Behavioral Responses of Bemisia tabaci Mediterranean Cryptic Species to Three Host Plants and Their Volatiles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth
2.2. Insect Rearing
2.3. Olfactory Choice Test with Y-Tube Olfactometer
2.4. Volatile Compounds Collection and Analysis
2.5. The Olfactory Responses of B. tabaci to Volatile Compound
2.6. Data Analysis
3. Results
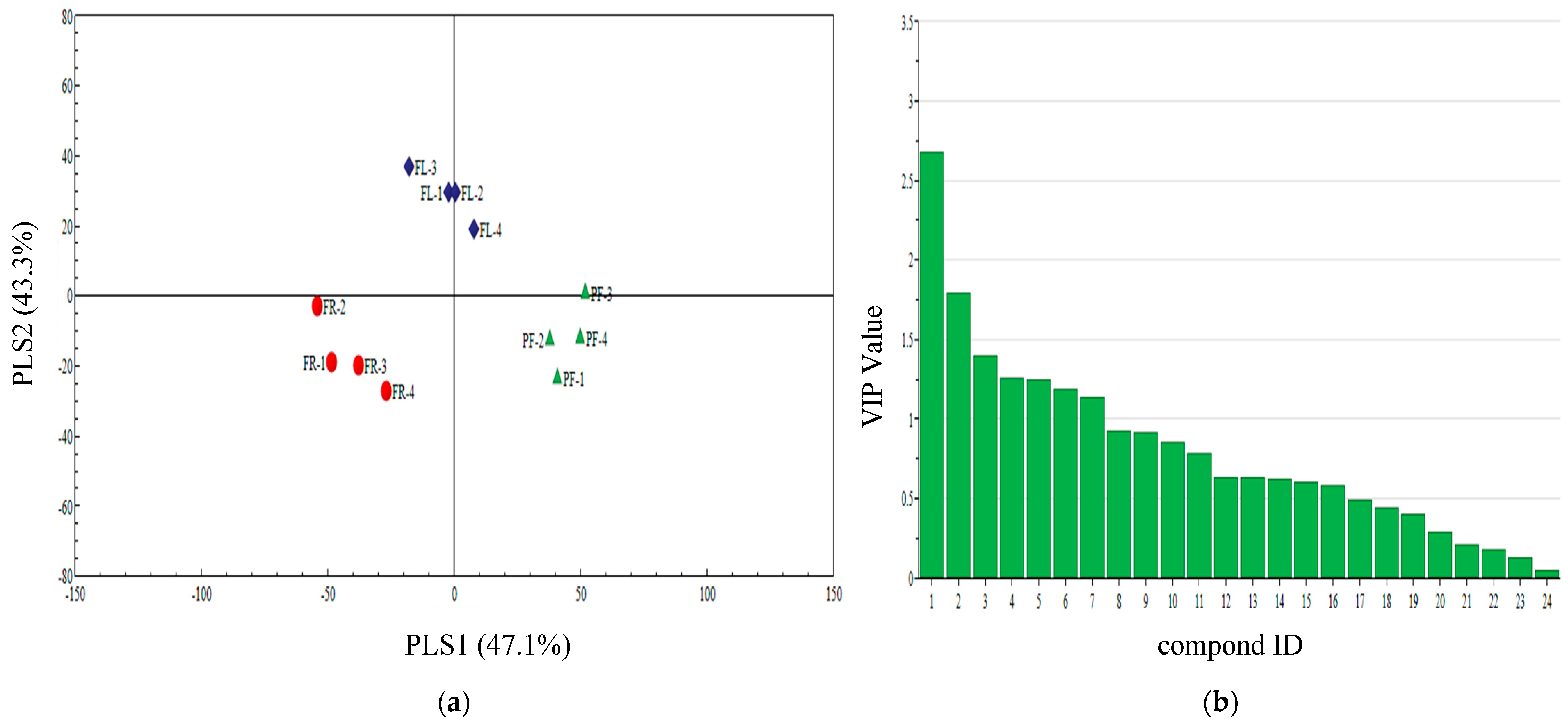
3.1. Response of B. tabaci to Volatiles of Pre-Flowering Plants
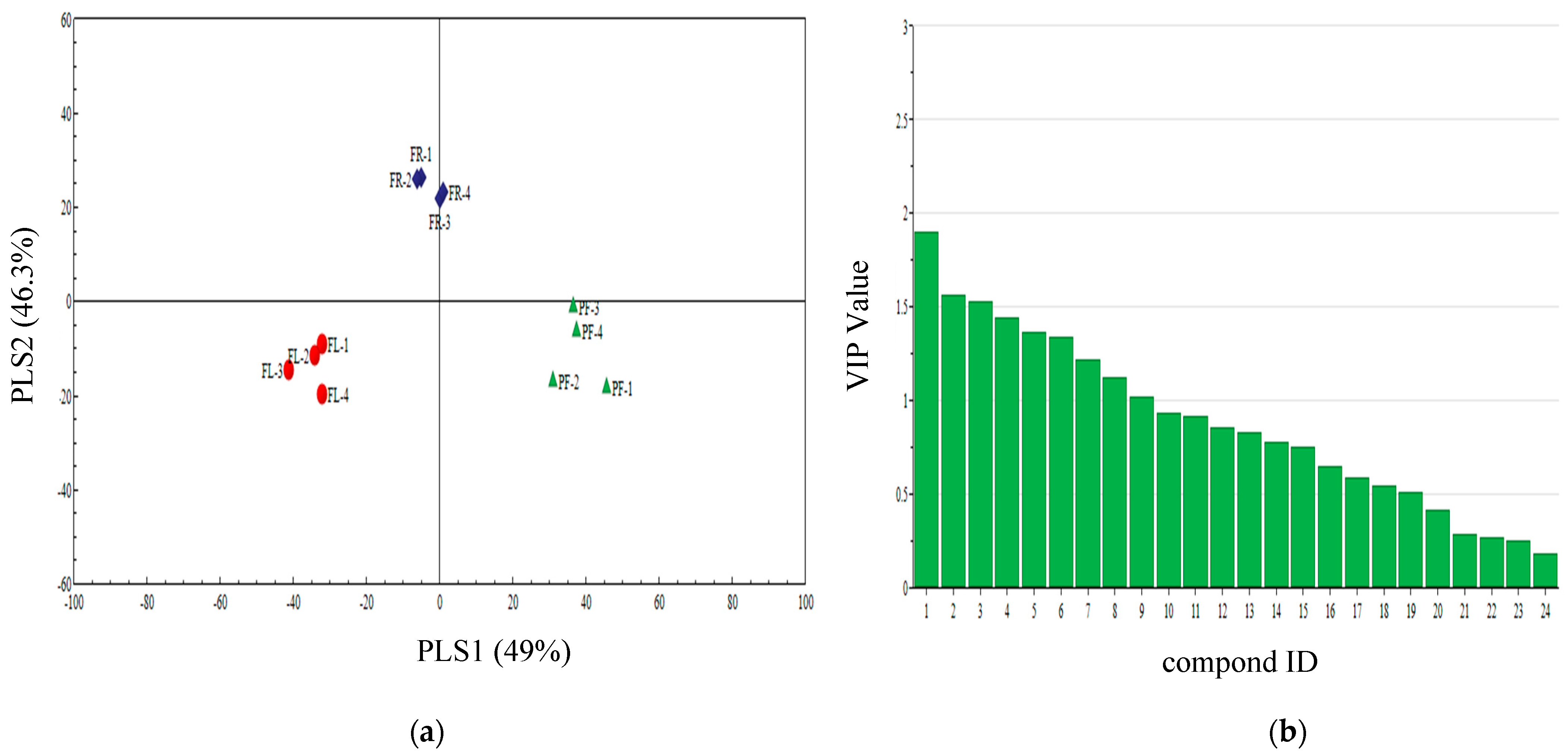
3.2. Response of B. tabaci to Volatiles of Florescence Plants
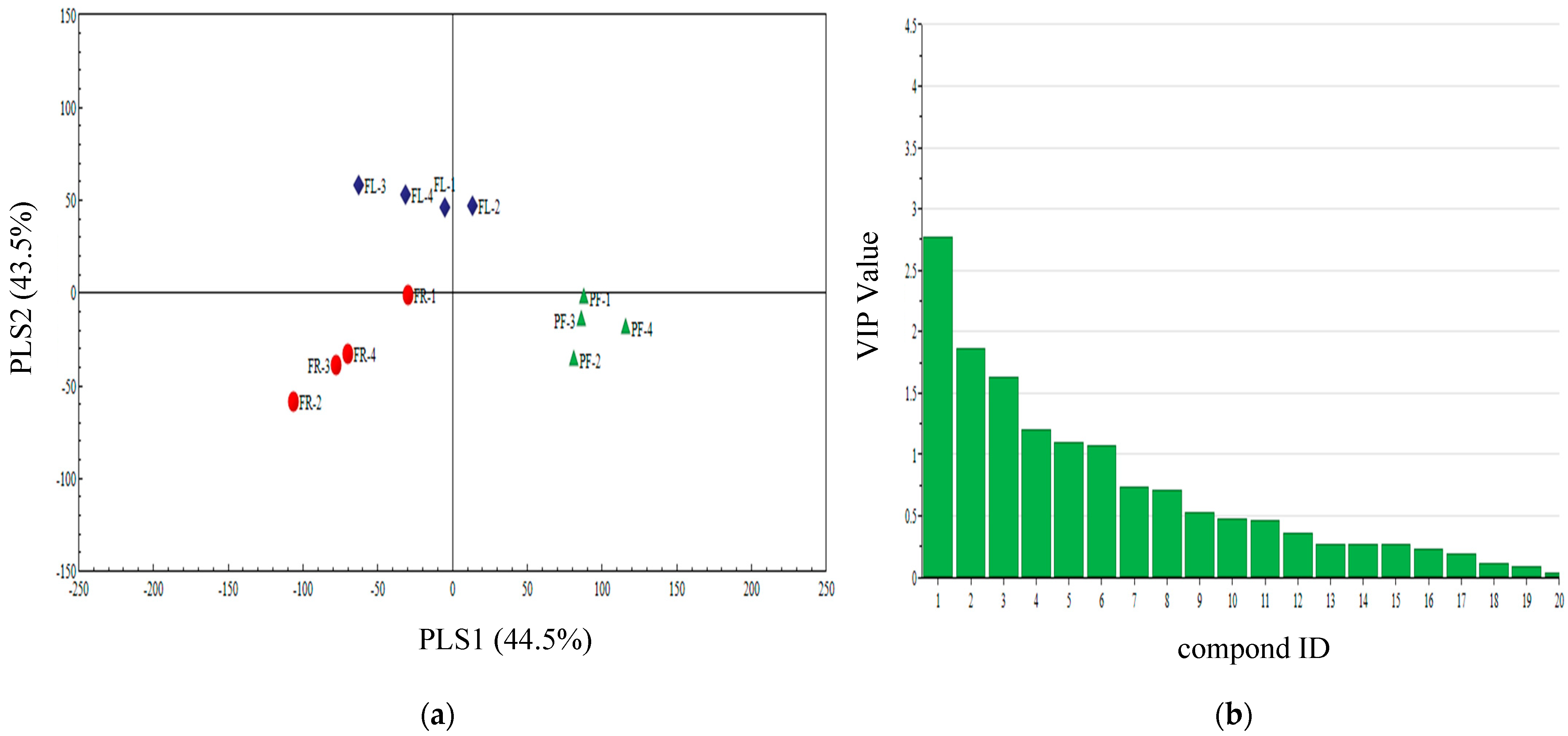
3.3. Response of B. tabaci to Volatiles of Fruiting Plants
3.4. Qualitative Analysis of Three Plant Volatiles in Different Periods
3.5. PLS-DA Analysis of Different Periods Volatiles of Three Plants
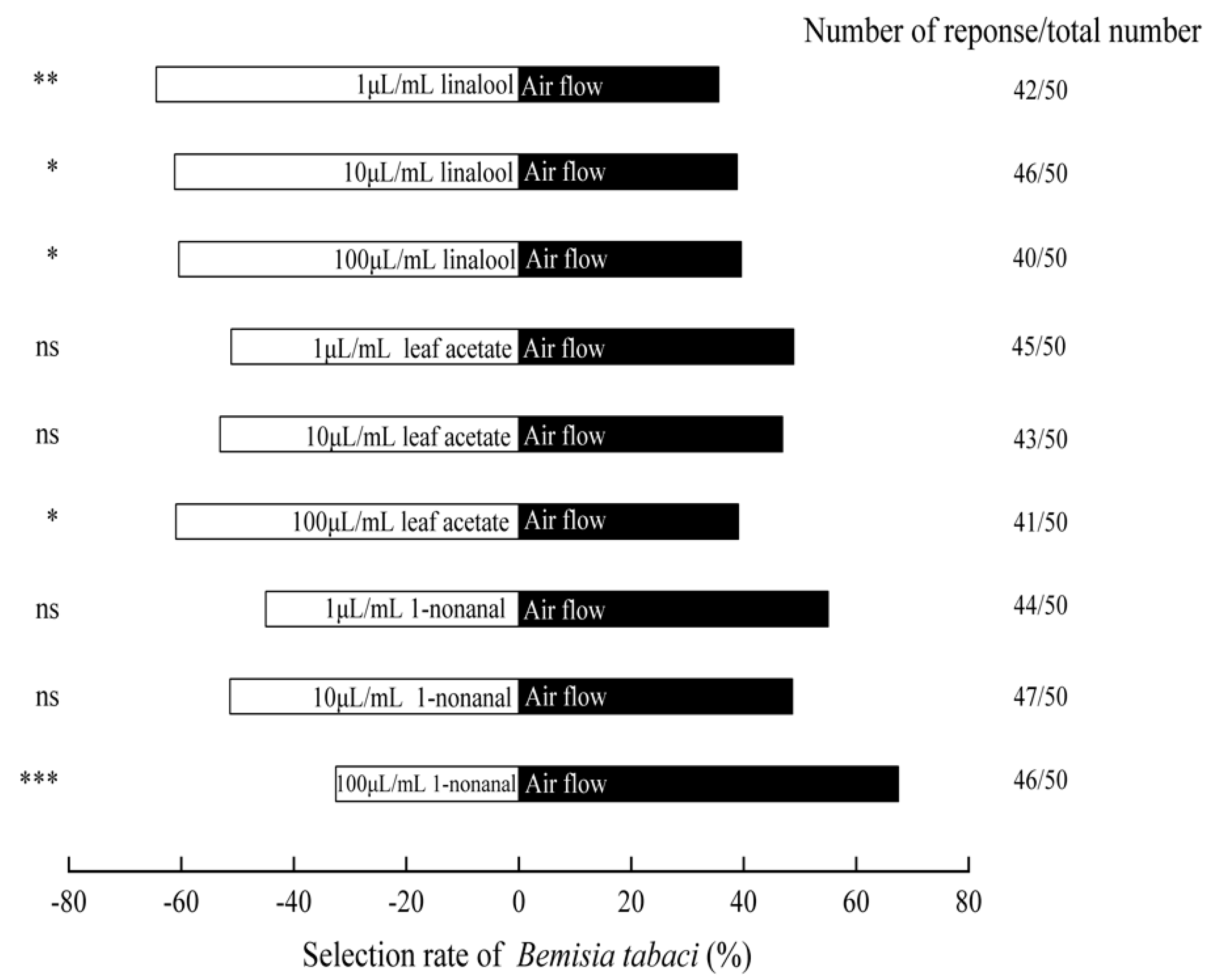
3.6. The Olfactory Responses of B. tabaci to Standard Samples of Volatile Compound
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.S. Push-pull strategy for control of sweet-potato whitefly, Bemisia tabaci (Hemiptera Aleyrodidae) in a tomato greenhouse. Korean Soc. Appl. Entomol. 2020, 58, 209–218. [Google Scholar]
- Brown, J.K.; Frohlich, D.R.; Rosell, R.C. The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 1995, 40, 511–534. [Google Scholar] [CrossRef]
- Gennadius, P. Disease of tobacco plantations in the Trikonia. The aleurodid of tobacco. Ellenike Ga. 1889, 5, 1–3. [Google Scholar]
- Dickey, A.M.; Osborne, L.S.; Shatters, R.; Hall, P.A.M.; McKenzie, C.L. Population genetics of invasive Bemisia tabaci (Hemiptera: Aleyrodidae) Cryptic species in the United States based on microsatellite markers. J. Econ. Èntomol. 2013, 106, 1355–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.M.; Jiao, X.G.; Yang, X.; Yang, N.N.; Zhang, Y.J. Effects of resistance to thiamethoxam on the feeding behavior of Bemisia tabaci biotype B (Hemiptera: Aleyrodidae). J. Plant Prot. 2016, 43, 175–176. [Google Scholar]
- Bernays, E.A.; Chapman, R.E. Host-Plant Selection by Phytophagous Insects; Springer: New York, NY, USA, 1994. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, C.; Beattie, G.A.; Zhang, X.; Cen, Y. Influence of different fertilizer types on life table parameters of citrus red mite, Panonychus citri (Acari: Tetranychidae). Syst. Appl. Acarol. 2019, 24, 2209–2218. [Google Scholar] [CrossRef]
- de Bruyne, M.; Baker, T.C. Odor detection in insects: Volatile codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef]
- Li, J.; Jin, Y.; Luo, Y.; Shen, Y.; Chen, H. Comparative analysis of volatile compounds from different host plants of Anoplophora glabripennis (Motsch.). J. Beijing For. Univ. 2002, 24, 165–169. [Google Scholar]
- Chen, W.B.; Chen, L.; Wang, L.L.; Su, H.H.; Yang, Y.Z. Host preference of Sylepta derogata between cotton and piemarker and active plant volatile determination. Xinjiang Agric. Sci. 2020, 57, 650–657. [Google Scholar]
- Hua, L.D.; Yang, Y.Z.; Ji, X.Y. Progress in research on the insecticidal activity of castor extracts. Chin. J. Appl. Entomol. 2013, 50, 1157–1162. [Google Scholar]
- Luo, J.Y.; Zhang, S.; Zhu, X.Z.; Ji, J.C.; Zhang, K.X.; Wang, C.Y.; Zhang, L.J.; Wang, L.; Li, C.H.; Cui, J.J. Effect of trap castor on cotton arthropod animal communities and biodiversity. Chin. Cotton 2018, 45, 9–11. [Google Scholar]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Èntomol. 2014, 59, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Seiter, N.J.; Reay-Jones, F.P.F.; Greene, J.K. Within-field spatial distribution of Megacopta cribraria (Hemiptera: Plataspidae) in soybean (Fabales: Fabaceae). Environ. Èntomol. 2013, 42, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Akol, A.M.; Njagi, P.G.N.; Sithanantham, S.; Mueke, J.M. Effects of two neem insecticide formulations on the attractiveness, acceptability and suitability of diamondback moth larvae to the parasitoid, Diadegma mollipla (Holmgren) (Hym., Ichneumonidae). J. Appl. Èntomol. 2003, 127, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Saad, K.A.; Mohamad Roff, M.N.; Hallett, R.H.; Idris, A.B. Aphid-induced defences in chilli affect preferences of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci. Rep. 2015, 5, 13697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Su, H.; Zhang, S.; Jing, T.; Liu, Z.; Yang, Y. The Effect of mirid density on volatile-mediated foraging behaviour of Apolygus lucorum and Peristenus spretus. Insects 2021, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Hasanuzzaman, A.T.M.; Zhang, Z.-F.; Zhang, Y.; Liu, T.-X. High level of nitrogen makes tomato plants releasing less volatiles and attracting more Bemisia tabaci (Hemiptera: Aleyrodidae). Front. Plant Sci. 2017, 8, 466. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Lin, K.J.; Huang, X.Z.; Geng, T.; Wei, H.Y.; Zhang, Y.J. EAG responses and taxis selection of green plant bug Apolygus lucorum to volatiles from seven species of kinds of Malvaceae plants. Chin. J. Biol. Control. 2016, 32, 135–141. [Google Scholar]
- Yu, H.-L.; Zhang, Y.-J.; Pan, W.-L.; Guo, Y.-Y.; Gao, X.-W. Identification of volatiles from field cotton plant under different induction treatments. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2007, 18, 859–864. [Google Scholar]
- Nyasembe, O.V.; Teal, P.E.; Mukabana, W.R.; Tumlinson, J.H.; Torto, B. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasites Vectors 2012, 5, 234. [Google Scholar] [CrossRef] [Green Version]
- Deconinck, E.; Aouadi, C.; Bothy, J.; Courselle, P. Detection and identification of multiple adulterants in plant food supplements using attenuated total reflectance—Infrared spectroscopy. J. Pharm. Biomed. Anal. 2018, 152, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.N.; Dinusha, N.U.; Lalithanjalie, D.A.; Priyani, A.P. Volatiles from host plant brinjal attract the brinjal fruit and shoot borer-Leucinodes orbonalis guenee. J. Asia-Pac. Entomol. 2021, 24, 695–703. [Google Scholar]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect odorscapes: From plant volatiles to natural Olfactory Scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef]
- Chen, L. Preference Evaluation of Sylepta Derogate Fabricius on Cotton and Abutilon Theophrasti Medic; Yangzhou University: Yangzhou, China, 2013. [Google Scholar]
- Rizwangul, A. Bioactivity Study on Two Species of Scarab Beetle to Three Host Plants. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2018. [Google Scholar]
- Li, S.; Zhao, J.; Zhang, X.M.; Wang, W.J.; Luo, C.; Wang, S. Behavioral responses of Bemisia tabaci MED to 13 plant volatiles. Plant Prot. 2016, 43, 105–110. [Google Scholar]
- Rizwangul, A.; Cao, Y.Z.; Zhang, S.; Yin, J.; Li, X.; Li, K.B. Feeding preference and taxis behavior of adult Holotrichia oblita (Coleoptera: Scarabaeidae) on three plants. Acta Entomol. Sin. 2018, 61, 585–595. [Google Scholar]
- Han, Y.; Liu, K.; Wu, J.H.; Tang, L.D. The Behavioral Response of Frankliniella intonsa (Trybom) to Eleven Different Chemicals. Chin. J. Trop. Crops 2015, 36, 1646–1649. [Google Scholar]
- Chen, C.; Song, Q.S.; Zhang, G.M.; Peng, Y.Q.; Wang, Q.Y.; Yang, D.R. Chemical attraction of fig volatiles to their pollinating fig wasps. Acta Ecol. Sin. 2004, 24, 2794–2798. [Google Scholar]
- Stevens, M.M.; Faulder, R.J.; Mo, J.; Mudford, E.M.; Morris, S.G. Attraction of Parastethorus nigripes and other insect species to methyl salicylate and (Z)-3-hexenyl acetate dispensers in a citrus grove and vineyard in Southeastern Australia. Phytoparasitica 2017, 45, 639–649. [Google Scholar] [CrossRef]
- Yu, H.L.; Zhang, Q.; Xu, H.L. (Z)-3-hexenyl acetate synergizes the response of oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae) to female sex pheromone. J. Fruit Sci. 2015, 32, 469–473. [Google Scholar]
- Yu, H.; Feng, J.; Zhang, Q.; Xu, H. (Z)-3-hexenyl acetate and 1-undecanol increase male attraction to sex pheromone trap in Grapholita molesta (Busck) (Lepidoptera: Tortricidae). Int. J. Pest Manag. 2014, 61, 30–35. [Google Scholar] [CrossRef]
- Borden, J.H.; Chong, L.J.; Gries, G.; Gries, R.; Huber, D.P.W.; Pierce, H.D.; Wilson, I.M. Use of Benzaldehyde, Benzyl Alcohol and Nonanal to Protect Coniferous Trees from Attack by Bark Beetles. Patent US6051612A, 18 April 2000. [Google Scholar]
- Yang, G.; Zhang, Y.N.; Gurr, G.M.; Vasseur, L.; You, M.S. Electroantennogram and behavioral responses of Cotesia plutellae to plant volatiles. Insect Sci. 2016, 23, 245–252. [Google Scholar] [CrossRef] [PubMed]
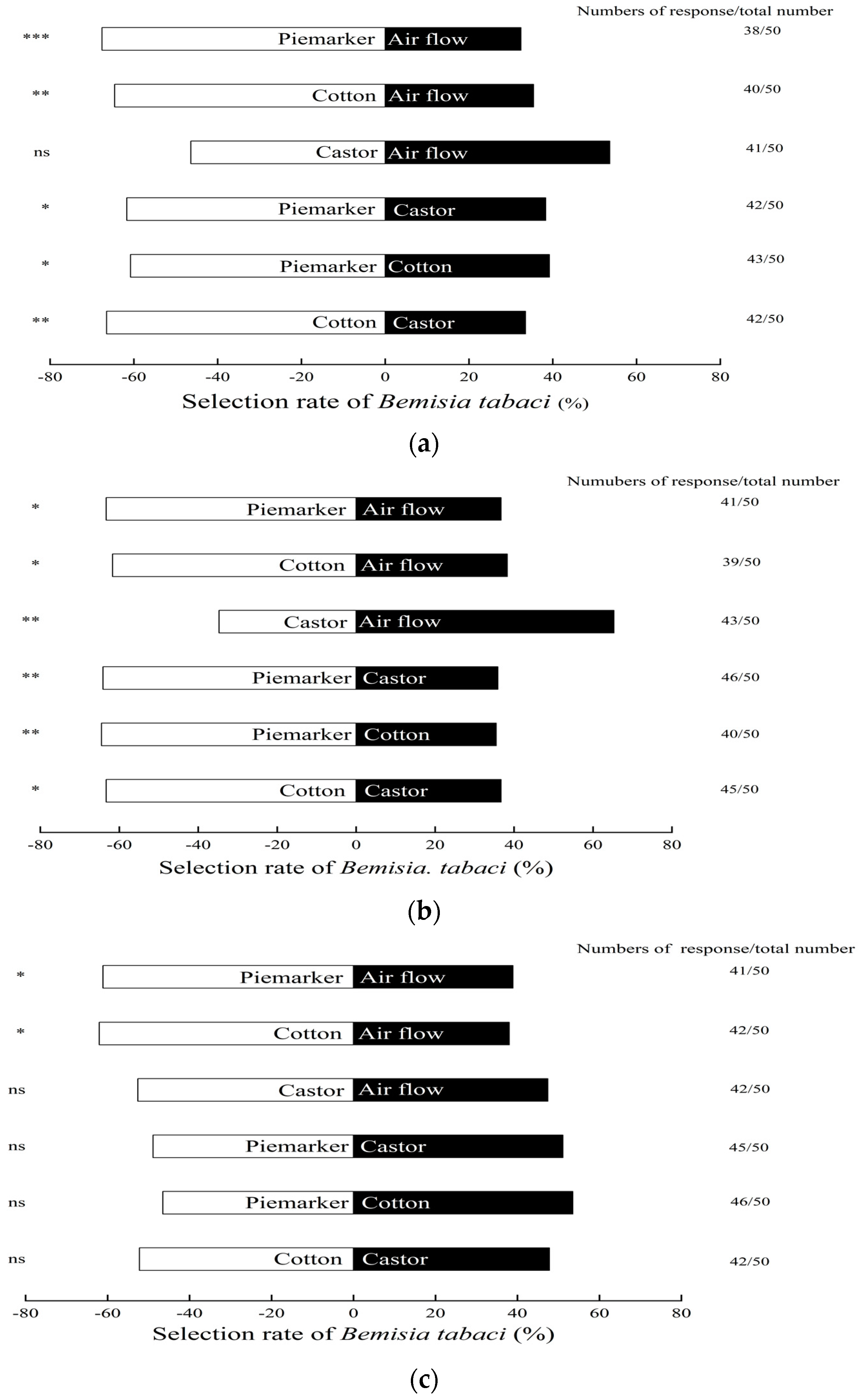
| Treatment | Treatment | Treatment | Treatment | ||
|---|---|---|---|---|---|
| pre-flowering piemarker | VS | air flow | pre-flowering piemarker | VS | pre-flowering cotton |
| pre-flowering cotton | VS | air flow | pre-flowering piemarker | VS | pre-flowering castor |
| pre-flowering castor | VS | air flow | pre-flowering cotton | VS | pre-flowering castor |
| florescence piemarker | VS | air flow | florescence piemarker | VS | florescence cotton |
| florescence cotton | VS | air flow | florescence piemarker | VS | florescence castor |
| florescence castor | VS | air flow | florescence cotton | VS | florescence castor |
| fruiting piemarker | VS | air flow | fruiting piemarker | VS | fruiting cotton |
| fruiting cotton | VS | air flow | fruiting piemarker | VS | fruiting castor |
| fruiting castor | VS | air flow | fruiting cotton | VS | fruiting castor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chen, W.; Zhang, S.; Chen, H.; Su, H.; Jing, T.; Yang, Y. Behavioral Responses of Bemisia tabaci Mediterranean Cryptic Species to Three Host Plants and Their Volatiles. Insects 2022, 13, 703. https://doi.org/10.3390/insects13080703
Liu Z, Chen W, Zhang S, Chen H, Su H, Jing T, Yang Y. Behavioral Responses of Bemisia tabaci Mediterranean Cryptic Species to Three Host Plants and Their Volatiles. Insects. 2022; 13(8):703. https://doi.org/10.3390/insects13080703
Chicago/Turabian StyleLiu, Zhe, Wenbin Chen, Shuai Zhang, Han Chen, Honghua Su, Tianxing Jing, and Yizhong Yang. 2022. "Behavioral Responses of Bemisia tabaci Mediterranean Cryptic Species to Three Host Plants and Their Volatiles" Insects 13, no. 8: 703. https://doi.org/10.3390/insects13080703
APA StyleLiu, Z., Chen, W., Zhang, S., Chen, H., Su, H., Jing, T., & Yang, Y. (2022). Behavioral Responses of Bemisia tabaci Mediterranean Cryptic Species to Three Host Plants and Their Volatiles. Insects, 13(8), 703. https://doi.org/10.3390/insects13080703






