Simple Summary
Chili thrips (Scirtothrips dorsalis) is an important pest of chili crops and a major vector of viral plant pathogens. Due to the widespread outbreak of thrips, chemical insecticides have been heavily used in the last few decades. To reduce the utilization of chemical pesticides, alternative biocontrol agents such as entomopathogenic fungi have been screened against the thrips. Laboratory screening revealed that 2 insect fungi isolates, Purpureocillium lilacinum TBRC 10638 and Beauveria bassiana BCC48145 were the most effective isolates against chili thrips. The fungus, P. lilacinum TBRC 10638 exhibited the highest efficacy against chili thrips in greenhouse and field trials and thus would be developed as a thrips control agent.
Abstract
In a laboratory assay, it was shown that B. bassiana BCC48145, BCC2660, and P. lilacinum TBRC10638 were the three strains that exhibited the highest insecticidal activity against chili thrips, causing 92.5% and 91.86% and 92.3% corrected mortality, respectively. The fungi B. bassiana BCC48145 and P. lilacinum TBRC10638 were selected for greenhouse spraying. Cytotoxicity test of the extracts from both fungi evaluated against 4 animal cell lines: KB; human oral cavity carcinoma, MCF7; human breast adenocarcinoma, NCI-H187; human small cell lung carcinoma and GFP-expressing Vero cells, showed none-cytotoxic to all cell lines. An efficacy validation in the greenhouse showed that P. lilacinum TBRC 10638 was more effective than B. bassiana BCC48145 and could control the thrips up to 80% when using the fungus at 108 spores/mL. The LC50 values of P. lilacinum TBRC 10638 against chili thrips based on total thrips count from two experiments were 1.42 × 108 and 1.12 × 107 spores/mL when the fungal spores were sprayed once a week. The optimal concentration of P. lilacinum TBRC 10638 spores for effective control of chili thrips was determined at 1.41 × 109 spores/mL. The average efficacy of P. lilacinum TBRC 10638 for thrips control from 3 field trials was 30.08%, 14.39%, and 29.92%. This result was not significantly different from that of the chemical insecticide treatment group, which showed efficacy at 19.27%, 14.92%, and 19.97%. Furthermore, there was no difference in productivity among the different treatment groups. Our results demonstrated that P. lilacinum TBRC 10638 is a promising biocontrol agent that could be used as an alternative to chemical insecticide for controlling chili thrips.
1. Introduction
Chili thrips (Scirtothrips dorsalis) is an important pest that affects more than 100 plant taxa around the world; including vegetable crops such as cucumber, pepper, and eggplant; fruit crops such as grape, lemon, and mangosteen; and ornamental crops such as rose and green buttonwood [1,2]. Chili thrips feed on all parts of plants, especially young leaves, buds, and fruits [3], on which the insect inflicts damage by extracting the contents of epidermal cells leading to necrosis of tissue. Thrips feeding on the leaves cause changes in tissue color from silver to brown and black. Chili thrips also transmit 7 viral pathogens including chili leaf curl (CLC) virus, peanut necrosis virus (PBNV) [4,5], and tobacco streak virus (TSV) in groundnut crops, as reported in India [6]. Recently, in Thailand chili thrips were reported as a vector of three tospoviruses i.e., melon yellow spot virus (MYSV), watermelon silver mottle virus (WsMoV), and capsicum chlorosis virus (CaCV) in field crops [7].
Many countries avoid insect pests by employing greenhouse cultivation, which nevertheless cannot prevent infestation by tiny insects such as whiteflies, mealybugs, and thrips. Normally, thrips are controlled by contact insecticides, but their habitats on plants especially on flowers or buds are well protected from chemical spray. Since it is difficult to achieve effective control of these insects, farmers are obligated to use high dosages of insecticides and increase spraying frequency [8]. As a consequence, insecticidal resistance has been reported in many countries. Thrips resistance to spinosad, which is a commonly used insecticide, has been reported in the USA [9], Australia [10], and China [11]. In Iran, it has been reported that Thrips tabaci populations are resistant to several classes of insecticides including diazinon and dichlorvos (organophosphorus), permethrin, and cypermethrin (pyrethroids), acetamiprid (neonicotinoids), spinosad (spinosyns) and azadirachtin (triterpenoids) [12].
Currently, Integrated Pest Management (IPM) systems for greenhouses utilize chemicals, parasites, predators, and entomopathogenic agents such as bacteria, viruses, and fungi. The entomopathogenic fungi are increasingly used for pest control in greenhouses, which provide a suitable environment for fungal growth. The entomopathogenic fungi that are widely used as biological controls include Beauveria bassiana against a broad range of insect pests such as diamondback moth (Plutella xylostella) [13], green peach aphid (Myzus persicae), whitefly (Bemisia tabaci), cicada (Lacobiasca formosana) [14] and cigarette beetle (Lasioderma serricorne) [15] Metarhizium anisopliae against sweet potato weevil (Cylas formicarius) [16], cotton bollworm (Helicoverpa armigera) [17] and termite (Odontotermes obesus) [18]; Paecilomyces fumosoroseus against whitefly (Bemisia argentifolii) [19] and tomato thrips (Ceratothripoides claratris) [20]; and Verticillium lecanii usually against aphid (Aphis gossypii) and whitefly (Trileurodes vaporariorum) [21]. For thrips control, there have been reports on the pesticidal activity of P. fumosorosea against C. claratris [20], M. anisopliae against T. tabaci [22], and B. bassiana and Purpureocillium lilacinus against Thrips palmi [23,24].
In this study, entomopathogenic fungi selected from the previous works were evaluated for the control of chili thrips (S. dorsalis) in laboratory, greenhouse, and field trials. The median lethal concentration (LC50) against the insect and cytotoxicity against mammalian cells were determined to assess the potential of these fungi for application in the field.
2. Materials and Methods
2.1. Entomopathogenic Fungal Isolates
Eight entomopathogenic fungal isolates selected from previous screening against tomato thrips (C. claratris) [20] and aphid (M. pericae) [25] (Table 1) were obtained from BIOTEC Culture Collection (BCC). They proved to be highly effective against both homopteran insects. The selected fungal isolates were cultured on Potato Dextrose Agar (DifcoTM, Sparks, MD, USA) at 25 °C for 7 days. Fungal spores were harvested from each fungal colony by adding 5 mL of 0.1% Tween 80 (Sigma-Aldrich, St. Louis, MO, USA) to the colony and scraping with a spatula. The spore suspension was transferred to a 15 mL centrifuge tube, mixed for 1 min, and then filtered using sterile gauze to separate hyphae from spore suspension. Spore concentration was adjusted to 2 × 108 spores/mL for bioassay against thrips.

Table 1.
List of fungal strains selected for laboratory screening against S. dorsalis.
2.2. Fungal Cultivation and Extraction
P. lilacinum TBRC10638 and Beauveria bassiana BCC48145 were maintained on potato dextrose agar at 25 °C for 14 days. Each agar culture was cut into 2 × 2 cm2 with a sterilized surgical knife and inoculated in a 250 mL Erlenmeyer flask containing 30 g rice grain added with 12 mL tap water. The solid cultures of both fungi were incubated at 2 °C for 14 days. Then, conidiospores of each strain were re-suspended in 25 mL of 0.1% Tween 80 (109 spores/mL) and these suspensions were diluted to the concentrations of 2 × 107–2 × 108 spores/mL as inoculum and starter culture.
The production of conidiospores and mycelia culture were conducted by using the same starter culture. For conidiospores preparation, 12 mL of starter culture was transferred into 10 plastic bags containing 200 g of rice grains autoclaved with 80 mL tap water and incubated at 25 °C for 10 days. Conidial spores were harvested and washed with 0.1% Tween 80 at least twice. Then, the wet spores were soaked in methanol for 2 days. Subsequently, the methanol extract of spores was extracted with ethyl acetate. Crude extracts were used for cytotoxicity test as described by Luangsa-ard et al., (2009) [26].
The mycelial cultivation of each fungal strain was carried out in potato dextrose broth (PDB). Twenty-five milliliters of starter culture were inoculated into a 1000 mL Erlenmeyer flask containing 250 mL PDB and incubated on a rotary shaker (200 rpm) at 25 °C for 7 days. Afterward, fungal culture was filtrated to separate wet mycelia and culture broth, which were then extracted for cytotoxicity test [26].
2.3. Cytotoxicity Test
The toxicity of P. lilacinum TBRC10638 and B. bassiana BCC48145 extracts was evaluated by resazurin microtiter plate assay [27] against animal cell lines: KB human oral cavity carcinoma; MCF7, human breast adenocarcinoma; NCI-H187, human small cell lung carcinoma; and GFP-expressing Vero cells, African green monkey kidney cell line transfected with plasmid carried gfp gene (pEGFP-N1, Clontech). KB (ATCC CCL-17), MCF7 (ATCC HTB-22), NCI-H187 (ATCC CRL-5804), and Vero (ATCC CCL-81) cell lines were obtained from the American Type Culture Collection (ATCC). KB and MCF7 cell lines were grown and maintained in Minimal Essential Medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum, 1 mM sodium pyruvate, 0.1 mg/mL insulin, 0.1 mM non-essential amino acid, and 1.5 g/L sodium bicarbonate. NCI-H187 cells were grown and maintained in RPMI-1640 supplemented with 15% heat-inactivated fetal bovine serum, 1 mM sodium pyruvate, 2.5 g/L Glucose, and 2.2 g/L sodium bicarbonate. GFP-Vero cells were grown and maintained in MEM supplemented with 10% heat-inactivated fetal bovine serum, 1 mM sodium pyruvate, 0.1 mM non-essential amino acid, 2.2 g/L sodium bicarbonate, and 0.8 mg/mL geneticin. All lines were incubated at 37 °C in a humidified incubator with 5% CO2.
Cytotoxicity assay was performed in 384-well plates in triplicate. Each well was added with 5 µL of fungal extract at 500 µg/mL in 5% dimethylsulfoxide (DMSO) and followed with 45 µ of KB, MCF7, NCI-H187, or GFP-Vero cell suspension at 2.2 × 104, 3.3 × 104, 6.7 × 104 or 3.3 × 104 cells/mL, respectively. In place of fungal extract, anti-cancer drugs were used as positive controls: ellipticine for GFP-Vero, ellipticine, and doxorubicin for KB and NCI-H187 and tamoxifen and doxorubicin for MCF7, whereas 5% DMSO was used as a negative control.
For GFP-Vero, cells were incubated at 37 °C in a humidified incubator with 5% CO2 for 4 days. Fluorescent signals were measured on day 0 and day 4 using the excitation and emission wavelengths of 485 and 535 nm., then the signal of day-4 was subtracted from that of day-0 before calculation. In the assays using other cell lines, cells were incubated at 37 °C in a humidified incubator with 5% CO2 for 3 days (for KB and MCF7) and 5 days (for NCI-H187). Afterward, 12.5 µL of 62.5 µg/mL resazurin solution was added to each well and the plates were further incubated at 37 °C for 4 h. Fluorescence was measured at 530 nm excitation and 590 nm emission wavelengths. The signals were then subtracted with a blank before calculation. The % cytotoxicity was calculated from subtracted fluorescent signals from extract-treated wells compared to the negative control. The % inhibition at 50% was used as a cut-off for cytotoxicity.
2.4. Insect Rearing
Chili thrips (S. dorsalis) colony was established with adults collected from a greenhouse at the Thailand Science Park and released on a 1-month-old potted plant of birds eye chili (Capsicum annuum) cultivars TVRV 758 that were covered with a 50 × 50 × 50 cm3 insect screen cages. The chili thrips would eventually migrate to the new plants and build up new colonies. The thrips would reach the second larval stage within 2 weeks and be ready for laboratory assays. New plants were replaced for thrips feeding every 2 weeks.
2.5. Laboratory Assay
Thrips assay in the laboratory was conducted in a 100 mm diameter Petri dish with a single chili leaf that was moisturized with cotton saturated with distilled water. Assays were performed in triplicate. In each replicate, 10 2nd instar larvae were gently released onto chili leaf using a small paint brush and fungal spore suspension was sprayed by a glass nozzle sprayer with 1 bar pressure. The Petri dish was then covered with a lid. For negative control, 0.1% Tween 80 was used instead of fungal spore suspension. Distilled water was added daily for fresh leaves reservation. Mortalities were scored after 5 days of incubation at 25 °C ± 1.85% relative humidity and 12 h daily photoperiod.
2.6. Greenhouse Test
Two best-performing fungal strains were selected from laboratory assay to compare efficacy in greenhouse test by spray application. The experiment was conducted in an evaporative cooling greenhouse at the Thailand Science Park, starting from April to November 2015. The potted plants of birds eye chili (C. annuum) cultivars TVRV 758 were used as hosts for chili thrips (S. dorsalis). Ten adults of thrips were released per plant and the insect populations would naturally increase within 2 weeks and ready for the experiments. All spray applications were made with the knapsack sprayer.
2.6.1. Comparison between B. bassiana (BCC48145) and P. lilacinum (TBRC10638)
Greenhouse tests were undertaken to compare the effects of r B. bassiana BCC48145 and P. lilacinum TBRC10638 application on the thrips population. Three treatments, including B. bassiana BCC48145 and P. lilacinum TBRC10638 spore suspension and spore diluent control, were compared using Randomized Complete Block Design (RCBD) with 3 replications. Ten potted plants infested with thrips were set for each replication in the greenhouse. The plants in each replication were sprayed with 15 mL of either fungal spore suspension containing 2 × 108 spores/mL in 0.1% Tween 80 containing 0.04% APSA (AMWAY®) or spore diluent (0.1% Tween 80 containing 0.04% APSA) for the control. The commercial surfactant APSA was added to help spread and protect fungal spores from UV radiation. Spraying was performed using a sprayer with 3 bar pressure. Spore suspension was applied weekly for 3 consecutive weeks before the determination of the insect population by direct counting. Results from the first greenhouse test were confirmed in another repeat of the experiment.
2.6.2. Median Lethal Concentration (LC50) Value Determination
For LC50 value determination, greenhouse tests using RCBD with five treatments and three replications were performed in two repeats of the experiment. Thrips-infested chili plants were prepared as described above. The treatments included spraying with 15 mL of P. lilacinum TBRC10638 spore suspensions at the concentrations of 2 × 105, 2 × 106, 2 × 107 and 2 × 108 spores/mL in 0.1%Tween 80 containing 0.04% APSA, and spore diluent (0.1%Tween 80 containing 0.04% APSA). Each treatment was applied in a single foliar spray for the estimation of control efficiency.
To evaluate the effects of treatments, pre-application sampling was made a day before each application and post-application sampling was made 7 days after spray application. Pre- and post-application samplings were carried out by direct counting of thrips on the three uppermost terminal leaves [28] from 30% of plants in each replication. Thrips populations were computed using the following formula to determine the control efficiency of the fungal strains according to Püntener (1981) [29].
where
Control efficiency = [1 − (Ta/Ca × Cb/Tb)] × 100
- Ta = Infestation in the treatment plot after application
- Tb = Infestation in the treatment plot before application
- Ca = Infestation in the control plot after application
- Cb = Infestation in the control plot before application
2.7. Field Trials
Two field trials were carried out to evaluate the effects of P. lilacinum TBRC10638 application on natural thrips infestation at the Tropical Vegetable Research Center (TVRC), Kasetsart University Kamphaeng Saen Campus, Nakhon Pathom Province, Thailand. Each experiment used approximately 975 m2 of land. One-month-old seedlings of C. annum cultivars TVRC 365 (spur chili) were transplanted in 20 5 × 5 m2 plots arranged in five columns and four rows with 2-m intervals. Each plot consisted of three 1.5 × 5 m2 rows, in which the seedlings were transplanted at 0.5 m apart from each other. Based on the LC99 values obtained from the greenhouse tests, fungal spores were prepared as suspensions containing 1.41 × 108, 1.41 × 109, 1.41 × 1010 spores/mL in 0.1% Tween 80 and 0.04% APSA. Five treatments including fungal spore suspensions at three concentrations, Imidacloprid (U dara 10®) diluted at the rate of 2 mL/L of water containing 0.04% APSA, and control (0.1% Tween 80 containing 0.04% APSA) were arranged in RCBD with 4 replications. The plants were sprayed one week after transplanting, and spraying was repeated weekly for 3 consecutive weeks. Spraying resumed when chili plants began to flower, once a week for 3 consecutive weeks. The same regimen was repeated at one-week intervals until harvesting. Two trials were conducted: the first trial in July-September 2017 and the second trial in October–December 2017.
Thrips populations were randomly counted from chili tips of 10 plants from each plot, 1 day before spraying and 7 days after spraying. Counting was made on a weekly basis until harvesting. Thrips populations were computed to determine the control efficiency of the fungal strains according to Püntener (1981) [28].
An assessment of the insecticidal effects of P. lilacinum TBRC 10638 and imidacloprid on the relative abundance of target and non-target insects present in the crop was carried out during the trials. The density of thrips (no identification), aphids, spiders, and parasitic wasps were monitored using a yellow sticky trap (7 × 11 inches). The traps were placed at both ends of plots [29]. At the end of each week for the entire cultivation period, the traps were collected for insect count and replaced by new traps. The numbers of non-target insects were counted under a stereo microscope.
2.8. Statistical Analysis
For laboratory assay, the corrected mortality [28] of each fungal strain was transformed by Arcsin transformation prior to one-way analysis of variance (ANOVA) (p value < 0.05), and means of corrected mortalities were compared using Duncan′s multiple range test ((DMRT) with SPSS11.5 program (SPSS for Windows v.11.5, IBM, www.ibm.com accessed on 1 February 2022). The strains exhibiting the highest efficacy against thrips were selected for greenhouse experiments to determine control efficiency, median lethal concentration (LC50), and 99% lethal concentration (LC99) values using Probit analysis.
For greenhouse experiments and field trials, the control efficiencies from each experiment were subjected to one-way ANOVA) (p value < 0.05) and mean comparison using Duncan′s multiple range test (DMRT) with an SPSS11.5 program.
3. Results
3.1. Evaluation of Entomopathogenic Fungi Insectidal Activity
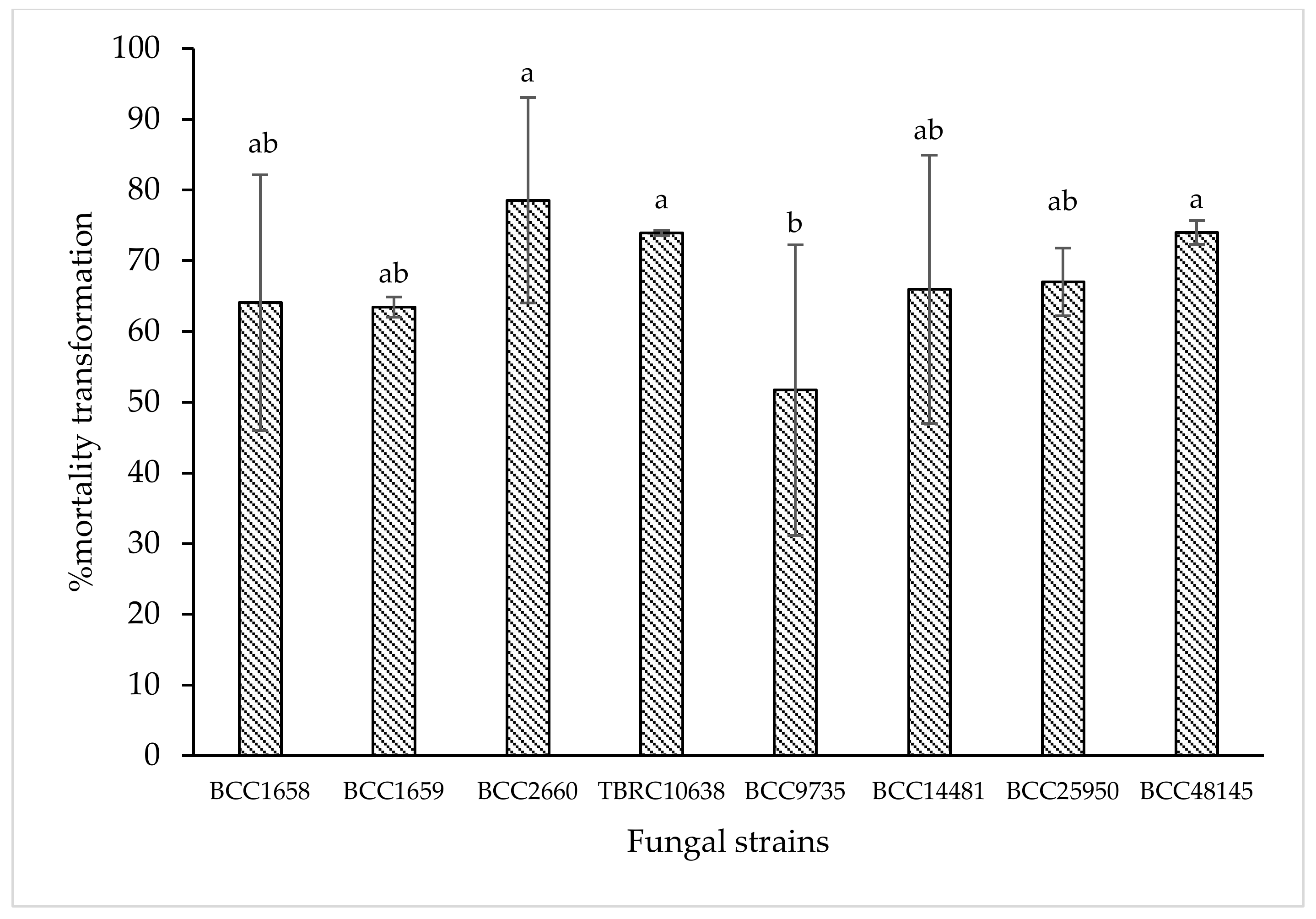
Data from laboratory assay showed that B. bassiana BCC2660, BCC48145, and P. lilacinum TBRC10638 were the three most effective strains that caused 78.55%, 73.98%, and 73.93% corrected mortality against chili thrips, while B. bassiana BCC25950, BCC14481, BCC1658, and I. fumosorosea BCC1659 were second ranking with 67.01%, 65.97%, 64.06%, and 63.45% corrected mortality, respectively (p < 0.05, Duncan in the repeated measures model). Based on these results, B. bassiana BCC48145 and P. lilacinum TBRC10638 were selected for further cytotoxicity and greenhouse tests.
3.2. Cytotoxicity Results
The yield of extract from P. lilacinum TBRC10638 spores produced by cultivation on rice grains was 169 mg/g spore, which was less than the extract yield of 519 mg/g from B. bassiana BCC48145 spores. From culture filtrates, B. bassiana BCC48145 provided extract yields of 12.3 mg/L in static condition and 13.2 mg/L in shaken condition, which were higher than extract yields of 6.3 mg/L from static culture and 3.5 mg/L shaken culture of P. lilacinum TBRC10638 (Table 2). All fungal extracts were tested against KB, MCF7, NCI-H187, and GFP-expressing Vero cell lines. Results indicated that none of these extracts showed cytotoxicity toward these cell lines when tested at the concentration of 50 µg/mL (Table 2). On the other hand, the anti-cancer drugs employed as positive controls exhibited different levels of cytotoxicity towards the four cell lines (footnotes of Table 2).

Table 2.
Cytotoxicity test of the extracts from P. lilacinum TBRC10638 and B. bassiana BCC48145 against 4 animal cell lines.
3.3. Comparing the Efficacy of B. bassiana (BCC48145) and P. lilacinum (TBRC10638) under Greenhouse Conditions
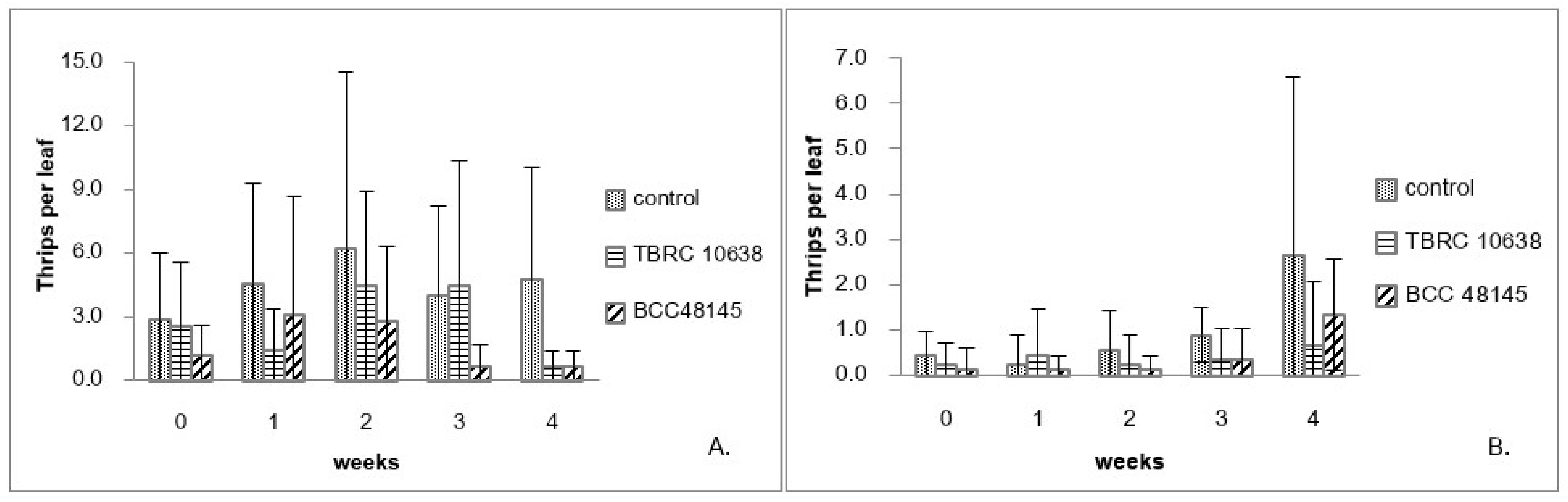
Results from the laboratory assay showed that the fungi B. bassiana BCC48145 and P. lilacinum TBRC10638 were highly virulent against chili thrips (Figure 1). Hence, they were chosen for greenhouse applications. At the beginning of the first experiment performed in April 2015, the mean of thrips populations from the control plants at approximately 2.9 insects/Leaf was higher than those of other treatments and increased to 6 insects/Leaf in the second week of the experiment. Afterward, chili plants were destroyed by thrips before they migrated to the new plants (Figure 2A). On the plants treated with B. bassiana BCC48145, the thrips population started at approximately 1.2 insects/Leaf and increased to 3 insects/Leaf after one week. Nevertheless, B. bassiana BCC48145 could control the thrips population in the following weeks (Figure 2A). On plants treated with P. lilacinum TBRC10638, the thrips population started from 2.6 insects/Leaf and gradually increased until reaching its peak at week 2 and then declined to 0.7 insects/Leaf by week 4. The control efficiency of each fungus was determined from the percentage of thrips population that was reduced after fungal treatment, using thrips population data normalized by those of the control group. Results showed that the control efficiency of P. lilacinum TBRC10638 (64.67%) was no different from that of B. bassiana BCC48145 (56.08%) (Table 3) under an average temperature of 28.33 °C ± 1.37 and humidity of 90.42% ± 7.68 in the greenhouse.
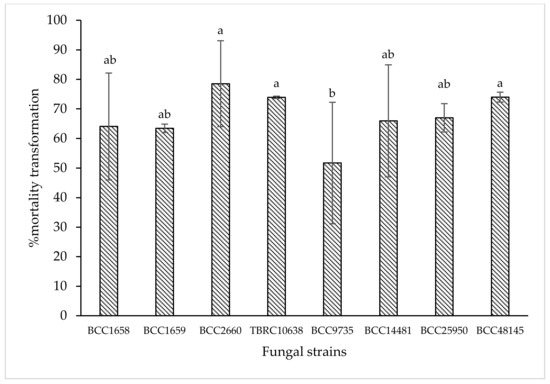
Figure 1.
Means of % corrected mortality obtained from insect mortality assay at 5 days after treatment with 8 entomopathogenic fungi against chili thrips (S. dorsalis) in a laboratory. Means indicated by the same letter are not significantly different according to Duncan′s multiple range test (p < 0.05).
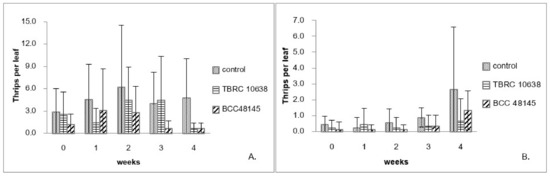
Figure 2.
Number of chili thrips (S. dorsalis) on chili plants sprayed with P. lilacinum TBRC10638 and B. bassiana BCC48145 in the greenhouse. (A). experiment 1 (April–May 2015) and (B). experiment 2 (June–July 2015).

Table 3.
Control efficacy of the fungi B. bassiana BCC48145 and P. lilacinum TBRC10638 against chili thrips (S. dorsalis) in the greenhouse experiment.
The second experiment was done in June 2015, the thrips population in all treatment groups was between 0.1–0.4 insects/plant at the start of this experiment. In the control treatment, the thrips population gradually increased until reaching 2.7 insects/Leaf in the last week (Figure 2B). On the other hand, P. lilacinum TBRC10638 application kept the thrips population below 0.7 insects/Leaf throughout the experiment, while B. bassiana BCC48145 application resulted in a thrips population of 1.3 insects/Leaf in the last week after the spraying program (Figure 2B). Consistent with results from the first experiment, the fungus B. bassiana BCC48145 exhibited lower control efficiency than P. lilacinum TBRC10638 (Table 3) under the average temperature of 28.35 °C ± 1.29 and humidity of 90.48% ± 7.57 in the greenhouse. Based on these results, the fungus P. lilacinum TBRC10638 was selected for the determination of LC50 values in the next step.
3.4. Determination of Lethal Concentration Values
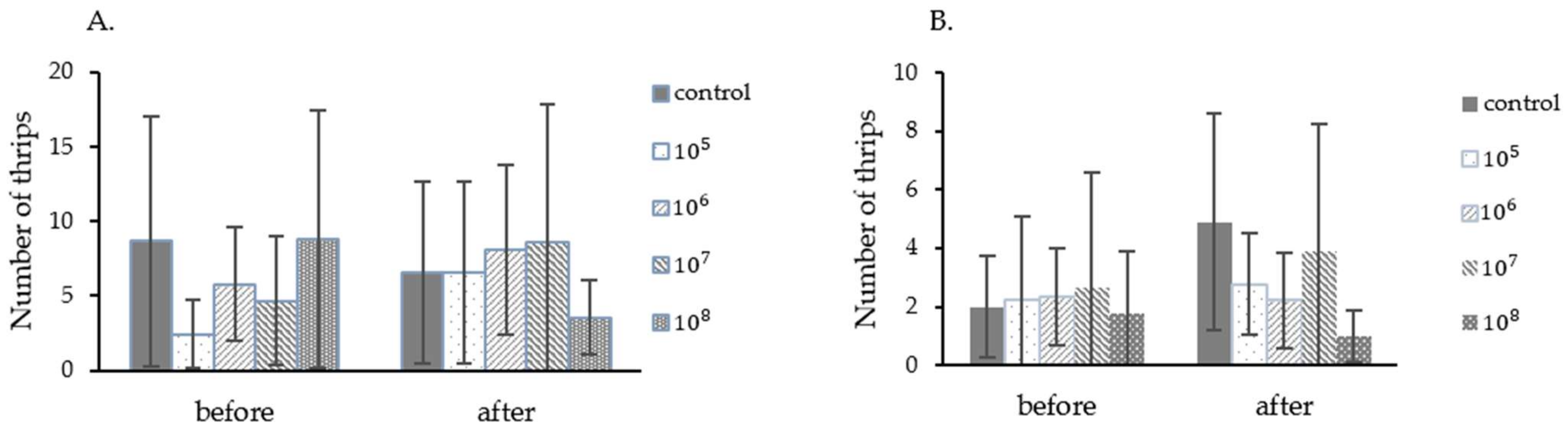
In this experiment, the fungus P. lilacinum TBRC10638 at the concentration of 2 × 105, 2 × 106, 2 × 107, 2 × 108 spores/mL, and 0.1% Tween 80 were applied in a single foliar spray in order to determine the LC50 value. In the first experiment, starting thrips populations in different treatment groups varied from 2.4 to 8.8 insects/Leaf (Figure 3A). After application, only in the treatment using 2 × 106 and 2 × 108 spores/mL did the fungus exhibit an ability to control the thrips population. Chili plants from this treatment also displayed less damage from thrips than those in other groups. In contrast, thrips populations in other treatments increased in one week. The control efficiency of each treatment was calculated from thrips population data and then submitted to probit analysis. The LC50 value obtained from this analysis was 1.42 × 108 spores/mL (Table 4) under the average temperature of 28.37 °C ± 1.45 and humidity of 83.48% ± 10.82 in the greenhouse.
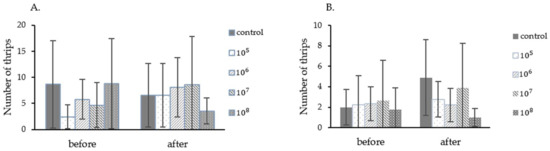
Figure 3.
Chili thrips populations on chili plants during the determination of P. lilacinum TBRC10638 LC50 values performed in the greenhouse. (A). Experiment 1 (September–October 2015), (B). Experiment 2 (October–November 2015).

Table 4.
Estimated lethal concentration values (spores/mL) of P. lilacinum TBRC10638 against chili thrips (S. dorsalis) in the greenhouse.
In the second experiment, starting chili thrips populations in different treatment groups varied from 1.78 to 2.67 insects/Leaf (Figure 3B). All treatments showed effectiveness against thrips at 7 days after spraying, with the control efficiency of 48.70%, 56.14%, 38.70%, and 68.56% when fungal spores at the concentration of 2 × 105, 2 × 106, 2 × 107, and 2 × 108 spores/mL were applied, respectively (data not shown). The LC50 value obtained after probit analysis were 1.24 × 107 spores/mL under the average temperature of 27.49 °C ± 1.49 and humidity of 83.85% ± 9.41 in the greenhouse. Notably, the LC50 value obtained from the second experiment was 10 times lower than that of the first experiment. As often the case for biological controls, the entomopathogenic fungus controls the thrips population more effectively at a lower than higher thrips infestation rate.
3.5. Evaluation of Fungal Spray Application in Field Trials
Thailand has a tropical climate with 3 distinct seasons; a hot season runs from March to mid-May, a rainy season from mid-May to October and a dry and relatively cool season normally runs from November to February (https://www.tmd.go.th/en/ accessed on 1 February 2022). The first field trial was conducted in the mid-rainy season (July–September 2017) with an annual rainfall of 3.4 mm (30 times/crop). The ambient temperatures were between 24.8–34.2 °C. The second field trial was conducted in the late rainy season (October–December 2017) with an average annual rainfall of 4.2 mm (22 times/crop). The ambient temperatures are between 22.8 °C and 31.0 °C.
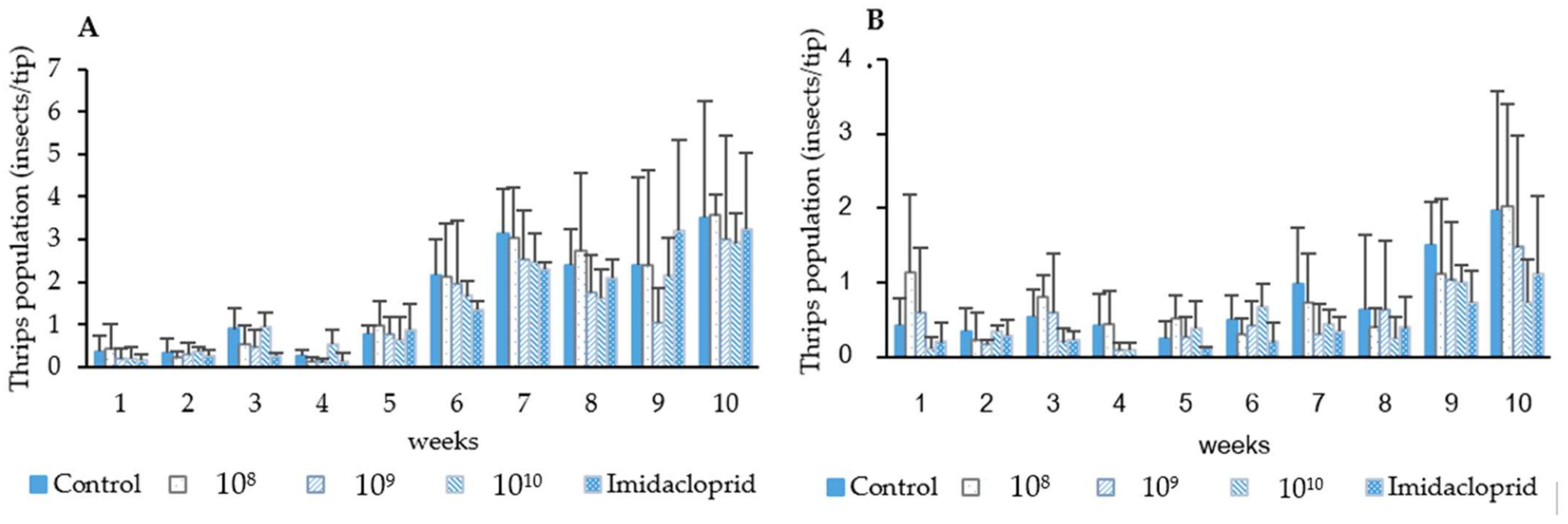
Thrips population samples taken before spray application indicated that during the first and second field trials thrips infestation rates did not exceed 1 thrips/chili tip. After spray applications, thrips densities were maintained in both the plots treated with chemical insecticide (imidacloprid) and P. lilacinum as compared with the untreated control during the two trials. Till the fifth week, the fungal control efficacy ranged between 0–51.47% in the first trial and up to 90% in the second trial. There was no significant difference in the efficacy between the fungal treatments and imidacloprid treatment in both the first and second trials (F = 9.28, df = 3, p = 0.05). Starting from week 6 (chili flowering stage), thrips populations began to increase in all treatment plots, from 1.33–2.17 thrips/chili tip. However, there were no significant difference in the efficacy between the fungal and insecticidal treatments until the week 10 (1st trial; F = 0.170, df = 3, p = 0.916, 2nd trial; F = 0.113, df = 3, p = 0.952). An exception was found in 1 treatment that was sprayed with fungal spores at the concentration of 1.41 × 108 spores/mL in the second trial, where the fungal spores were less effective than insecticide (F = 7.32, df = 4, p = 0.05). The control efficacy from weeks 6–10 was between 0–39.97% in the first trial and 0–54.11% in the second trial (Figure 4 and Table 5).
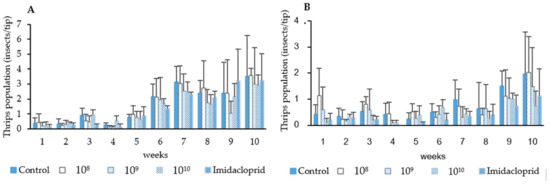
Figure 4.
Effect of P. lilacinum TBRC10638 and imidacloprid on chili thrips (S. dorsalis) populations during the first (A) and second trial (B).

Table 5.
Control efficiency and chili yield (Kg/rai) of the chili plots sprayed with P. lilacinum TBRC10638 at the concentration of 1.4 × 108–1.4 × 1010 spores/mL and imidacloprid during the first and second trials.
Application of three concentrations of P. lilacinum BCC10638 and imidacloprid significantly reduced the severity of damage by S. dorsalis on chili as recorded by visual scores during the two trials. Different treatments showed no significant increase in yield of chili fruit during the first trial; 121.36 ± 15.98, 143.08 ± 32.32, 128.45 ± 28.49, 105.14 ± 25.55 and 137.20 ± 41.61 kg/rai (F = 0.243; df = 4, 15; p = 0.909) and the second trial; 293.93 ± 46.94, 277.55 ± 39.16, 303.90 ± 50.49, 344.78 ± 41.67 and 335.10 ± 42.09 kg/rai (F = 0.406; df = 4, 15; p = 0.801), respectively (Table 5).
Effects of fungal spores and imidaclopid application on non-target insects were monitored by using sticky traps. Results showed the presence of insect pests; thrips and aphids, natural enemies; and spiders and parasitic wasps. An increase in the number of thrips and aphids in the test area was observed after application. Moreover, there were no significant differences with 95% confidence in the densities of spiders and parasitic wasps among the different treatments. Spray application of all fungal spore suspensions and chemicals did not affect the populations of these natural enemies (Table 6). Results of both trials suggested that the natural enemies would increase their populations according to the number of pests that entered the test area.

Table 6.
Impact assessment of P. lilacinum TBRC 10638 and imidacloprid applications on the number of non-target insects (mean ± SE) during the first and second trials.
4. Discussion
Screening results showed that B. bassiana BCC48145 and BCC2660 and P. lilacinum TBRC10638 were the most virulent strains against chili thrips (S. dorsalis). This was not surprising since B. bassiana is generally used for insect pest control especially for insects such as chili thrips (S. dorsalis) [30] and aphid (M. persicae) [31], although it should be noted that the insect host of B. bassiana BCC2660 was an adult of Coleopteran insect. The fungus P. lilacinum TBRC10638 has been used for biocontrol of nematode pests including Radopholus similis, Heterodera spp., Globodeera spp. [32,33,34] and shown to kill many insect species such as T. palmi in orchid farms [24], Mediterranean fruit fly (Ceratitis capitate), Nettle caterpillar (Setora nitens), Cotton aphids (A. gossypi), and Winchuka (Triatoma infestans) [35]. Thus, both B. bassiana and P. lilacinum are considered broad-spectrum fungi.
This study highlighted the potential use of entomopathogenic fungi against chili thrips (S. dorsalis) in the greenhouse by comparing the two fungal strains, P. lilacinum TBRC10638 and B. bassiana BCC48145, which were the two out of the three most effective strains against chili thrips according to the laboratory screening results. Although B. bassiana had been reported to provide effective control against thrips in the greenhouse [36], its efficacy was lower than P. lilacinum TBRC10638. The fungus Purpureocillium lilacinum is the new genus name of Paecilomyces lilacinus that has increasingly been reported as the causal agent of infections in man and other vertebrates [37]. Therefore, a preliminary test on the toxicity of this fungal strain toward mammalian cell lines was conducted. Results confirmed that extracts from spores and mycelial culture of P. lilacinum TBRC10638 displayed no cytotoxic effect on all cell lines tested, consistent with the report on Paecilomyces lilacinus strain 251, which is not toxic or pathogenic to mammals and approved for residential use [38]. Results from cytotoxicity tests suggested that P. lilacinum TBRC10638 and B. bassiana BCC48145 are safe to be used as thrips control.
The fungus P. lilacinum TBRC10638 provided better control against thrips in both greenhouse experiments than B. bassiana BCC48145, which showed the control efficiency of 64.67% and 72.46% for in the first and second experiment, respectively. In the 2nd experiment, due to the surge in the thrips population, the control efficacy of B. bassiana BCC48145 was quite low. We observed the high variability of results within and between the first and second experiments, which could be attributed to the movement of thrips between plants as reinfestation by the new migrant thrips has been described elsewhere [36].
Effective mycoinsecticides are expected to stay for a long period on the leaves and provide rapid control of the insect pest. P. lilacinum TBRC10638 exhibited the ability to promptly control the thrips population. According to Arthurs et al., (2013) [36], two mycoinsecticides and other bio-rational insecticides applied at 7 to 14 days intervals reduced overall S. dorsalis populations on pepper plants C. annuum cv. California Wonder in the greenhouse. B. bassiana GHA reduced S. dorsalis population by 81–94% and I. fumosorosea PFR-97 by 62–66%. Relatively more species and strains of entomopathogenic fungi had been tested against F. occidentalis. Sengonca et al., (2006) [39] tested 41 entomopathogenic fungi isolates from Thailand belonging to 25 species and 11 genera against first instar F. occidentalis larvae on bean leaves. Among the 14 most virulent isolates, LC50 values of Beauveria spp. ranged from 2.4 × 104 to 5.9 × 106 conidia/mL, Metarhizium spp. from 2.0 × 104 to 5.0 × 105 conidia/mL, and Isaria spp. from 3.9 × 104 to 5.5 × 106 conidia/mL.
In each field trial, the control efficiencies of fungi and chemicals fluctuated as the tests were conducted during the rainy season. The regular rainfall may wash away the fungal suspension, despite the addition of a commercial sticker. Control efficacies of fungal suspensions and chemicals of approximately 10–15% were not statistically different. However, more thrips mortality was observed in the second crop, which took place in the year with less rainfall. The influence of climate variation on trial results was also mentioned by Kirk (1997) and Maniania et al., (2003) [22,40].
In conclusion, mycoinsecticides may be used to control S. dorsalis and provide proper solutions for chemical insecticide resistance. Mycoinsecticides may be most effective in pest management programs integrated with beneficial insect pests, or in greenhouse crops where favorable environmental conditions (high humidity and low UV exposure) can be manipulated [37]. Additional research to optimize the utilization of entomopathogenic fungi—for example, through spore yield optimization, spray formulation, and storage formulation—is considered important for the improvement of insect pest control strategy.
Author Contributions
Conceptualization, C.P. and V.V.; Investigation, C.P., S.S., S.V., R.C. and T.B.; Methodology, C.P., S.S. and S.V.; Visualization, C.P. and V.V.; Writing—original draft, C.P.; writing—review and editing, C.P. and V.V.; project administration, C.P.; funding acquisition, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from National Science and Technology Development Agency (NSTDA), Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Information presented in this study can be obtained from Related authors. The information is not publicly available due to contractual obligations with research funding sources.
Acknowledgments
We thank Tipvadee Attathom and Boonhiang Promdonkoy for help with editing the manuscript. This research was supported by National Science and Technology Development Agency (NSTDA) and National Center for Genetic Engineering and Biotechnology (BIOTEC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seal, D.R.; Ciomperlik, M.; Richards, M.L.; Klassen, W. Comparative effectiveness of chemical insecticides against the chili thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), on pepper and their compatibility with natural enemies. Crop Prot. 2006, 25, 949–955. [Google Scholar] [CrossRef]
- Arthurs, S.; McKenzie, C.L.; Chen, J.; Dogramaci, M.; Brennan, M.; Houben, K.; Osborne, L. Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chili thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol. Control 2009, 49, 91–96. [Google Scholar] [CrossRef]
- Dogramaci, M.; Arthurs, S.P.; Chen, J.; McKenzie, C.; Irrizary, F.; Osborne, L. Management of chili thrips Scirtothrips dorsalis (Thysanoptera: Thripidae) on peppers by Amblyseius swirskii (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae). Biol. Control 2011, 59, 340. [Google Scholar] [CrossRef]
- Mound, L.A.; Palmer, J.M. Identification, distribution and host plants of the pest species of Scirtothrips (Thysanoptera: Thripidae). Bull. Entomol. Res. 1981, 71, 467–479. [Google Scholar] [CrossRef]
- Ananthakrishnan, T.N. Bioecology of Thrips; Indira Publishing House: Oak Park, MI, USA, 1984; p. 223. [Google Scholar]
- Rao, P.R.D.V.J.; Reddy, A.S.; Reddy, S.V.; Thirumala-Devi, K.; Chander Rao, S.; Manoj Kumar, V.; Subramaniam, K.; Yellamanda Reddy, T.; Nigam, S.N.; Reddy, D.V.R. The host range of Tobacco streak virus in India and transmission by thrips. Annals Appl. Biol. 2003, 142, 365–368. [Google Scholar]
- Chiemsombat, P.; Gajanandana, O.; Warin, N.; Hongprayoon, R.; Bhunchoth, A.; Pongsapich, P. Biological and molecular characterization of tospoviruses in Thailand. Arch. Virol. 2008, 153, 571–577. [Google Scholar] [CrossRef]
- Rebek, E.J.; Schnelle, M.A. Arthropod Pest Management in Greenhouses and Interiorscapes. 2013. Available online: https://www.researchgate.net/publication/280948324 (accessed on 1 February 2022).
- Irnmaraju, J.A.; Paine, T.D.; Bethke, J.A.; Robb, K.L.; Newman, J.P. Western flower thrips (Thysanoptera: Thripidae) resistance to insecticides in coastal Californian greenhouses. Econ. Entomol. 1992, 85, 9–14. [Google Scholar] [CrossRef]
- Herron, G.A.; James, T.M. Insecticideresistance in Australian populations of western flower thrips, Frankliniella occidentalis Pergande (Thysanoptera: Thripidae). Gen. Appl. Entomol. 2007, 36, 1–6. [Google Scholar]
- Zhang, S.Y.; Kono, S.; Murai, T.; Miyata, T. Mechanisms of resistance to spinosad in the western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Insect Sci. 2008, 15, 125–132. [Google Scholar] [CrossRef]
- Nazemi, J.; Khajehali, T.; Leeuwen, V. Incidence and characterization of resistance to pyrethroid and organophosphorus insecticides in Thrips tabaci (Thysanoptera: Thripidae) in onion fields in Isfahan, Iran. Pestic. Biochem. Physiol. 2016, 129, 28–35. [Google Scholar] [CrossRef]
- Duarte, R.T.; Gonc¸alves, K.C.; Espinosa, D.J.L.; Moreira, L.F.; Bortoli, S.A.; De Humber, R.A.; Polanczyk, R.A. Potential of Entomopathogenic Fungi as Biological Control Agents of Diamondback Moth (Lepidoptera: Plutellidae) and Compatibility with Chemical Insecticides. J. Econ. Entomol. 2016, 109, 594–601. [Google Scholar] [CrossRef]
- Ali Bugti Ghulam, G.A.; Bin, W.; Na, C.; Feng, L.H. Pathogenicity of Beauveria bassiana strain 202 against sapsucking insect pests. Plant Protect. Sci. 2017, 54, 111–117. [Google Scholar] [CrossRef]
- Saeed, M.B.E.E.E.M.; Laing, M.D.; Miller, R.M.; Bancole, B. Ovicidal, larvicidal and insecticidal activity of strains of Beauveria bassiana (Balsamo) Vuillemin against the cigarette beetle, Lasioderma serricorne Fabricius (Coleoptera: Anobiidae), on rice grain. J. Stored Prod. Res. 2017, 74, 78–86. [Google Scholar] [CrossRef]
- Dotaona, R.; Wilsonb, B.A.L.; Ashb, G.J.; Hollowayc, J.; Stevens, M.M. Sweetpotato weevil, Cylas formicarius (Fab.) (Coleoptera: Brentidae) avoids its host plant when a virulent Metarhizium anisopliae isolate is present. J. Invertebr. Pathol. 2017, 148, 67–72. [Google Scholar] [CrossRef]
- Jarrahi, A.; Safavi, S.A. Effects of pupal treatment with Proteus (R) and Metarhizium anisopliae sensu lato on functional response of Habrobracon hebetor parasitising Helicoverpa armigera in an enclosed experiment system. Biocontrol Sci. Technol. 2016, 26, 1–15. [Google Scholar] [CrossRef]
- Balachander, M.; Remadevi, O.K.; Sasidharan, T.O. Dissemination of Metarhizium anisopliae infection among the population of Odontotermes obesus (Isoptera: Termitidae) by augmenting the fungal conidia with attractants. J. Asia-Pac. Entomol. 2013, 16, 199–208. [Google Scholar] [CrossRef]
- James, R.R.; Buckner, J.S.; Freeman, T.P. Cuticular lipids and silverleaf whitefly stage affect conidial germination of Beauveria bassiana and Paecilomyces fumosoroseus. J. Invertebr. Pathol. 2003, 84, 67–74. [Google Scholar] [CrossRef]
- Panyasiri, C.; Attathom, T.; Poehling, H.-M. Pathogenicity of entomopathogenic fungi-potential candidates to.control insect pests on tomato under protected cultivation in Thailand. J. Plant Dis. Prot. 2007, 114, 278–287. [Google Scholar] [CrossRef]
- Kikuchi, O.; Satoh, K. Effect of Fungicides on Entomopathogenic Fungus, Verticillium lecanii. Jpn. J. Appl. Entomol. Zool. 1998, 42, 107–113. [Google Scholar] [CrossRef]
- Maniania, N.K.; Sithanantham, S.; Ekesi, S.; Ampong-Nyarko, K.; Baumgartner, J.; Lohr, B.; Matoka, C.M. A field trial of the entomopathogenous fungus Metarhizium anisopliae for control of onion Thrips Tabaci. Crop Prot. 2003, 22, 553–559. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ugineb, T.A.; Ramosc, M.E.; Sanderson, J.P. Efficacy of spray applications of entomopathogenic fungi against western flower thrips infesting greenhouse impatiens under variable moisture conditions. Biol. Control 2016, 97, 31–47. [Google Scholar] [CrossRef]
- Hotaka, D.; Amnuaykanjanasin, A.; Maketon, C.; Siritutsoontorn, S.; Maketon, M. Efficacy of Purpureocillium lilacinum CKPL-053 in controlling Thrips palmi (Thysanoptera: Thripidae) in orchid farms in Thailand. Appl. Entomol. Zool. 2015, 50, 317–329. [Google Scholar] [CrossRef]
- Amnuaykanjanasin, A.; Jirakkakul, J.; Panyasiri, C.; Panyarakkit, P.; Nounurai, P.; Chantasingh, D.; Eurwilaichitr, L.; Cheevadhanarak, S.; Tanticharoen, M. Erratum to: Infection and colonization of tissues of the aphid Myzus persicae and cassava mealybug Phenacoccus manihoti by the fungus Beauveria Bassiana. Biol. Control 2012, 58, 393–396. [Google Scholar] [CrossRef][Green Version]
- Luangsa-ard, J.J.; Berkaew, P.; Ridkaew, R.; Hywel-Jones, N.; Isaka, M. A beauvericin hot spot in the genus Isaria. Mycol. Res. 2009, 113, 1389–1395. [Google Scholar] [CrossRef]
- O′Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Püntener, W. Evaluation of trial-Calculation of efficacy. In Manual for Field trials in Plant Protection, Agricultural Division; Ciba-Geigy Limited: Basel, Switzerland, 1981. [Google Scholar]
- Broughton, S.; Harrison, J. Evaluation of monitoring methods for thrips and the effect of trap colour and semiochemicals on sticky trap capture of thrips (Thysanoptera) and beneficial insects (Syrphidae, Hemerobiidae) in deciduous fruit trees in Western Australia. Crop Prot. 2012, 42, 156–163. [Google Scholar] [CrossRef]
- Seal, D.R.; Kumar, V. Biological response of chili thrips, Scirtothrips dorsalis Hood (thysanoptera: Thripidae), to various regimes of chemical and biorational insecticides. Crop Prot. 2010, 29, 1241–1247. [Google Scholar] [CrossRef]
- Lee, W.W.; Shin, T.Y.; Bae, S.M.; Woo, S.D. Screening and evaluation of entomopathogenic fungi against the green peach aphid, Myzus persicae, using multiple tools. J. Asia-Pac. Entomol. 2015, 18, 607–615. [Google Scholar] [CrossRef]
- Sharma, S.; Trivedi, P.C. Application of Paecilomyces lilacinus for the Control of Meloidogyne incognita Infecting Vigna radiate. Indian J. Nematol. 2012, 42, 1–4. [Google Scholar]
- Kannan, R.; Veeravel, R. Effect of Different Dose and Application Methods of Paecilomyces lilacinus (Thom.) Samson against Root Knot Nematode, Meloidogyne incognita (Kofoidand White) Chitwood in Okra. J. Agric. Sci. 2012, 4, 119–127. [Google Scholar] [CrossRef]
- Lopez-Lima, D.; Carrion, G.; Núñez-Sánchez, Á.E. Isolation of fungi associated with Criconemoides sp. and their potential use in the biological control of ectoparasitic and semiendoparasitic nematodes in sugar cane. Aust. J. Crop Sci. 2014, 8, 389–396. [Google Scholar]
- Castillo Lopez, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The Entomopathogenic Fungal Endophytes Purpureocillium lilacinum (Formerly Paecilomyces lilacinum) and Beauveria bassiana Negatively Affect Cotton Aphid Reproduction under Both Greenhouse and Field Conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, S.P.; Aristizábal, L.F.; Avery, P.B. Evaluation of entomopathogenic fungi against chili thrips, Scirtothrips dorsalis. J. Insect Sci. 2013, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Luangsa-ard, J.J.; Houbraken, J.; van Doorn, T.; Hong, S.-B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a newgenus for themedically important Paecilomyces lilacinus. FEMS Microbio. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Kiewnick, S. Effect of temperature on growth, germination, germ-tube extension and survival of Paecilomyces lilacinus strain 251. Biocontrol Sci. Technol. 2006, 16, 535–546. [Google Scholar] [CrossRef]
- Sengonca, C.; Thungrabeab, M.; Blaeser, P. Potential of different isolates of entomopathogenic fungi from Thailand as biological control agents against western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). J. Plant. Dis. Prot. 2006, 113, 74–80. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Distribution, abundance and population dynamics. In Thrips as Crop Pests; Lewis, T., Ed.; CABI International: Wallingford, UK, 1997; pp. 217–227. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).