One Species, Hundreds of Subspecies? New Insight into the Intraspecific Classification of the Old World Swallowtail (Papilio machaon Linnaeus, 1758) Based on Two Mitochondrial DNA Markers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subspecies Concept
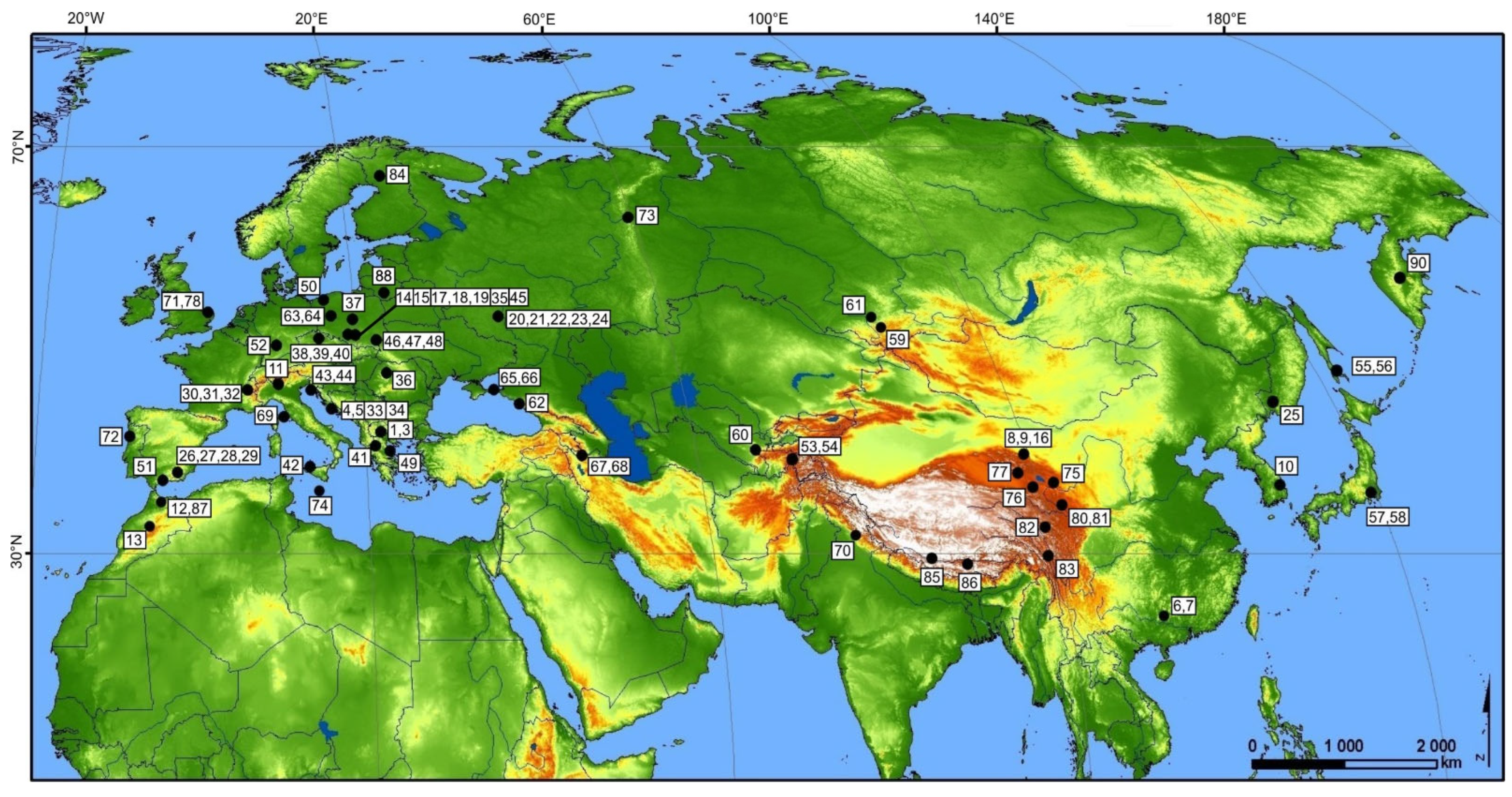
2.2. Specimen Sampling
2.3. DNA Extraction and Amplification
2.4. Molecular Analysis
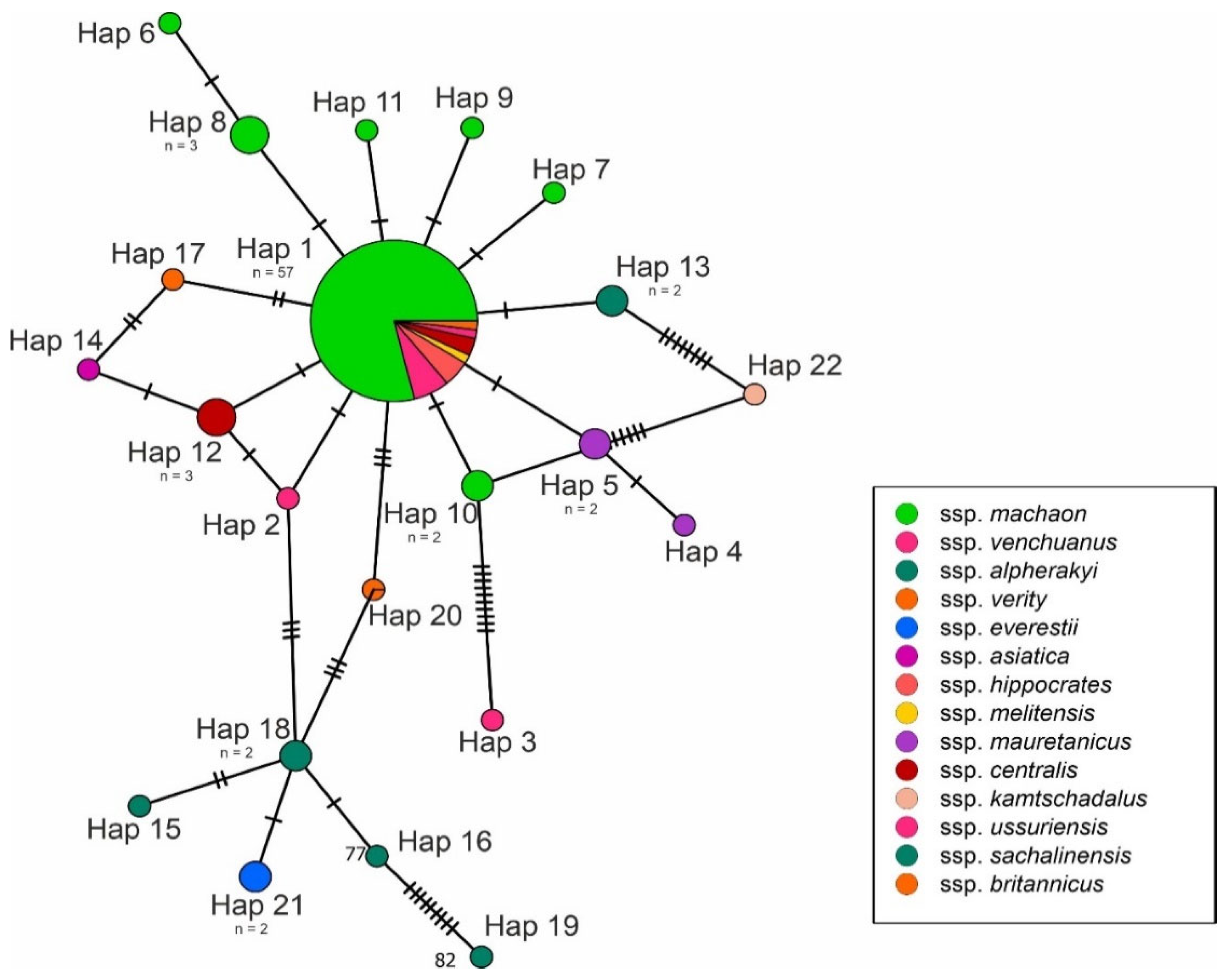
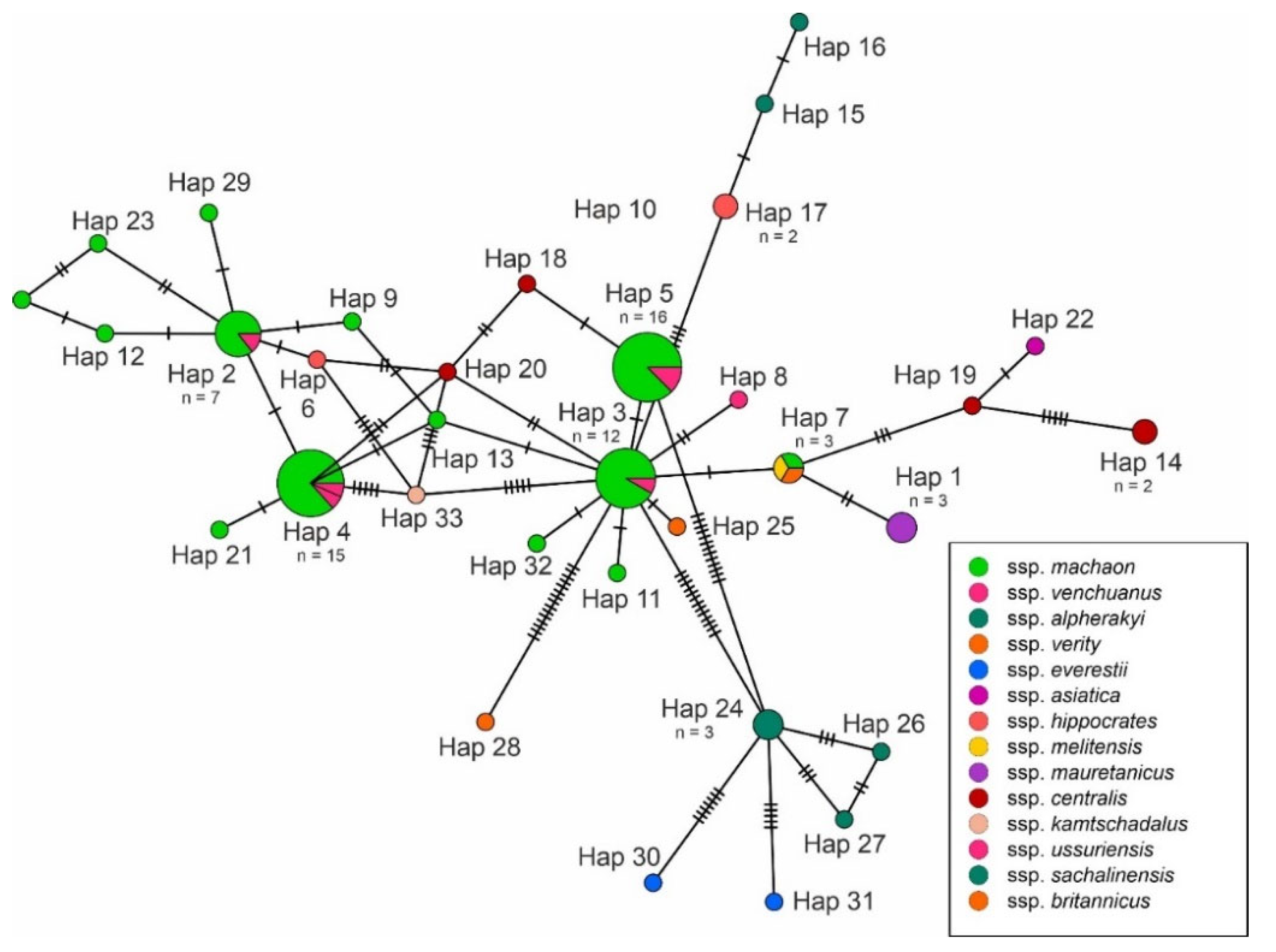
3. Results
4. Discussion
4.1. The British Isles
4.2. North Africa
4.3. Asia (Japan, Russia)
4.4. Asia (China)
4.5. Asia (India, Tajikistan and Uzbekistan)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eller, K. Die Rassen von Papilio machaon L.; E.J. Brill: Leiden, The Netherlands, 1936; 96p. [Google Scholar]
- Seyer, H. Versuch einer revision der Papilio machaon-subspezies in der westlichen Paläarktis. Mitt. Entomol. Ges. Basel 1974, 24, 64–90. [Google Scholar]
- Sturm, R. Butterflies of the World, Part 45: Papilionidae XVI: Illustrated Checklist of Papilio machaon—Group, Iphiclides podalirius, and Papilio alexanor; Goecke & Evers: Keltern, Germany, 2017; 45p. [Google Scholar]
- Rothschild, W. A revision of the Papilios of the eastern hemisphere, exclusive of Africa. Novit. Zool. 1895, 2, 167–463. [Google Scholar]
- Eimer, G.H.T. Die Artbildung und Verwandtschaft bei den Schmetterlingen. II. Teil Eine systematische Darstellung der Abanderungen, Abarten und Arten der Schwalbenschwanz-ahnlichen Formen der Gattung Papilio; Gustav Fischer: Jena, Germany, 1895; 153p. [Google Scholar]
- Seitz, A. Die Großschmetterflinge der Erde: Die Großschmetterlinge des Palaearktischen Faunengebietes, 1. Band: Die Palaearktischen Tagfalter; Alfred Kernen: Stuttgart, Germany, 1906; 379p. [Google Scholar]
- Seyer, H. Versuch einer revision der Papilio machaon-subspezies in der westlichen Paläarktis. Mitt. Entomol. Ges. Basel 1974, 24, 93–117. [Google Scholar]
- Seyer, H. Versuch einer revision der Papilio machaon-subspezies in der ostlichen Paläarktis. Mitt. Entomol. Ges. Basel 1976, 26, 65–87. [Google Scholar]
- Seyer, H. Versuch einer revision der Papilio machaon-subspezies in der ostlichen Paläarktis. Mitt. Entomol. Ges. Basel 1976, 26, 97–145. [Google Scholar]
- Seyer, H. Entomologische Notizen. Mitt. Entomol. Ges. Basel 1977, 27, 28. [Google Scholar]
- Seyer, H. 2. Nachtrag zum Versuch einer revision der Papilio machaon-subspezies in der Paläarktis und 1. beitrag zur Nearktis. Mitt. Entomol. Ges. Basel 1977, 27, 105–115. [Google Scholar]
- Seyer, H. 3. Nachtrag zum Versuch einer revision der Papilio machaon-subspezies in der Paläarktis und Nearktis. Mitt. Entomol. Ges. Basel 1980, 30, 55–58. [Google Scholar]
- Seyer, H. 4. Nachtrag zum Versuch einer revision der Papilio machaon L. Subspezies in der Paläarktis. Mitt. Entomol. Ges. Basel 1987, 37, 128–131. [Google Scholar]
- Seyer, H. Aus den drei Papilio machaon-Untergruppen O-, S- u. SO-Asiens meines Revisionsversuches von 1976 sind drei neue Arten entstanden! Papilio hippocrates, P. asiatica und P. chinensis (Lepidoptera: Papilionidae). Ber. Kr. Nürnbg. Ent. galathea 1992, 8, 141–147. [Google Scholar]
- Fujioka, T.; Tsukiyama, H.; Chiba, H. Japanese Butterflies and Their Relatives in the World I; Shuppan-Geijutu: Tokyo, Japan, 1997; 301p. (In Japanese) [Google Scholar]
- Nakae, M.M.; Nishiyama, Y.; Cotton, A. Papilionidae of the World; Roppon-Ashi Entomological Books: Tokyo, Japan, 2021; 336p. (In Japanese) [Google Scholar]
- Sperling, F.A.H. Evolution of the Papilio machaon species group in western Canada (Lepidoptera: Papilionidae). Quaest. Entomol. 1987, 23, 198–315. [Google Scholar]
- Sperling, F.A.H. Mitochondrial DNA phylogeny of the Papilio machaon species group (Lepidoptera: Papilionidae). Mem. Entomol. Soc. Can. 1993, 165, 233–242. [Google Scholar] [CrossRef]
- Sperling, F.A.H.; Harrison, R.G. Mitochondrial DNA variation within and between species of the Papilio machaon group of swallowtail butterflies. Evolution 1994, 48, 408–422. [Google Scholar] [CrossRef]
- Aubert, J.; Legal, L.; Descimon, H.; Michel, F. Molecular Phylogeny of Swallowtail Butterflies of the Tribe Papilionini (Papilionidae, Lepidoptera). Mol. Phylogenet. Evol. 1999, 12, 156–167. [Google Scholar] [CrossRef]
- Caterino, M.S.; Sperling, F.A.H. Papilio phylogeny based on mitochondrial cytochrome oxidase I and II genes. Mol. Phylogenet Evol. 1999, 11, 122–137. [Google Scholar] [CrossRef]
- Dupuis, J.R.; Sperling, F.A.H. Repeated Reticulate Evolution in North American Papilio machaon Group Swallowtail Butterflies. PLoS ONE 2015, 10, e0141882. [Google Scholar] [CrossRef]
- Miyakawa, M.; Ito-Harashima, S.; Yagi, T. Unique phylogenetic position of the Japanese Papilio machaon population in the subgenus Papilio (Papilionidae: Papilio) inferred from mitochondrial DNA sequences. Lepid. Sci. 2017, 68, 121–128. [Google Scholar]
- Miyakawa, M.; Hosoi, M.; Kawakita, A.; Ito-Harashima, S.; Yagi, T.; Ishihara, M. Genetic variations and phylogeography of the swallowtail butterfly Papilio machaon on the Japanese Islands. Entomol. Sci. 2018, 21, 248–259. [Google Scholar] [CrossRef]
- Dincă, V.; Dapporto, L.; Somervuo, P.; Vodă, R.; Cuvelier, S.; Gascoigne-Pees, M.; Huemer, P.; Mutanen, M.; Hebert, P.D.N.; Vila, R. High resolution DNA barcode library for European butterflies reveals continental patterns of mitochondrial genetic diversity. Commun. Biol. 2021, 4, 315. [Google Scholar] [CrossRef]
- Coues, E. A Checklist of North American Birds; Naturalists Agency: Salem, MA, USA, 1873; 137p. [Google Scholar]
- Amadon, D. The seventy-five percent rule for subspecies. Condor 1949, 51, 250–258. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Mayr, E. Bureaucratic mischief: Recognizing endangered species and subspecies. Science 1991, 251, 1187–1188. [Google Scholar] [CrossRef]
- Braby, M.F.; Eastwood, R.; Murray, N. The subspecies concept in butterflies: Has its application in taxonomy and conservation biology outlived its usefulness? Biol. J. Linn. Soc. 2012, 106, 699–716. [Google Scholar] [CrossRef]
- Gilbert, M.T.P.; Moore, W.; Melchior, L.; Worobey, M. DNA Extraction from Dry Museum Beetles without Conferring External Morphological Damage. PLoS ONE 2007, 2, e272. [Google Scholar] [CrossRef]
- Lis, J.A.; Ziaja, D.J.; Lis, P. Recovery of mitochondrial DNA for systematic studies of Pentatomoidea (Hemiptera: Heteroptera): Successful PCR on early 20th century dry museum specimens. Zootaxa 2011, 2748, 18–28. [Google Scholar] [CrossRef]
- Nazari, V.; Schmidt, B.C.; Prosser, S.; Hebert, P.D.N. Century-Old DNA Barcodes Reveal Phylogenetic Placement of the Extinct Jamaican Sunset Moth, Urania sloanus Cramer (Lepidoptera: Uraniidae). PLoS ONE 2016, 11, e0164405. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequence and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Simmons, R.B.; Weller, S.J. Utility and evolution of cytochrome b in insects. Mol. Phylogenet Evol. 2001, 20, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-Joining Networks for Inferring Intraspecific Phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Tajima, F. Statistical methods to test for nucleotide mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, 232–235. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Todisco, V.; Gratton, P.; Cesaroni, D.; Sbordoni, V. Phylogeography of Parnassius apollo: Hints on taxonomy and conservation of a vulnerable glacial butterfly invader. Biol. J. Linn. Soc. 2010, 101, 169–183. [Google Scholar] [CrossRef]
- Korb, S.K.; Fric, Z.F.; Bartoňová, A. Phylogeography of Koramius charltonius (Gray, 1853) (Lepidoptera: Papilionidae): A case of too many poorly circumscribed subspecies. Nota Lepidopterol. 2016, 39, 169–191. [Google Scholar] [CrossRef]
- Dapporto, L. Speciation in Mediterranean refugia and post-glacial expansion of Zerynthia polyxena (Lepidoptera, Papilionidae). J. Zool. Syst. Evol. Res. 2009, 48, 229–237. [Google Scholar] [CrossRef]
- Vandewoestijne, S.; Baguette, M.; Brakefield, P.M.; Saccheri, I.J. Phylogeography of Aglais urticae (Lepidoptera) based on DNA sequences of the mitochondrial COI gene and control region. Mol. Phylogenet Evol. 2004, 31, 630–646. [Google Scholar] [CrossRef]
- Masłowski, J.; Fiołek, K. Motyle Świata, Paziowate—Papilionidae; Wydawnictwo Koliber: Nowy Sącz, Poland, 2010; 232p. (In Polish) [Google Scholar]
- Tarnawski, D.; Kadej, M.; Smolis, A.; Malkiewicz, A. Niepylak Apollo Parnassius apollo (Linnaeus, 1758) Monografia Gatunku; Fundacja EkoRozwoju: Wrocław, Poland, 2012; 139p. (In Polish) [Google Scholar]
- Standfuss, M. Handbuch der Paläarktischen Gross-Schmetterlinge für Forscher und Sammler; Gustav Fischer: Jena, Germany, 1896; 392p. [Google Scholar]
- Dockery, M.; Logunov, D.V. David Longsdon (1864–1937) and his collection of swallowtail butterflies (Papilionidae) at the Manchester Museum. Russ. Entomol. J. 2015, 24, 155–179. [Google Scholar] [CrossRef][Green Version]
- Domagała, P.J.; Dobosz, R. Urania sloanus (Cramer, 1779) (Lepidoptera: Uraniidae), an Enigmatic Extinct Species in Polish Museum Collections. Ann. Zool. 2019, 69, 697–702. [Google Scholar] [CrossRef]
- Verity, R. Rhopalocera Palaearctica. Iconographie et Description des Papilionides Diurnes de la Région Paléarctique; Roger Verity: Florence, Italy, 1911; Volume 26–29, pp. 295–299. [Google Scholar]
- Leraut, P. Butterflies of Europe and Neighbouring Regions; N.A.P. Editions: Verrières-le-Buisson, France, 2016; 1116p. [Google Scholar]
- Tshikolovets, V. Butterflies of Europe and Mediterranean Area; Tshikolovets: Kiev, Ukraine, 2011; 544p. [Google Scholar]
- Tolman, T.; Lewington, R. Butterflies of Britain and Europe; Harper Collins: London, UK, 1997; 320p. [Google Scholar]
- Aguilar, L.; Miller, J.Y.; Sarto, V. A new lepidopteran family for the European fauna. SHILAP Rev. Lepidopterol. 2001, 29, 86–87. [Google Scholar]
- González, J.M.; Domagala, P.; Larysz, A. The Giant Butterfly-Moths (Lepidoptera Castniidae) of the Upper Silesian Museum (Muzeum Górnośląskie) in Bytom, Poland, with notes on the history of the Museum. Biodivers. J. 2013, 4, 219–228. [Google Scholar]
- Kenis, M.; Nacambo, S.; Leuthardt, F.L.G.; Domenico, F.D.; Haye, T. The box tree moth, Cydalima perspectalis, in Europe: Horticultural pest or environmental disaster? Aliens Invasive Species Bull. 2013, 33, 38–41. [Google Scholar]
- Oberthür, C. Etudes D’Entomologie Faunes Entomologiques: Descriptions D’insectes Nouveaux ou Peu Connus. IV.-Catalogue Raisonne Des Papilionidae De la Collection de Ch. Oberthur; Imprimerie Oberthur et Fils: Rennes, France, 1879; 130p. [Google Scholar]
- Pittaway, A.R.; Larsen, T.B.; Clarke, C.A.; Smith, C.R.; Crnjar, R.; Clarke, F.M.M. Papilio saharae Oberthur, 1879, specifically distinct from Papilio machaon Linnaeus, 1758 (Lepidoptera: Papilionidae). Entomol. Gaz. 1994, 45, 223–249. [Google Scholar]
- Moonen, J.J.M. Notes on the Papilio machaon group (Lepidoptera: Papilionidae) from the Palaearctic Papilionidae collection of the Zoological Museum of Amsterdam. Entomol. Ber. 2012, 72, 184–186. [Google Scholar]
- Wiemers, M.; Gottsberger, B. Discordant patterns of mitochondrial and nuclear differentiation in the Scarce Swallowtail Iphiclides podalirius feisthamelii (Duponchel, 1832) (Lepidoptera: Papilionidae). Entomol. Z. 2010, 120, 111–115. [Google Scholar]
- Dincă, V.; Montagud, S.; Talavera, G.; Hernández-Roldán, J.; Munguira, M.L.; García-Barros, E.; Hebert, P.D.N.; Vila, R. DNA barcode reference library for Iberian butterflies enables a continental-scale preview of potential cryptic diversity. Sci. Rep. 2015, 5, 1239. [Google Scholar] [CrossRef]
- Eller, K. Versuch einer historischen und geographischen Analyse zur Rassen- und Artbildung (Auf Grund von Untersuchungen in der Papilio machaon-Gruppe Lep. Rhop.). Z. Indukt. Abstamm.—Vererb. 1939, 77, 135–171. [Google Scholar]
- Tuzov, V.K.; Dewatkin, A.L.; Kabak, L.V.; Korolev, V.A.; Murzin, V.S.; Samodurov, G.D.; Tarasov, E.A.; Bogdanov, P.V. Guide to the Butterflies of Russia and Adjacent Territories (Lepidoptera, Rhopalocera). Volume 1: Hesperiidae, Papilionidae, Pieridae, Satyridae; Pensoft: Sofia, Bulgaria; Moscow, Russia, 1997; 480p. [Google Scholar]
- Tshikolovets, V.; Yakovlev, R.; Kosterin, O. The Butterflies of Altai, Sayans and Tuva (South Siberia); Konvoj Ltd.: Kyiv, Ukraine; Pardubice, Czech Republic, 2009; 374p. [Google Scholar]
- Hemming, A.F. On the identity of four species of rhopalocera described by Johannes Gistel in 1857. Stylops 1935, 4, 121–123. [Google Scholar]
- Lee, C.L. A revision of the Chinese species of Papilio machaon L. and their geographical distribution. Acta Entomol. Sin. 1980, 23, 427–431. [Google Scholar]
- Gaonkar, H. Type specimens of Papilionidae and Pieridae (Lepidoptera) in the Zoological Survey of India Collection, Calcutta. J. South Asian Nat. Hist. 1999, 4, 219–244. [Google Scholar]
- Huang, H. A list of butterflies collected from Nujiang (Lou Tse Kiang) and Dulongjiang, China with descriptions of new species, new subspecies and revisional notes. Neue Entomol. Nachr. 2003, 55, 3–114. [Google Scholar]
- Staudinger, O. Centralasiatische Lepidopteren. Stettin. Entomol. Ztg. 1886, 47, 193–215. [Google Scholar]
- Kato, Y.; Yagi, T. Biogeography of the subspecies of Parides (Byasa) alcinous (Lepidoptera: Papilionidae) based on a phylogenetic analysis of mitochondrial ND5 sequences. Syst. Entomol. 2004, 29, 1–9. [Google Scholar] [CrossRef]
- Hundertmark, K.J.; Shields, G.F.; Udina, I.G.; Bowyer, R.T.; Danilkin, A.A.; Schwartz, C.C. Mitochondrial phylogeography of Moose (Alces alces): Late Pleistocene divergence and population expansion. Mol. Phylogenet. Evol. 2002, 22, 375–387. [Google Scholar] [CrossRef]
- Fry, A.J.; Zink, R.M. Geographic analysis of nucleotide diversity and song sparrow (Aves: Emberizidae) population history. Mol. Ecol. 1998, 7, 1303–1313. [Google Scholar] [CrossRef]
- Stajich, J.E.; Hahn, M.W. Disentangling the Effects of Demography and Selection in Human History. Mol. Biol. Evol. 2005, 22, 63–73. [Google Scholar] [CrossRef]
- Kocot-Zalewska, J.; Domagała, P.J.; Lis, B. Living in isolation for almost 40 years: Molecular divergence of the 28S rDNA and COI sequences between French and Polish populations of the cave beetle Speonomus normandi hydrophilus (Jeannel, 1907). Subterr. Biol. 2021, 37, 75–88. [Google Scholar] [CrossRef]


| Subspecies * | n | H | uH | hd | S | V | ND | TM | k | Dist |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 87 | 22 | - | 0.5704 | 39 | 39 | 0.00424 | 41 | 1.982 | - |
| ssp. machaon | 54 | 7 | 6 | 0.3054 | 6 | 6 | 0.00077 | 6 | 0.361 | 0.00077 |
| ssp. alpherakyi | 5 | 4 | 3 | 0.9000 | 11 | 11 | 0.01026 | 11 | 4.800 | 0.01026 |
| ssp. asiatica | 1 | 1 | 1 | - | - | - | - | - | - | - |
| ssp. britanicus | 2 | 2 | 1 | 1.0000 | 2 | 2 | 0.00427 | 2 | 2.000 | 0.00427 |
| ssp. centralis | 5 | 2 | 1 | 0.6000 | 1 | 1 | 0.00128 | 1 | 0,600 | 0.00128 |
| ssp. everestii | 2 | 1 | 2 | 0.0000 | 0 | 0 | 0.00000 | 0 | 0.000 | 0 |
| ssp. hippocrates | 3 | 1 | 0 | 0.0000 | 0 | 0 | 0.00000 | 0 | 0.000 | 0 |
| ssp. kamtschadalus | 1 | 1 | 1 | - | - | - | - | - | - | - |
| ssp. mauretanicus | 3 | 2 | 2 | 0.6670 | 1 | 1 | 0.00142 | 1 | 0.667 | 0.00142 |
| ssp. melitensis | 1 | 1 | 0 | - | - | - | - | - | - | - |
| ssp. sachalinensis | 2 | 1 | 1 | 0.0000 | 0 | 0 | 0.00000 | 0 | 0.000 | 0 |
| ssp. ussuriensis | 1 | 1 | 0 | - | - | - | - | - | - | - |
| ssp. venchuanus | 6 | 3 | 2 | 0.6000 | 12 | 12 | 0.00855 | 12 | 4.000 | 0.00855 |
| ssp. verity | 1 | 1 | 1 | - | - | - | - | - | - | - |
| Subspecies * | n | H | uH | hd | S | V | ND | TM | k | Dist |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 87 | 33 | - | 0.9137 | 55 | 55 | 0.02691 | 59 | 9.229 | - |
| ssp. machaon | 54 | 14 | 9 | 0.833 | 10 | 10 | 0.00606 | 10 | 2.079 | 0.00606 |
| ssp. alpherakyi | 5 | 3 | 2 | 0.700 | 4 | 4 | 0.00583 | 4 | 2.000 | 0.00583 |
| ssp. asiatica | 1 | 1 | 1 | - | - | - | - | - | - | - |
| ssp. britanicus | 2 | 2 | 1 | 1.000 | 2 | 2 | 0.00583 | 2 | 2.000 | 0.00583 |
| ssp. centralis | 5 | 4 | 4 | 0.900 | 9 | 9 | 0.01516 | 9 | 5.200 | 0.01516 |
| ssp. everestii | 2 | 2 | 2 | 1.000 | 8 | 8 | 0.02332 | 8 | 8.000 | 0.02330 |
| ssp. hippocrates | 3 | 2 | 2 | 0.667 | 7 | 7 | 0.01361 | 7 | 4.667 | 0.01360 |
| ssp. kamtschadalus | 1 | 1 | 1 | - | - | - | - | - | - | - |
| ssp. mauretanicus | 3 | 1 | 1 | 0.000 | 0 | 0 | 0 | 0 | 0 | 0.00000 |
| ssp. melitensis | 1 | 1 | 0 | - | - | - | - | - | - | - |
| ssp. sachalinensis | 2 | 2 | 2 | 1.000 | 1 | 1 | 0.00292 | 1 | 1.000 | 0.00292 |
| ssp. ussuriensis | 1 | 1 | 0 | - | - | - | - | - | - | - |
| ssp. venchuanus | 6 | 6 | 1 | 0.933 | 6 | 6 | 0.00758 | 6 | 2.600 | 0.00758 |
| ssp. verity | 1 | 1 | 1 | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domagała, P.J.; Lis, J.A. One Species, Hundreds of Subspecies? New Insight into the Intraspecific Classification of the Old World Swallowtail (Papilio machaon Linnaeus, 1758) Based on Two Mitochondrial DNA Markers. Insects 2022, 13, 752. https://doi.org/10.3390/insects13080752
Domagała PJ, Lis JA. One Species, Hundreds of Subspecies? New Insight into the Intraspecific Classification of the Old World Swallowtail (Papilio machaon Linnaeus, 1758) Based on Two Mitochondrial DNA Markers. Insects. 2022; 13(8):752. https://doi.org/10.3390/insects13080752
Chicago/Turabian StyleDomagała, Paweł J., and Jerzy A. Lis. 2022. "One Species, Hundreds of Subspecies? New Insight into the Intraspecific Classification of the Old World Swallowtail (Papilio machaon Linnaeus, 1758) Based on Two Mitochondrial DNA Markers" Insects 13, no. 8: 752. https://doi.org/10.3390/insects13080752
APA StyleDomagała, P. J., & Lis, J. A. (2022). One Species, Hundreds of Subspecies? New Insight into the Intraspecific Classification of the Old World Swallowtail (Papilio machaon Linnaeus, 1758) Based on Two Mitochondrial DNA Markers. Insects, 13(8), 752. https://doi.org/10.3390/insects13080752







