Nat. Commun. 2016, 7, 13699. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Liu, X.; Dong, Y.; Yan, Z.; Lv, D.; Wang, P.; Li, Y. Comparison of Gut Bacterial Communities of Grapholita molesta (Lepidoptera: Tortricidae) Reared on Different Host Plants. Int. J. Mol. Sci. 2021, 22, 6843. [Google Scholar] [CrossRef]
- Moo, P.S.; Rosales, M.; Ibarra-Laclette, E.; Desgarennes, D.; Huerta, C.; Lamelas, A. Diversity and Composition of the Gut Microbiota in the Developmental Stages of the Dung Beetle Copris incertus Say (Coleoptera, Scarabaeidae). Front. Microbiol. 2020, 11, 1698. [Google Scholar]
- Koichiro, T.; Glen, S.; Sudhir, K. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 7, 3022–3027. [Google Scholar]
- Li, P.; Xue, Y.; Shi, J.; Pan, A.; Tang, X.; Ming, F. The response of dominant and rare taxa for fungal diversity within different root environments to the cultivation of Bt and conventional cotton varieties. Microbiome 2018, 6, 184. [Google Scholar] [CrossRef]
- Xiong, C.; He, J.-Z.; Singh, B.; Wang, J.; Li, P.-P.; Zhang, Q.-B.; Han, L.-L.; Shen, J.-P.; Ge, A.-H.; Wu, C.-F.; et al. Rare taxa maintain the stability of crop mycobiomes and ecosystem functions. Environ. Microbiol. 2020, 23, 1907–1924. [Google Scholar] [CrossRef]
- Kaeslin, M.; Pfister-Wilhelm, R.; Lanzrein, B. Influence of the parasitoid Chelonus inanitus and its polydnavirus on host nutritional physiology and implications for parasitoid development. J. Insect Physiol. 2005, 51, 1330–1339. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A.; De Luna-Santillana, E.J.; Rodríguez-Perez, M.A. Parasitism by the endoparasitoid, Cotesia flavipes induces cellular immunosuppression and enhances susceptibility of the sugar cane borer, Diatraea saccharalis to Bacillus thuringiensis. J. Insect Sci. 2011, 11, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B.; Lin, J.; Wang, Y.; Song, F.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Guidotti Pinto, C.; Walker, A.; Robinson, S.; King, G.; Rossi, G. Proteotranscriptomics reveals the secretory dynamics of teratocytes, regulators of parasitization by the endoparasitoid wasp Cotesia flavipes. J. Insect Physiol. 2022, 139, 104395. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef] [Green Version]
- White, J.A.; Richards, N.K.; Laugraud, A.; Saeed, A.; Curry, M.M.; McNeill, M.R. Endosymbiotic Candidates for Parasitoid Defense in Exotic and Native New Zealand Weevils. Microb. Ecol. 2015, 70, 274–286. [Google Scholar] [CrossRef]
- Dillon, R. Chemical barriers to gut infection in the desert locust: In vivo production of antimicrobial phenols associated with the bacterium Pantoea agglomerans. J. Invertebr. Pathol. 1995, 66, 72–75. [Google Scholar] [CrossRef]
- Nakamatsu, Y.; Kuriya, K.; Harvey, J.A.; Tanaka, T. Influence of nutrient deficiency caused by host developmental arrest on the growth and development of a koinobiont parasitoid. J. Insect Physiol. 2006, 52, 1105–1112. [Google Scholar] [CrossRef]
- Becchimanzi, A.; Avolio, M.; Di Lelio, I.; Marinelli, A.; Varricchio, P.; Grimaldi, A.; Eguileor, M.; Pennacchio, F.; Caccia, S. Host regulation by the ectophagous parasitoid wasp Bracon nigricans. J. Insect Physiol. 2017, 101, 73–81. [Google Scholar] [CrossRef]
- Lin, X.; Pan, Q.; Tian, H.; Douglas, A.; Liu, T. Bacteria abundance and diversity of different life stages of Plutella xylostella (Lepidoptera: Plutellidae), revealed by bacteria culture-dependent and PCR-DGGE methods. Insect Sci. 2015, 22, 375–385. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Huang, J.; Wang, Q.; You, M.; Xia, X. Changes in the Host Gut Microbiota during Parasitization by Parasitic Wasp Cotesia vestalis. Insects 2022, 13, 760. https://doi.org/10.3390/insects13090760
Zhang S, Huang J, Wang Q, You M, Xia X. Changes in the Host Gut Microbiota during Parasitization by Parasitic Wasp Cotesia vestalis. Insects. 2022; 13(9):760. https://doi.org/10.3390/insects13090760
Chicago/Turabian StyleZhang, Shuaiqi, Jieling Huang, Qiuping Wang, Minsheng You, and Xiaofeng Xia. 2022. "Changes in the Host Gut Microbiota during Parasitization by Parasitic Wasp Cotesia vestalis" Insects 13, no. 9: 760. https://doi.org/10.3390/insects13090760
APA StyleZhang, S., Huang, J., Wang, Q., You, M., & Xia, X. (2022). Changes in the Host Gut Microbiota during Parasitization by Parasitic Wasp Cotesia vestalis. Insects, 13(9), 760. https://doi.org/10.3390/insects13090760




