Simple Summary
It is the first investigation of drosophilid seasonal population changes considering their biotope association, abundance and species diversity in European Russia. The material was collected using beer traps in different forest biotopes. Beer is an attractive component for fruit flies. Two species were most common (Drosophila obscura and Drosophila histrio). We found three groups of mass species with a significant correlation of seasonal dynamics.
Abstract
(1) Background: Seasonal dynamics of the abundance and species diversity of various insect groups is of great importance for understanding their life cycles; (2) Methods: In our study, Drosophilidae species and their seasonal changes in Mordovia State Nature Reserve were explored. We collected the flies by crown fermental traps in five types of forests (birch, aspen, linden, pine and oak) since May to October in 2019. (3) Results: A total of 4725 individuals belonging to 9 genera and 30 species of drosophilid flies were identified, among them 15 species in 3 genera are new to the Republic of Mordovia. Drosophila obscura and D. histrio were the most abundant species in traps, the other mass species are D. kuntzei, D. testacea, D. phalerata, S. rufifrons, D. bifasciata, A. semivirgo, and L. quinquemaculata. (4) Conclusions: We found three groups of mass species with significant correlation of seasonal dynamics, e.g., D.obscura and D. bifasciata; D. histrio, D. kuntzei, D. phalerata, and D. testacea, and, finally, A. semivirgo and S. rufifrons. Apparently, the similarity observed in the seasonal dynamics of these drosophilid species is influenced at a high degree by their food preferences and rearing sites.
1. Introduction
Most aspects of body physiology, metabolism, and behavior are controlled by the clock and lead to daily or seasonal strategies. The relationship between the timing of life cycle events and seasonal climatic changes (i.e., phenology) is a fundamental biological process in natural systems. Phenology is the main factor determining population dynamics, species interaction, animal movement, and the evolution of life history [1,2]. The timing of phenological events is gradually changing as a result of climate change [3,4,5]. Along with other adaptive mechanisms, plasticity in phenology is essential for maintaining many aspects of biodiversity in a changing environment, such as species demography, species interaction, and species distribution [6,7,8]. In response to seasonal natural changes, the species composition of populations and the number of species in them undergoes significant fluctuations [9,10,11].
The rhythms of the vital activity of insects as poikilothermic animals are also adapted to seasonal environmental changes. Insects are particularly sensitive to an increase or decrease in temperatures above or below their optimum, to frost and drought, as well as to a decrease in the availability of resources, particularly food [4,12]. Therefore, seasonal rhythms of insect activity depend on a variety of environmental factors, most often on temperature, photoperiod, and humidity [10,13,14]. In this regard, the observed climate changes lead to clear shifts in the phenology of species, changes in their life cycles of development and reproduction [15,16,17,18,19,20]. Thus, the seasonal aspects of insect biology are key processes that can link climate change to population conservation and possibly to community composition [21].
Especially clear seasonal rhythms were found in a wide variety of insect groups living in temperate latitudes. In particular, the seasonal activity of species of Carabidae [22,23], Staphylinidae [24], Mordellidae [25], Scarabaeidae [26], Cerambycidae [27], Elateridae [28], and many others. The phenological features of Lepidoptera and Hymenoptera of temperate climate have been well studied [29,30,31,32]. No less interesting are the seasonal dynamics of individual families and species of Diptera. In the forest zone of Russia in the second half of September, there was a gradual increase in the number of Diptera with a peak in mid-October. The autumn increase in the number of Diptera in different biotopes exceeded the summer peak several times [33]. Based on the analysis of the activity of 194 Syrphidae species, ten phenological groups were identified, which differed in peaks of activity during the season [34]. Many parasitic Diptera species depend on the seasonal activity of their hosts, which serve for the development of larvae [35]. Anisopodidae activity occurs at the end of August and autumn [36]. The seasonal activity of Stomoxys calcitrans shows one large peak at the end of summer and a second smaller peak just before the end of the flight season [37]. The phenological phases of Ceratitis capitata development depended on the abundance of food items—various fruits [38]. Generally known as fruit flies, family Drosophilidae consists of approximately 4000 species worldwide [39,40]. The majority of adult drosophilids feed on the bacteria and yeasts arising from the fermentation of various plant substrates (fruit, tree sap, rotting leaves, etc.). Their larvae also prefer the bacteria and yeasts arising from the fermentation of carbohydrates [41] but some species feed on living mushrooms, living plant tissues as miners, etc., [42]. The aim of the research was to study the species diversity and seasonal dynamics of drosophilids in various forest biotopes of the center of the European part of Russia. The objectives of the research were: (1) study of the species diversity of drosophilids in various biotopes using beer traps; (2) study of the seasonal dynamics of mass species of drosophilids.
2. Materials and Methods
2.1. Study Area
The study was carried out in the Mordovia State Nature Reserve (European Russia), located on the southern boundary of the taiga zone (54°42′–54°56′ N, 43°04′–43°36′ E; up to 190 m a.s.l.). The Mordovia State Nature Reserve contains natural ecosystems in the center of European Russia, acknowledged as a hotspot for biodiversity [43,44]. The total area of the protected area is 321.62 km2 with forest communities covering 89.3% of this area. Pine (Pinus sylvestris L.) is the main forest tree species where it forms pure or mixed forest communities. Most of these places are artificial pine plantings of different ages. Birch (Betula pendula Roth) is the second commonest tree species and forms predominantly secondary forest communities on old logging or burnt areas. In mixed forests, birch is the main component of the second tier of the forest. Small-leaved linden (Tilia cordata Mill.) forms pure stands in the northern part of the Mordovia State Nature Reserve, as well as being important in the development of an undergrowth layer in pine stands and mixed forests. Oak (Quercus robur L.) forests occupy relatively small areas mainly on the floodplain of the Moksha River in the western part of the Mordovia State Nature Reserve. Sections of oak forests have also been preserved along the shores of some lakes in the southwestern part of the protected area. The mean annual precipitation is 406.6–681.3 mm. The reserve is located in a temperate zone with a predominance of forest-steppe type of climate. The average annual air temperature ranges from 3.5 to 4.0 °C. The average temperature of the coldest month (January) varies between −11.5–−12.3 °C; temperature drops to −47 °C are noted. The average temperature of the warmest month—July—18.9–19.8 °C. Extreme temperatures in summer reach 37 °C. We collected the flies from May to October in 2019 when average day temperatures allowed insects to be active.
2.2. Sampling
Each trap was a plastic 5 L container with a window cut out in it on one side at a distance of 10 cm from the bottom [45]. Two traps were installed in each biotope at a distance of 5 m from each other. The traps were suspended on tree trunks in the crown at a height of 7–8 m. Fermented liquid (beer with added sugar) was used as a luring liquid. The fermentation period of the liquid was one day. The sampling period ranged from 6 to 17 days. All studies in biotopes were carried out by A.B. Ruchin.
The definition of the flies was performed by N.G. Gornostaev with the use of drosophilid key [46]. The systematics of Drosophilidae is interpreted by Grimaldi [47]. Species new to the region are marked with an asterisk “*”.
2.3. Statistical Analysis
To estimate correlated changes in the number of species by months and biotopes, Spearman rank order correlations were used according to the percentage of the number of each species in the sample obtained for this species for the entire period of accounting in this biotope. Estimates were obtained using the Statistica 12 program [48]. Diagrams of seasonal dynamics of species are constructed in the Excel program according to the corresponding values of the percentage of the number of this species from the total number in this biotope for the entire accounting season.
To compare the drosophilid fauna in five biotopes, we calculated the Shannon–Weaver biodiversity index and the Simpson dominance index (based on the data in Appendix A).
3. Results
3.1. Faunistic Composition
Until recently, the fauna of the Drosophilidae of the Republic of Mordovia was totally unknown. The first paper with a short regional drosophilid faunistic list considered ecological questions of insect post-fire forest recovery [49]. This preliminary faunistic list includes 15 species in 6 genera of Drosophildae. Here we give an addition with a new list of Drosophilidae of the Republic of Mordovia consisting of 30 species in 9 genera.
Among the flies collected in beer traps we found 4 genera and 9 species of subfamily Steganinae and 5 genera and 21 species of subfamily Drosophilinae:
Steganinae
- Amiota (Amiota) albilabris (Roth in Zetterstedt, 1860)
- Amiota (Amiota) alboguttata (Wahlberg, 1839)
- Amiota (Amiota) rufescens (Oldenberg, 1914)
- *Amiota (Amiota) subtusradiata Duda, 1934
- Amiota (Phortica) semivirgo Maca, 1977
- Gitona distigma Meigen, 1830
- Leucophenga maculata (Dufour, 1839)
- Leucophenga quinquemaculata Strobl, 1893
- *Stegana (Steganina) coleoptrata (Scopoli, 1763)
Drosophilinae
- 10.
- *Chymomyza amoena (Loew, 1862)
- 11.
- *Chymomyza caudatula Oldenberg, 1914
- 12.
- Chymomyza costata (Zetterstedt, 1838)
- 13.
- *Chymomyza fuscimana (Zetterstedt, 1838)
- 14.
- *Drosophila (Dorsilopha) busckii Coquillett, 1901
- 15.
- *Drosophila (Drosophila) funebris (Fabricius, 1787)
- 16.
- Drosophila (Drosophila) histrio Meigen, 1830
- 17.
- *Drosophila (Drosophila) hydei Sturtevant, 1921
- 18.
- *Drosophila (Drosophila) immigrans Sturtevant, 1921
- 19.
- *Drosophila (Drosophila) kuntzei Duda, 1924
- 20.
- Drosophila (Drosophila) phalerata Meigen, 1830
- 21.
- Drosophila (Drosophila) testacea von Roser, 1840
- 22.
- Drosophila (Drosophila) transversa Fallen, 1823
- 23.
- Drosophila (Sophophora) bifasciata Pomini, 1940
- 24.
- *Drosophila (Sophophora) melanogaster Meigen, 1830
- 25.
- Drosophila (Sophophora) obscura Fallen, 1823
- 26.
- *Drosophila (Sophophora) tristis Fallen, 1823
- 27.
- *Hirtodrosophila confusa (Staeger, 1844)
- 28.
- *Hirtodrosophila trivittata (Strobl, 1893)
- 29.
- Scaptodrosophila rufifrons (Loew, 1873)
- 30.
- *Scaptomyza (Hemiscaptomyza) unipunctum (Zetterstedt, 1847)
3.2. Seasonal Dynamics of Drosophilidae
As a result of the study, 4725 individuals from 9 genera and 30 species were detected in 2019 (Table 1).

Table 1.
Number of Drosophilidae flies collected in traps.
As we can conclude from our results, nine drosophild species (D. obscura, D. histrio, D. kuntzei, D. testacea, D. phalerata, S. rufifrons, D. bifasciata, A. semivirgo and L. quinquemaculata) were the most abundant in 2019, e.g., each of them with total number of flies caught in traps more than 100 exemplars. The amount of flies belonging to these 9 species is 4496 exemplars, which is 95.15% of total drosophilid number in our collection. We consider the other 21 species collected in amounts less than 100 flies as relatively rare or weakly attracted to this type of traps.
Interestingly, the most abundant species of Drosophilidae demonstrate different patterns of seasonal dynamics. Six species, e.g., D. obscura, D. histrio, D. kuntzei, D. testacea, D. phalerata, and D. bifasciata, show very strong increases in collected drosophilid numbers in October. However, among this group, D. obscura and D. bifasciata show additional moderate summer increases in July, and D. histrio in May, August, and September. On the contrary, two species, S. rufifrons and A. semivirgo, show low numbers in May–June increasing in July up to maximum values in August followed by decreases in September–October. One species, L. quinquemaculata, demonstrates similar maximal numbers in May and October, decreases in June, noticeable increases in July, and minimal equal numbers in August–September.
3.3. Species diversity of Drosophildae
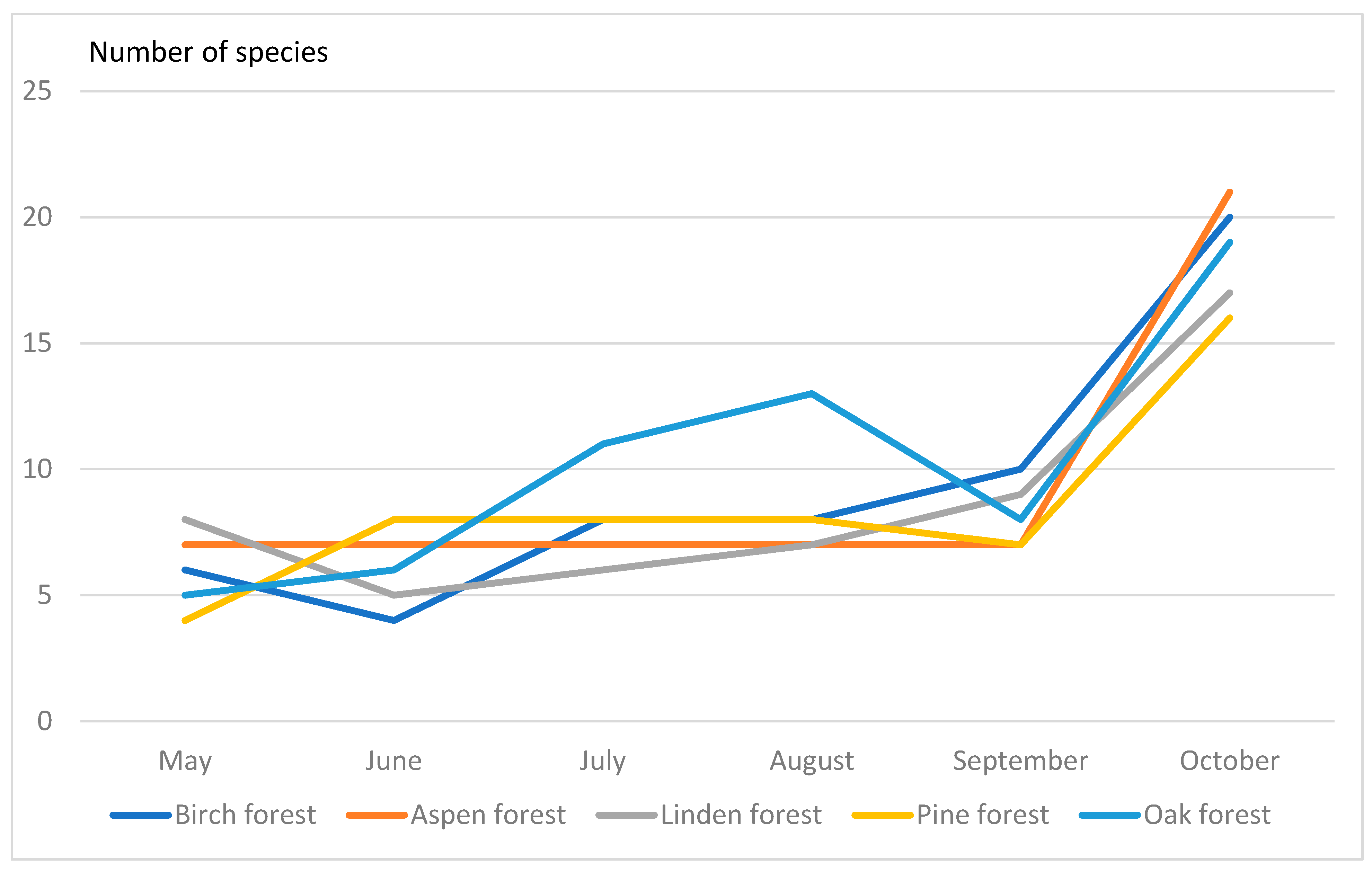
The drosophilid species diversity, e.g., number of collected species, varied between different types of forest since May to October (Figure 1). We found that species diversity have maximal values in October in all types of forest examined.
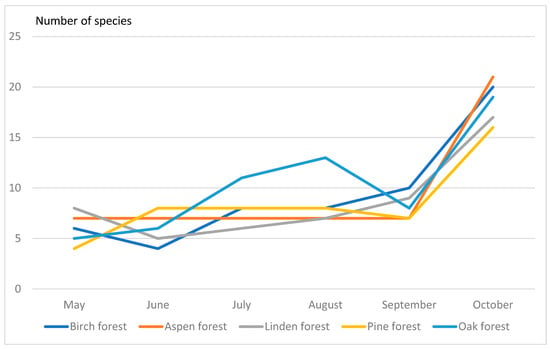
Figure 1.
Seasonal changes of species diversity of Drosophildae.
In birch and linden forests, the number of drosophilid species was 20, in pine—19 species, in aspen—21 species. The greatest species diversity was observed in oak forest (23 species). At the same time, the calculated indices showed interesting results. Thus, according to the Shannon–Weaver index, the most diverse communities were in the linden forest (index 2.11), and the least diverse in the oak forest (index 1.87). In other communities, this index was intermediate and very similar (1.95–1.99). The Simpson index showed that the dominance of one or two drosophilid species is maximal in the oak forest (0.31). At the same time, in the linden forest, the dominance of species is the least pronounced (0.15), i.e., here the community is more aligned (Table A10).
3.4. Seasonal Dynamics of Drosophilidae in Five Biotopes
We studied seasonal dynamics of Drosophilidae in five types of forest. We found that the drosophilid abundance was as follows: maximum value was in birch forest (1322) and the lowest in oak forest (640). Interestingly, the number of females exceeded the number of males in traps in all types of forest.
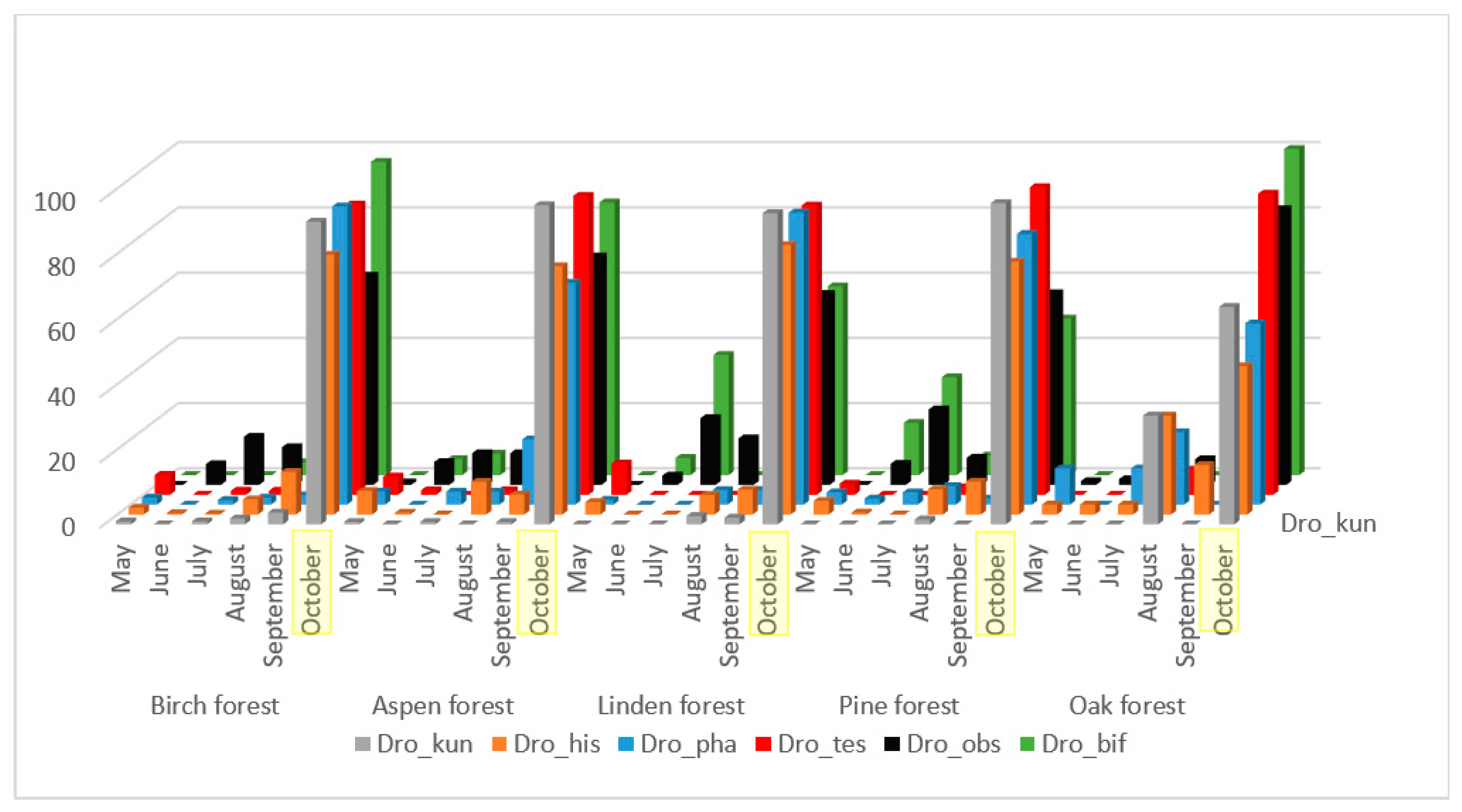
The majority of the mass species presented in Figure 2, with the exception of L. quinquemaculata, have a significant correlation of population fluctuations throughout the entire accounting season, from May to October (Table 2). These species are characterized by low representation in June, an increase in numbers in July–September, and maximum representation in October. Some differences in seasonal dynamics by biotopes are caused by a small intermediate peak in the abundance of D. kuntzei, D. histrio, and D. phalerata species in August in oak forest collections, and in D. obscura and D. bifasciata species in linden and pine forests.
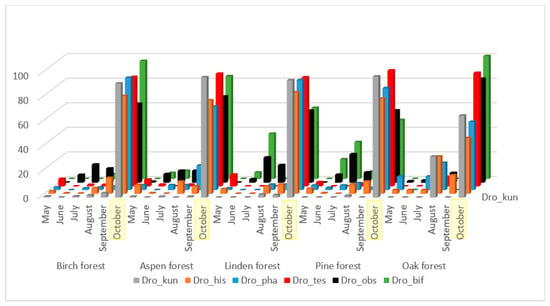
Figure 2.
Dynamics of changes in the number of species D. obcura, D. bifasciata, D. histrio, D. kuntzei, D. phalerata, D. testacea from May to October as a percentage of the total number for the entire period of accounting in biotope.

Table 2.
Correlation of seasonal dynamics of the number of species D.obcura, D.bifasciata, D. histrio, D. kuntzei, D. phalerata, D. testacea, L. quinquemaculata from May to October in five forest biotopes (Spearman rank order correlations). Significant correlation coefficients are highlighted in red, with values greater than 0.6 in bold.
We found the highest significant correlation of seasonal dynamics between closely related species D. obscura and D. bifasciata (Table 2). They are typical xylosaprobionts, their larvae live mainly in the tissues under the bark and in the fermenting tree sap [42]. The second group with high significant correlation of seasonal dynamics consists of D. histrio, D. kuntzei, D. phalerata, and D. testacea. All these species are mycetobionts, their larvae live in various fungi.
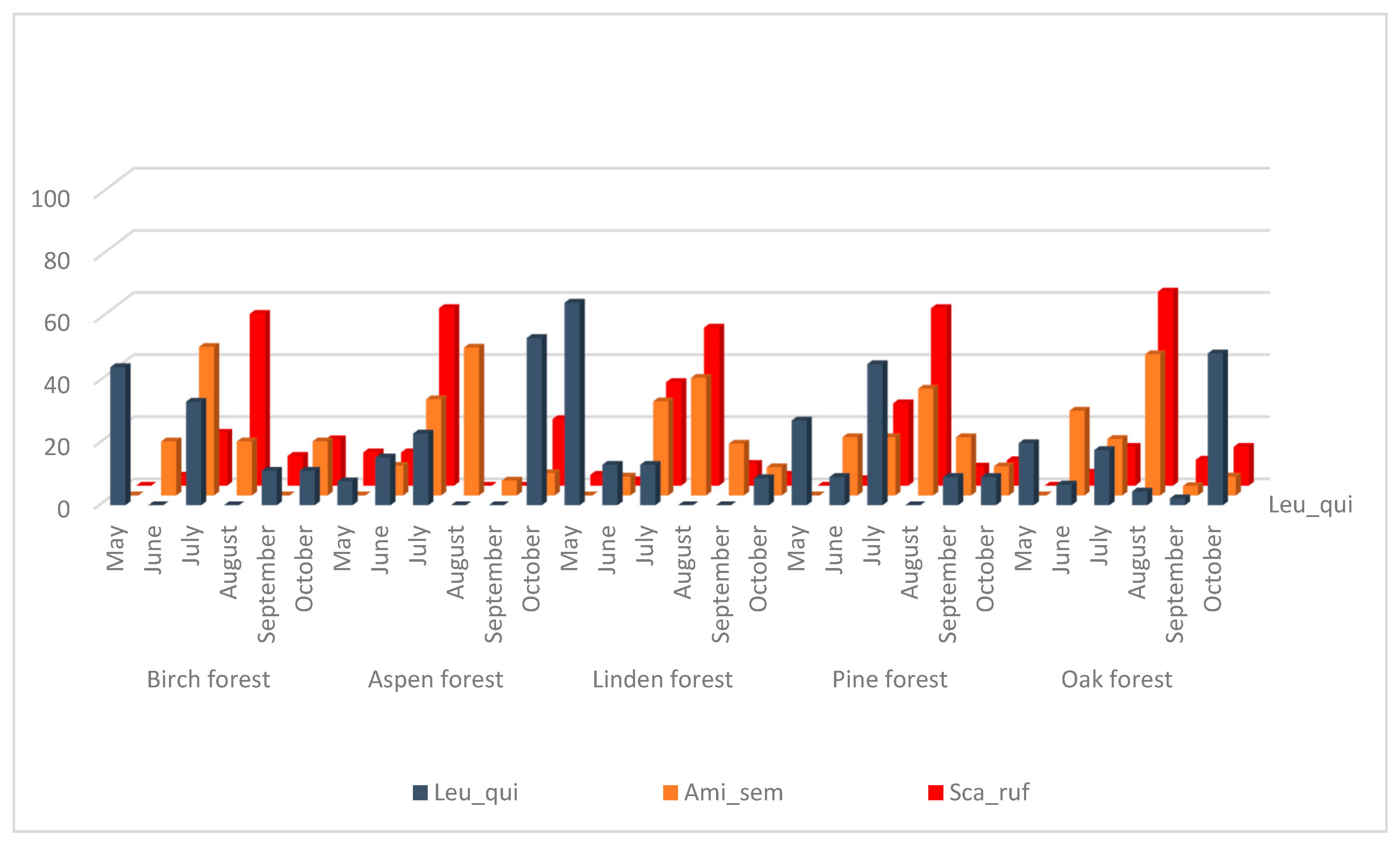
The species Amiota semivirgo and S. rufifrons also have significantly correlated seasonal dynamics (Figure 3, Table 3) but their main peak is observed in July–August in all biotopes, and in September–October, the number of collected flies decreases sharply. These species are also xylosaprobionts. Seasonal fluctuations in the number of L. quinquemaculata species do not show a significant correlation with any of drosophilid species and show a maximum in May and July in birch and pine forests, in May only in the linden forest, and in October in aspen and oak forests. The larvae of L. quinquemaculata could be found mainly in bracket fungi so they occupy a rather separate and specific ecological niche.
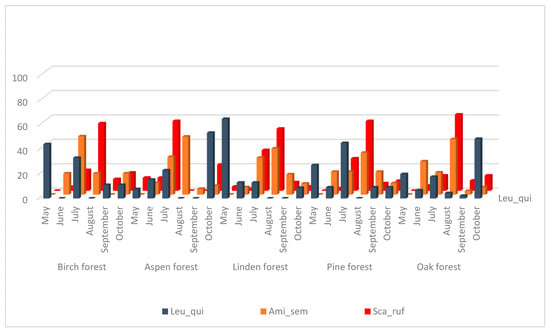
Figure 3.
Dynamics of changes in the number of species L. quinquemaculata, A. semivirgo, S. rufifrons from May to October as a percentage of the total number for the entire period of accounting in biotope.

Table 3.
Correlation of seasonal dynamics of the number of species L. quinquemaculata, A. semivirgo, S. rufifrons from May to October in five forest biotopes (Spearman’s rank order correlations). Significant correlation coefficients are highlighted in red, with values greater than 0.6 in bold.
The group of mycetobionts developing mainly in various species of basidiomycetes includes mass species D. histrio, D. kuntzei, D. phalerata, D. testacea (Table A2, Table A3, Table A4 and Table A5), and L. quinquemaculata rearing in bracket fungi (Table A9), which were found in an amount of more than 100 specimens (Table 4).

Table 4.
Total collected specimens of mycetobiont drosophilid species in five biotopes.
The second large ecological group of drosophila includes xylosaprobionts (D. obscura, D. bifasciata, S. rufifrons, A. semivirgo) (Table A1, Table A6, Table A7 and Table A8); their larvae live mainly in tissues under the bark and in fermenting tree sap (Table 5).

Table 5.
Total collected specimens of xylosaprobiont drosophilid species in five biotopes.
4. Discussion
The influence of seasonal changes on the abundance of Drosophilidae has been studied mainly in tropical and temperate climatic zones. Their abundance in tropical regions is affected by precipitation, and in regions with a temperate climate, temperature fluctuations are most affected [49,50,51,52,53]. Our studies have shown that Drosophilidae in central Russia have one peak in numbers, which begins at the end of September with a maximum in mid-October. At this time, daytime temperatures were recorded at no higher than 15 °C, and at night—no more than 10 °C. At the same time, throughout the season, the number of this family in traps was more or less constant without sharp peaks or lows. Similar dynamics were found in experiments in Uşak province, Turkey [54]. The average temperature of October and November with the highest numbers of Drosophilidae was from 5 to 10 °C. At the same time, in September, when the temperature was more favorable for fruit flies, the amount of catch was less [54].
Our work is the first study considering seasonal dynamics of Drosophilidae in European Russia. A total of 4725 individuals belonging to 9 genera and 30 species of drosophilid flies were identified in Mordovia State Nature Reserve. D. obscura and D. histrio were the most abundant species in beer traps. At the same time, seven more species (D. bifasciata, D. kuntzei, D. phalerata, D. testacea, L. quinquemaculata) were observed in traps with high numbers.
Among the 30 species of drosophila collected in Republic of Mordovia, 5 species of the genus Drosophila (D. busckii, D. funebris, D. hydei, D. immigrans, D. melanogaster) are synanthropic, i.e., closely related to humans and their activities. They live and breed in places where they can find fermenting and rotting fruits and vegetables, wine, beer and juices [42,55,56,57,58]. These species occur in small numbers in wild biotopes, apparently, due to migration attempts or wind transport. Most of the other drosophilid species (24 in our collections) are typical forest dwellers, which rarely occur far from the forest or groups of trees. The larvae of these drosophilids develop in moist tissues under the bark of deciduous trees, in fermenting tree sap, and in various fungi, including ascomycetes and tinders [59,60,61]. The larvae of the last species in our faunistic list, Gitona distigma Mg., according to the literature, are phytophages living in inflorescences of family Asteraceae plants, e.g., Sonchus and Crepis species [42]. Therefore, G. distigma may occur in different biotopes, not only in forests, sometimes even in people’s houses.
Here we compare the drosophilid fauna of the Republic of Mordovia with other regions of European Russia, we used data for the Moscow region—35 species [62], Voronezh region—18 species [62,63], Samara region—13 species [62,64], and North Karelia—19 species [65] (Table 6).

Table 6.
Comparison of drosophilid fauna in five regions of European Russia.
As can be seen from Table 6, the largest number of drosophilid species was observed in the Moscow region and Republic of Mordovia; this is a consequence of the special studies of this family conducted in these regions. Nevertheless, by now the degree of similarity is about 2/3 of the total number of species, we have found 21 common species for the fauna of the Republic of Mordovia and the Moscow region.
We studied seasonal dynamics of Drosophilidae in five types of forest (birch, aspen, linden, pine, and oak). Interestingly, the highest abundance of drosophilids was found in October in all types of the forests examined. We found that the drosophilid abundance demonstrated maximum value in birch forest and the lowest value in oak forest. In our collection we found representatives of two main ecological groups—mycetobionts and xylosaprobionts.
The total number of mass mycetobionts (2493) is 55.45% of the total number of drosophilid mass species (4496) and 52.76% of the total number of collected flies. At the same time, the larvae of D. histrio, D. kuntzei, D. phalerata and D. testacea develop mainly in the fruit bodies of basidiomycetes, and the larvae of L. quinquemaculata develop in the bracket fungi. As can be seen from Table 4, the number of imagos of D. histrio, D. kuntzei, D. phalerata and D.testacea collected in the oak forest is minimal, and several times less than in other biotopes. On the contrary, L. quinquemaculata imagos were collected in maximum quantity in the oak forest. We suggest that this is due to noticeable differences in the composition of the mycoflora of oak forests and other types of forests. Apparently, the number of basidiomycetes growing in the oak forest was minimal or their species composition was less attractive for these drosophilid mycetobionts (D. histrio, D. kuntzei, D. phalerata, and D. testacea). On the contrary, bracket fungi, apparently, occur most often in the oak forest, which explains the largest number of L.quinquemaculata collected here. The question of the relationship of various drosophilid species with fungi in Republic of Mordovia has not been studied yet but perhaps deserves a separate investigation.
The total number of mass xylosaprobionts (2003) is 44.55% of the total number of drosophilid mass species (4496) and 42.39% of the total number of flies collected. As can be seen from Table 5, xylosaprobionts demonstrate the maximum abundance in oak and birch forests. Apparently, this is due to the greatest number of wounds on tree trunks in these biotopes, which attract drosophilids of these species (D. obscura, D. bifasciata, S. rufifrons, A. semivirgo).
We found the highest significant correlation of seasonal dynamics between closely related xylosaprobiont species D. obscura and D. bifasciata. The second group with high significant correlation of seasonal dynamics consists of mycetobiont species D. histrio, D. kuntzei, D. phalerata, and D. testacea. The third group includes xylosaprobiont species A. semivirgo and S. rufifrons. Apparently, the similarity observed in the seasonal dynamics of some drosophilid species is influenced at a high degree by their food preferences and rearing sites.
We also analyzed species communities in five biotopes by calculating the Shannon–Weaver index and the Simpson index. It turned out that the greatest differences were found between oak and linden forests: the most diverse species community lives in the linden forest and the least diverse in the oak forest. On the contrary, the dominance of drosophilid species in the linden forest is the least pronounced, and in the oak forest it is the largest among all biotopes (Table A10).
In addition, according to our data, the mass species of drosophilids of the Republic of Mordovia show a different picture of seasonal population peaks. They can be divided into different types: species with summer–autumn peaks of abundance (D. obscura and D. bifasciata), with spring–autumn peaks (D. histrio, D. testacea), only with summer peaks (A. semivirgo and S. rufifrons), only with autumn peaks (D. kuntzei, D. phalerata), and with three peaks of abundance (L. quinquemaculata) (Table 7). Therefore, we can conclude that the presence of two or three peaks in numbers of abundance suggests the presence of two or three generations in these drosophilids. However, the presence of one peak number in our collections does not negate the possibility of having two generations, for example, in D. kuntzei and D. phalerata. Perhaps, for these mycetobiont species, beer traps become less attractive in the summer during the mushroom abundance season. Interestingly, for six mass species of drosophilids, the autumn peak of abundance is the maximum.

Table 7.
Total month-to-month numbers of Drosophilidae in 2019.
5. Conclusions
In our study, Drosophilidae species and their seasonal changes in Mordovia State Reserve were explored. It is the first investigation of drosophilid seasonal population changes considering their biotope association, abundance and species diversity in European Russia. We collected the flies by crown fermental traps in five types of forests (birch, aspen, linden, pine, and oak) from May to October in 2019. A total of 4725 individuals belonging to 9 genera and 30 species of drosophilid flies were identified, among them 15 species in 3 genera are new to Republic of Mordovia. Drosophila obscura Fll. and D. histrio Mg. were the most abundant species in traps, the other mass species are D. kuntzei, D. testacea, D. phalerata, S. rufifrons, D. bifasciata, A. semivirgo, and L. quinquemaculata. Interestingly, the highest abundance of drosophilids and their species diversity was found in October in all types of the forests examined. We found the highest significant correlation of seasonal dynamics between closely related species D. obscura and D. bifasciata, the second group with high significant correlation of seasonal dynamics consists of D. histrio, D. kuntzei, D. phalerata, and D. testacea, and finally the third group consists of A. semivirgo and S. rufifrons. Apparently, the similarity observed in the seasonal dynamics of these drosophilid species is influenced at high degree by their food preferences and rearing sites.
Author Contributions
Conceptualization, N.G.G.; methodology, N.G.G. and A.B.R.; software, A.M.K.; validation, N.G.G. and A.B.R.; formal analysis, A.M.K.; investigation, A.B.R. and M.N.E.; resources, M.N.E.; data curation, A.B.R.; writing—original draft preparation, N.G.G.; writing—review and editing, N.G.G. and A.B.R.; visualization, N.G.G. and A.B.R.; supervision, N.G.G.; project administration, A.B.R.; funding acquisition, A.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
The work of NGG and AMK was conducted under the IDB RAS Government basic research program in 2022 No 0088-2021-0019. The manuscript was prepared partly due to the financing of the Russian Science Foundation (grant number 22-14-00026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Here we present the seasonal dynamics for the most abundant (>100 flies collected) drosophilid species for every type of forest (Table A1, Table A2, Table A3, Table A4, Table A5, Table A6, Table A7, Table A8 and Table A9) and total numbers of drosophilid specimens collected in five biotopes with calculated Shannon–Weaver and Simpson indexes (Table A10):

Table A1.
Seasonal dynamics of Drosophila obscura in five biotopes.
Table A1.
Seasonal dynamics of Drosophila obscura in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 0 | 16 | 37 | 29 | 8 | 161 | 251 |
| Aspen forest | 2 | 18 | 25 | 25 | 7 | 180 | 257 |
| Linden forest | 0 | 6 | 43 | 30 | 8 | 123 | 210 |
| Pine forest | 0 | 14 | 50 | 18 | 7 | 127 | 216 |
| Oak forest | 4 | 6 | 12 | 27 | 4 | 290 | 343 |
| Total amount | 6 | 60 | 167 | 129 | 34 | 881 | 1277 |

Table A2.
Seasonal dynamics of Drosophila histrio in five biotopes.
Table A2.
Seasonal dynamics of Drosophila histrio in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 10 | 2 | 1 | 22 | 62 | 379 | 476 |
| Aspen forest | 13 | 1 | 0 | 18 | 11 | 137 | 180 |
| Linden forest | 7 | 0 | 0 | 11 | 14 | 152 | 184 |
| Pine forest | 14 | 2 | 0 | 26 | 34 | 262 | 338 |
| Oak forest | 1 | 1 | 1 | 10 | 5 | 15 | 33 |
| Total amount | 45 | 6 | 2 | 87 | 126 | 945 | 1211 |

Table A3.
Seasonal dynamics of Drosophila kuntzei in five biotopes.
Table A3.
Seasonal dynamics of Drosophila kuntzei in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 1 | 0 | 1 | 2 | 4 | 103 | 111 |
| Aspen forest | 1 | 0 | 1 | 0 | 1 | 137 | 140 |
| Linden forest | 0 | 0 | 0 | 5 | 4 | 187 | 196 |
| Pine forest | 0 | 0 | 0 | 1 | 0 | 66 | 67 |
| Oak forest | 0 | 0 | 0 | 1 | 0 | 2 | 3 |
| Total amount | 2 | 0 | 2 | 9 | 9 | 495 | 517 |

Table A4.
Seasonal dynamics of Drosophila testacea in five biotopes.
Table A4.
Seasonal dynamics of Drosophila testacea in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 5 | 0 | 1 | 1 | 2 | 73 | 82 |
| Aspen forest | 4 | 1 | 0 | 0 | 0 | 66 | 71 |
| Linden forest | 11 | 0 | 0 | 0 | 2 | 102 | 115 |
| Pine forest | 3 | 0 | 0 | 2 | 0 | 83 | 88 |
| Oak forest | 1 | 0 | 0 | 1 | 0 | 12 | 14 |
| Total amount | 24 | 1 | 1 | 4 | 4 | 336 | 370 |

Table A5.
Seasonal dynamics of Drosophila phalerata in five biotopes.
Table A5.
Seasonal dynamics of Drosophila phalerata in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 3 | 0 | 2 | 3 | 4 | 128 | 140 |
| Aspen forest | 1 | 0 | 1 | 1 | 5 | 17 | 25 |
| Linden forest | 1 | 0 | 0 | 3 | 3 | 60 | 67 |
| Pine forest | 2 | 1 | 2 | 3 | 1 | 44 | 53 |
| Oak forest | 0 | 0 | 1 | 2 | 0 | 5 | 8 |
| Total amount | 7 | 1 | 6 | 12 | 13 | 254 | 293 |

Table A6.
Seasonal dynamics of Scaptodrosophila rufifrons in five biotopes.
Table A6.
Seasonal dynamics of Scaptodrosophila rufifrons in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 0 | 3 | 16 | 52 | 9 | 14 | 94 |
| Aspen forest | 3 | 3 | 16 | 11 | 4 | 6 | 43 |
| Linden forest | 2 | 1 | 19 | 29 | 4 | 2 | 57 |
| Pine forest | 0 | 1 | 13 | 28 | 3 | 4 | 49 |
| Oak forest | 0 | 1 | 3 | 15 | 2 | 3 | 24 |
| Total amount | 5 | 9 | 67 | 135 | 22 | 29 | 267 |

Table A7.
Seasonal dynamics of Drosophila bifasciata in five biotopes.
Table A7.
Seasonal dynamics of Drosophila bifasciata in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 0 | 0 | 0 | 3 | 0 | 73 | 76 |
| Aspen forest | 0 | 3 | 4 | 3 | 0 | 51 | 61 |
| Linden forest | 0 | 1 | 7 | 0 | 0 | 11 | 19 |
| Pine forest | 0 | 8 | 15 | 3 | 0 | 24 | 50 |
| Oak forest | 0 | 0 | 0 | 0 | 0 | 57 | 57 |
| Total amount | 0 | 12 | 26 | 9 | 0 | 216 | 263 |

Table A8.
Seasonal dynamics of Amiota semivirgo in five biotopes.
Table A8.
Seasonal dynamics of Amiota semivirgo in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 0 | 4 | 11 | 4 | 0 | 4 | 23 |
| Aspen forest | 0 | 4 | 13 | 20 | 2 | 3 | 42 |
| Linden forest | 0 | 4 | 20 | 25 | 11 | 6 | 66 |
| Pine forest | 0 | 6 | 6 | 11 | 6 | 3 | 32 |
| Oak forest | 0 | 9 | 6 | 15 | 1 | 2 | 33 |
| Total amount | 0 | 27 | 56 | 75 | 20 | 18 | 196 |

Table A9.
Seasonal dynamics of Leucophenga quinquemaculata in five biotopes.
Table A9.
Seasonal dynamics of Leucophenga quinquemaculata in five biotopes.
| Biotopes | May | June | July | August | September | October | Total Amount |
|---|---|---|---|---|---|---|---|
| Birch forest | 4 | 0 | 3 | 0 | 1 | 1 | 9 |
| Aspen forest | 1 | 2 | 3 | 1 | 0 | 7 | 14 |
| Linden forest | 15 | 3 | 3 | 0 | 0 | 2 | 23 |
| Pine forest | 3 | 1 | 5 | 0 | 1 | 1 | 11 |
| Oak forest | 9 | 3 | 8 | 2 | 1 | 22 | 45 |
| Total amount | 32 | 9 | 22 | 3 | 3 | 33 | 102 |

Table A10.
Drosophilid specimens collected in five biotopes with calculated Shannon–Weaver and Simpson indexes.
Table A10.
Drosophilid specimens collected in five biotopes with calculated Shannon–Weaver and Simpson indexes.
| Birch Forest | Aspen Forest | Linden Forest | Pine Forest | Oak Forest | |
|---|---|---|---|---|---|
| Amiota (Phortica) semivirgo Maca, 1977 | 23 | 42 | 66 | 32 | 33 |
| Amiota (Amiota) albilabris (Roth in Zetterstedt, 1860) | 0 | 0 | 0 | 0 | 6 |
| Amiota (Amiota) alboguttata (Wahlberg, 1839) | 0 | 0 | 0 | 0 | 7 |
| Amiota (Amiota) rufescens (Oldenberg, 1914) | 0 | 0 | 0 | 2 | 2 |
| Amiota (Amiota) subtusradiata Duda, 1934 | 0 | 0 | 1 | 0 | 3 |
| Gitona distigma Meigen, 1830 | 1 | 1 | 1 | 1 | 23 |
| Leucophenga maculata (Dufour, 1839) | 1 | 2 | 4 | 1 | 10 |
| Leucophenga quinquemaculata Strobl, 1893 | 9 | 14 | 23 | 11 | 45 |
| Stegana (Steganina) coleoptrata (Scopoli, 1763) | 0 | 0 | 1 | 0 | 1 |
| Chymomyza amoena (Loew, 1862) | 4 | 2 | 0 | 3 | 6 |
| Chymomyza caudatula Oldenberg, 1914 | 0 | 0 | 1 | 0 | 0 |
| Chymomyza costata (Zetterstedt, 1838) | 0 | 0 | 1 | 0 | 0 |
| Chymomyza fuscimana (Zetterstedt, 1838) | 0 | 1 | 0 | 0 | 0 |
| Drosophila (Dorsilopha) busckii Coquillett, 1901 | 1 | 1 | 0 | 1 | 0 |
| Drosophila (Drosophila) funebris (Fabricius, 1787) | 6 | 1 | 4 | 0 | 2 |
| Drosophila (Drosophila) histrio Meigen, 1830 | 476 | 180 | 184 | 338 | 33 |
| Drosophila (Drosophila) hydei Sturtevant, 1921 | 4 | 1 | 0 | 1 | 0 |
| Drosophila (Drosophila) immigrans Sturtevant, 1921 | 23 | 2 | 14 | 13 | 11 |
| Drosophila (Drosophila) kuntzei Duda, 1924 | 111 | 140 | 196 | 67 | 3 |
| Drosophila (Drosophila) phalerata Meigen, 1830 | 140 | 25 | 67 | 53 | 8 |
| Drosophila (Drosophila) testacea von Roser, 1840 | 82 | 71 | 115 | 88 | 14 |
| Drosophila (Drosophila) transversa Fallen, 1823 | 8 | 2 | 1 | 10 | 2 |
| Drosophila (Sophophora) melanogaster Meigen, 1830 | 1 | 2 | 3 | 0 | 0 |
| Drosophila (Sophophora) bifasciata Pomini, 1940 | 76 | 61 | 19 | 50 | 57 |
| Drosophila (Sophophora) obscura Fallen, 1823 | 251 | 257 | 210 | 216 | 343 |
| Drosophila (Sophophora) tristis Fallen, 1823 | 0 | 0 | 0 | 0 | 1 |
| Hirtodrosophila confusa (Staeger, 1844) | 10 | 3 | 3 | 3 | 5 |
| Hirtodrosophila trivittata (Strobl, 1893) | 1 | 1 | 0 | 1 | 0 |
| Scaptodrosophila rufifrons (Loew, 1873) | 94 | 43 | 57 | 49 | 24 |
| Scaptomyza (Hemiscaptomyza) unipunctum (Zetterstedt, 1847) | 0 | 0 | 0 | 0 | 1 |
| Shannon–Weaver index | 1.98 | 1.99 | 2.11 | 1.95 | 1.87 |
| Simpson index | 0.20 | 0.19 | 0.15 | 0.21 | 0.31 |
References
- Chambers, L.E.; Altwegg, R.; Barbraud, C.; Barnard, P.; Beaumont, L.J.; Crawford, R.J.M.; Durant, J.M.; Hughes, L.; Keatley, M.R.; Low, M.; et al. Phenological changes in the southern hemisphere. PLoS ONE 2013, 8, e75514. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, K. Diapause research in insects: Historical review and recent work perspectives. Entomol. Exp. Appl. 2019, 167, 27–36. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Singer, M.C.; Parmesan, C. Phenological asynchrony between herbivorous insects and their hosts: Signal of climate change or pre-existing adaptive strategy? Philos. Trans. B 2010, 365, 3161–3176. [Google Scholar] [CrossRef]
- Damien, M.; Tougeron, K. Prey–predator phenological mismatch under climate change. Curr. Opin. Insect Sci. 2019, 35, 60–68. [Google Scholar] [CrossRef]
- Åkesson, A.; Curtsdotter, A.; Eklöf, A.; Ebenman, B.; Norberg, J.; Barabás, G. The importance of species interactions in eco-evolutionary community dynamics under climate change. Nat. Commun. 2021, 12, 4759. [Google Scholar] [CrossRef]
- van Dijk, J.; De Baets, K. Biodiversity and Host–Parasite (Co)Extinction. In The Evolution and Fossil Record of Parasitism; Topics in Geobiology; De Baets, K., Huntley, J.W., Eds.; Springer: Cham, Switzerland, 2021; Volume 50. [Google Scholar] [CrossRef]
- Altermatt, F. Temperature-related shifts in butterfly phenology depend on the habitat. Glob. Chang. Biol. 2012, 18, 2429–2438. [Google Scholar] [CrossRef]
- Blackshaw, R.P.; Esbjerg, P. Statistical indicators for insects caught in the development trap. J. Appl. Entomol. 2017, 142, 272–276. [Google Scholar] [CrossRef]
- Chick, L.D.; Strickler, S.; Perez, A.; Martin, R.A.; Diamond, S.E. Urban heat islands advance the timing of reproduction in a social insect. J. Therm. Biol. 2019, 80, 119–125. [Google Scholar] [CrossRef]
- Berger, D.; Olofsson, M.; Friberg, M.; Karlsson, B.; Wiklund, C.; Gotthard, K. Intraspecific variation in body size and the rate of reproduction in female insects—Adaptive allometry or biophysical constraint? J. Anim. Ecol. 2012, 81, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Pau, S.; Wolkovich, E.M.; Cook, B.I.; Davies, T.J.; Kraft, N.J.B.; Bolmgren, K.; Betancourt, J.L.; Cleland, E.E. Predicting phenology by integrating ecology, evolution and climate science. Glob. Chang. Biol. 2011, 17, 3633–3643. [Google Scholar] [CrossRef]
- Minin, A.A.; Ananin, A.A.; Buyvolov, Y.A.; Larin, E.G.; Lebedev, P.A.; Polikarpova, N.V.; Prokosheva, I.V.; Rudenko, M.I.; Sapelnikova, I.I.; Fedotova, V.G.; et al. Recommendations to unify phenological observations in Russia. Nat. Conserv. Res. 2020, 5, 89–110. [Google Scholar] [CrossRef]
- Visser, M.E.; Caro, S.P.; van Oers, K.; Schaper, S.V.; Helm, B. Phenology, seasonal timing and circannual rhythms: Towards a unified framework. Philos. Trans. R. Soc. Lond. 2010, 365, 3113–3127. [Google Scholar] [CrossRef]
- Sawoniewicz, M. Seasonal dynamics of saproxylic beetles (Coleoptera) occurring in decaying birch (Betula spp.) wood in the Kampinos National Park. For. Res. Pap. 2015, 76, 213–220. [Google Scholar] [CrossRef][Green Version]
- Afonina, E.Y.; Tashlykova, N.A.; Kuklin, A.P.; Tsybekmitova, G.T. Environmental features and dynamics of plankton communities in a mountain glacial moraine lake (Baikal Lake basin, Russia). Nat. Conserv. Res. 2020, 5, 23–36. [Google Scholar] [CrossRef]
- Vitasse, Y.; Ursenbacher, S.; Klein, G.; Bohnenstengel, T.; Chittaro, Y.; Delestrade, A.; Monnerat, C.; Rebetez, M.; Rixen, C.; Strebel, N.; et al. Phenological and elevational shifts of plants, animals and fungi under climate change in the European Alps. Biol. Rev. 2021, 96, 1816–1835. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Wilson, R.J. Intra- and interspecific variation in the responses of insect phenology to climate. J. Anim. Ecol. 2021, 90, 248–259. [Google Scholar] [CrossRef]
- Hill, G.M.; Kawahara, A.Y.; Daniels, J.C.; Bateman, C.C.; Scheffers, B.R. Climate change effects on animal ecology: Butterflies and moths as a case study. Biol. Rev. 2021, 96, 2113–2126. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Hoye, T.T.; Inouye, D.W.; Post, E. The effects of phenological mismatches on demography. Philos. Trans. R. Soc. Lond. 2010, 365, 3177–3186. [Google Scholar] [CrossRef]
- Kašák, J.; Foit, J.; Hučín, M. Succession of ground beetle (Coleoptera: Carabidae) communities after windthrow disturbance in a montane Norway spruce forest in the Hrubý Jeseník Mts. (Czech Republic). Cent. Eur. For. J. 2017, 63, 180–187. [Google Scholar] [CrossRef][Green Version]
- Zamotajlov, A.S.; Serdyuk VYu Khomitskiy, E.E.; Belyi, A.I. New data on distribution and biology of some rare ground beetles (Coleoptera, Carabidae) in South Russia. Nat. Conserv. Res. 2019, 4, 81–90. [Google Scholar] [CrossRef]
- Nasir, S.; Akram, W.; Ahmed, F. The population dynamics, ecological and seasonal activity of Paederus fuscipes Curtis (Staphylinidae; Coleoptera) in the Punjab, Pakistan. APCBEE Procedia 2012, 4, 36–41. [Google Scholar] [CrossRef][Green Version]
- Zemoglyadchuk, A.V.; Ruchin, A.B.; Egorov, L.V. An annotated checklist of the tumbling flower beetles (Coleoptera, Mordellidae) of the Republic of Mordovia, with a short review of the family in European Russia. Entomol. Rev. 2020, 100, 771–787. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Seasonal activity of Coleoptera attracted by fermental crown traps in forest ecosystems of Central Russia. Ecol. Quest. 2021, 32, 37–53. [Google Scholar] [CrossRef]
- Handley, K.; Hough-Goldstein, J.; Hanks, L.M.; Millar, J.G.; D’Amico, V. Species richness and phenology of cerambycid beetles in urban forest fragments of Northern Delaware. Ann. Entomol. Soc. Am. 2015, 108, 251–262. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Semishin, G.B. Fauna of click beetles (Coleoptera: Elateridae) in the interfluve of Rivers Moksha and Sura, Republic of Mordovia, Russia. Biodiversitas 2018, 19, 1352–1365. [Google Scholar] [CrossRef]
- Kuussaari, M.; Heliölä, J.; Luoto, M.; Pöyry, J. Determinants of local species richness of diurnal Lepidoptera in boreal agricultural landscapes. Agric. Ecosyst. Environ. 2007, 122, 366–376. [Google Scholar] [CrossRef]
- Teder, T. Phenological responses to climate warming in temperate moths and butterflies: Species traits predict future changes in voltinism. Oicos 2020, 129, 1051–1060. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Soon, V.; Hanula, J.L. Vertical distribution and seasonality of predatory wasps (Hymenoptera: Vespidae) in a temperate deciduous forest. Fla. Entomol. 2011, 94, 1068–1070. [Google Scholar] [CrossRef]
- Ruchin, A.B. Seasonal dynamics and spatial distribution of lepidopterans in selected locations in Mordovia, Russia. Biodiversitas 2021, 22, 2569–2575. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Esin, M.N. Seasonal dynamics of Diptera in individual biotopes in the center of the European part of Russia. Biosyst. Divers. 2021, 29, 374–379. [Google Scholar] [CrossRef]
- Pestov, S.V. Seasonal dynamics of hoverfly (Diptera, Syrphidae) activity in the taiga zone of the Komi Republic. Entomol. Rev. 2010, 90, 718–723. [Google Scholar] [CrossRef]
- Paur, J.; Gray, D.A. Seasonal dynamics and overwintering strategy of the tachinid fly (Diptera: Tachinidae), Ormia ochracea (Bigot) in southern California. Terr. Arthropod Rev. 2011, 4, 145–156. [Google Scholar] [CrossRef]
- Dvořák, L.; Dvořáková, K.; Oboňa, J.; Ruchin, A.B. Selected Diptera families caught with beer traps in the Republic of Mordovia (Russia). Nat. Conserv. Res. 2020, 5, 65–77. [Google Scholar] [CrossRef]
- Semelbauer, M.; Mangová, B.; Barta, M.; Kozánek, M. The factors influencing seasonal dynamics and spatial distribution of stable fly Stomoxys calcitrans (Diptera, Muscidae) within stables. Insects 2018, 9, 142. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Katsoyannos, B.I.; Carey, J.R.; Kouloussis, N.A. Seasonal and annual occurrence of the Mediterranean fruit fly (Diptera: Tephritidae) in Northern Greece. Ann. Entomol. Soc. Am. 2001, 94, 41–50. [Google Scholar] [CrossRef]
- Yassin, A. Phylogenetic classification of the Drosophilidae Rondani (Diptera): The role of morphology in the postgenomic era. Syst. Entomol. 2013, 38, 349–364. [Google Scholar] [CrossRef]
- Miller, E.M.; Marshall, S.A.; Grimaldi, D.A. A Review of the Species of Drosophila (Diptera: Drosophilidae) and Genera of Drosophilidae of Northeastern North America. Can. J. Arthropod Identif. 2017, 31, 1–232. [Google Scholar]
- Silva, N.M.; Fantinel, C.C.; Valente, V.L.S.; Valiati, V.H. Population dynamics of the invasive species Zaprionus indianus (Gupta) (Diptera: Drosophilidae) in communities of drosophilids of Porto Alegre City, Southern of Brazil. Neotrop. Entomol. 2005, 34, 363–374. [Google Scholar] [CrossRef]
- Gornostaev, N.G. Ecological classification of drosophilid flies (Diptera, Drosophilidae). Entomol. Obozr. 1996, 75, 698–705. [Google Scholar]
- Ruchin, A.B.; Khapugin, A.A. Red data book invertebrates in a protected area of European Russia. Acta Zool. Acad. Sci. Hung. 2019, 65, 349–370. [Google Scholar] [CrossRef]
- Khapugin, A.A.; Silaeva, T.B. The arrangement of threatened plants in Mordovia: The role of biodiversity research centers. Écoscience 2020, 27, 157–164. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Gornostaev, N.G. A key to the drosophilid flies (Diptera, Drosophilidae) from European Russia and neighbouring countries. Entomol. Obozr. 2001, 80, 908–915. (In Russian) [Google Scholar]
- Grimaldi, D.A. A phylogenetic revised classificationof genera in the Drosophilidae (Diptera). Bull. Am. Mus. Nat. Hist. 1990, 197, 1–139. [Google Scholar]
- Hill, T.; Lewicki, P. Statistics: Methods and Applications; StatSoft: Tulsa, OH, USA, 2007; 719p. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvorak, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.R.; Madi-Ravazzi, L. Seasonal variation in natural populations of Drosophila spp. (Diptera) in two woodlands in the State of São Paulo, Brazil. Iheringia Sér. Zool. 2006, 96, 437–444. [Google Scholar] [CrossRef]
- Poppe, J.L.; Valente, V.L.S.; Schmitz, H.J. Population dynamics of Drosophilids in the Pampa biome in response to temperature. Neotrop. Entomol. 2013, 42, 269–277. [Google Scholar] [CrossRef]
- Prigent, S.R.; Le Gall, P.; Mbunda, S.W.; Veuille, M. Seasonal and altitudinal structure of drosophilid communities on Mt Oku (Cameroon volcanic line). Comptes Rendus Geosci. 2013, 345, 316–326. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Montes, M.; Oliveira, G.; De Carvalho-Neto, F.; Rohde, C.; Garcia, A. Effects of seasonality on drosophilids (Insecta, Diptera) in the northern part of the Atlantic Forest, Brazil. Bull. Entomol. Res. 2017, 107, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Zengin, E. Occurrence of invasive species and seasonal dynamics of fruit flies (Diptera: Drosophilidae) species in Uşak province, Turkey. Rev. Soc. Entomol. Argent. 2020, 79, 21–30. [Google Scholar] [CrossRef]
- Bächli, G.; Rocha Pité, M.T. Family Drosophilidae. In Catalogue of Palaearctic Diptera; Clusiidae–Chloropidae; Akadémiai Kiadó: Budapest, Hungary, 1984; Volume 10, pp. 186–220. [Google Scholar]
- Nartshuk, E.P. Fruit flies (Diptera: Drosophilidae) of the Russian Arctic. Zoosystematica Ross. 2014, 23, 256–263. [Google Scholar] [CrossRef]
- Toda, M.J.; Sidorenko, V.S.; Watabe, H.; Kholin, S.K.; Vinokurov, N.N. A revisionof the Drosophilidae (Diptera) in East Siberia and Russian Far East: Taxonomy and biogeography. Zool. Sci. 1996, 13, 455–477. [Google Scholar] [CrossRef]
- Beuk, P.L.T. The species of the Drosophila repleta group in Northwestern Europe with special reference to the Netherlands (Diptera: Drosophilidae). Ent. Ber. Amst. 1993, 53, 96–98. [Google Scholar]
- Krivosheina, N.P. Macromycete fruit bodies as a habitat for dipterans (Insecta, Diptera). Entmol. Rev. 2008, 88, 778. [Google Scholar] [CrossRef]
- Yoshimoto, J.; Kakutani, T.; Nishida, T. Influence of resource abundance on the structure of the insect community attracted to fermented tree sap. Ecol. Res. 2005, 20, 405–414. [Google Scholar] [CrossRef]
- Lachance, M.A.; Gilbert, D.G.; Starmer, W.T. Yeast communities associated with Drosophila species and related flies in an eastern oak-pine forest: A comparison with western communities. J. Ind. Microbiol. 1995, 14, 484–494. [Google Scholar] [CrossRef]
- Gornostaev, N.G. Addition to the fauna of drosophilid flies (Diptera, Drosophilidae) of Russia. Russian Entomol. J. 1997, 6, 113–118. [Google Scholar]
- Negrobov, O.P. (Ed.) Cadastre of Invertebrates of the Voronezh Region; Springer: Voronezh, Russia, 2005; 825p. [Google Scholar]
- Rosenberg, G.S. (Ed.) Cadastre of Invertebrates of Samarskaya Luka: Tutorial; Springer: Samara, Russia, 2007; 471p. [Google Scholar]
- Gornostaev, N.G.; Kulikov, A.M. New data on the drosophilid fauna (Diptera, Drosophilidae) of North Karelia, Russia. Euroasian Entomol. J. 2018, 17, 100–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).