Comparative Tolerance Levels of Maize Landraces and a Hybrid to Natural Infestation of Fall Armyworm
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Maize Genotypes
2.2. Management Practices and Experimental Model
2.3. Data Collection
2.4. Data Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karjagi, C.G.; Sekhar, J.C.; Lakshmi, S.P.; Suby, S.B.; Kaur, J.; Mallikarjuna, M.G.; Kumar, P. Breeding for Resistance to Insect Pests in Maize. In Breeding Insect Resistant Crops for Sustainable Agriculture; Arora, R., Sandhu, S., Eds.; Springer Singapore: Singapore, 2017; pp. 201–229. [Google Scholar]
- Pimentel, D. Pesticides and Pest Control. In Integrated Pest Management: Innovation Development; Peshi, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 83–87. [Google Scholar]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the Global Extent of Invasion of the Cereal Pest Spodoptera frugiperda, the Fall Armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef]
- Sparks, A.N. A Review of the Biology of the Fall Armyworm. Florida Entomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Cui, Y.; Ren, Y.; Lyu, M.; Zheng, S.; Feng, Q.; Xiang, H. Genomic Divergences Between the Two Polyphagous Spodoptera Relatives Provide Cues for Successful Invasion of the Fall Armyworm. Insect Sci. 2020, 27, 1257–1265. [Google Scholar] [CrossRef]
- Gouin, A.; Bretaudeau, A.; Nam, K.; Gimenez, S.; Aury, J.-M.; Duvic, B.; Hilliou, F.; Durand, N.; Montagné, N.; Darboux, I.; et al. Two Genomes of Highly Polyphagous Lepidopteran Pests (Spodoptera frugiperda, Noctuidae) with Different Host-Plant Ranges. Sci. Rep. 2017, 7, 11816. [Google Scholar] [CrossRef]
- Toepfer, S.; Fallet, P.; Kajuga, J.; Bazagwira, D.; Mukundwa, I.P.; Szalai, M.; Turlings, T.C.J. Streamlining Leaf Damage Rating Scales for the Fall Armyworm on Maize. J. Pest Sci. 2021, 94, 1075–1089. [Google Scholar] [CrossRef]
- Jing, D.P.; Guo, J.F.; Jiang, Y.Y.; Zhao, J.Z.; Sethi, A.; He, K.L.; Wang, Z.Y. Initial Detections and Spread of Invasive Spodoptera frugiperda in China and Comparisons with Other Noctuid Larvae in Cornfields Using Molecular Techniques. Insect Sci. 2020, 27, 780–790. [Google Scholar] [CrossRef]
- Otim, M.H.; Tay, W.T.; Walsh, T.K.; Kanyesigye, D.; Adumo, S.; Abongosi, J.; Ochen, S.; Sserumaga, J.; Alibu, S.; Abalo, G.; et al. Detection of Sister-Species in Invasive Populations of the Fall Armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) from Uganda. PLoS ONE 2018, 13, e0194571. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae), a Wew Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Chormule, A.; Shejawal, N.; Sharanabasappa, C.M.; Asokan, R.; Swamy, H.M.; Studies, Z. First Report of the Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae) on Sugarcane and Other Crops from Maharashtra, India. J. Entomol. Zool. Stud. 2019, 7, 114–117. [Google Scholar]
- Sharanabasappa, D.; Kalleshwaraswamy, C.M.; Asokan, R.; Mahadeva Swamy, H.M.; Maruthi, M.S.; Pavithra, H.B.; Hegde, K.; Navi, S.; Prabhu, S.T.; Goergen, G. First report of the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018, 24, 23–29. [Google Scholar]
- Tambo, J.A.; Day, R.K.; Lamontagne-Godwin, J.; Silvestri, S.; Beseh, P.K.; Oppong-Mensah, B.; Phiri, N.A.; Matimelo, M. Tackling Fall Armyworm (Spodoptera frugiperda) Outbreak in Africa: An Analysis of Farmers’ Control Actions. Int. J. Pest Manag. 2020, 66, 298–310. [Google Scholar] [CrossRef]
- Banerjee, R.; Hasler, J.; Meagher, R.; Nagoshi, R.; Hietala, L.; Huang, F.; Narva, K.; Jurat-Fuentes, J.L. Mechanism and DNA-Based Detection of Field-Evolved Resistance to Transgenic Bt Corn in Fall Armyworm (Spodoptera frugiperda). Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boaventura, D.; Ulrich, J.; Lueke, B.; Bolzan, A.; Okuma, D.; Gutbrod, O.; Geibel, S.; Zeng, Q.; Dourado, P.M.; Martinelli, S.; et al. Molecular Characterization of Cry1F Resistance in Fall Armyworm, Spodoptera frugiperda from Brazil. Insect Biochem. Mol. Biol. 2020, 116, 103280. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; Dos Santos, A.C.; Omoto, C. Field-Evolved Resistance to Cry1F Maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 150–158. [Google Scholar] [CrossRef]
- Flagel, L.; Lee, Y.W.; Wanjugi, H.; Swarup, S.; Brown, A.; Wang, J.; Kraft, E.; Greenplate, J.; Simmons, J.; Adams, N.; et al. Mutational Disruption of the ABCC2 Gene in Fall Armyworm, Spodoptera frugiperda, Confers Resistance to the Cry1Fa and Cry1A.105 Insecticidal Proteins. Sci. Rep. 2018, 8, 7255. [Google Scholar] [CrossRef]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.O.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a Ryanodine Receptor Target-Site Mutation in Diamide Insecticide Resistant Fall Armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2020, 76, 47–54. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the Molecular Mechanisms of Organophosphate and Pyrethroid Resistance in the Fall Armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef]
- Do Nascimento, A.R.B.; Farias, J.R.; Bernardi, D.; Horikoshi, R.J.; Omoto, C. Genetic Basis of Spodoptera frugiperda (Lepidoptera: Noctuidae) Resistance to the Chitin Synthesis Inhibitor Lufenuron. Pest Manag. Sci. 2016, 72, 810–815. [Google Scholar] [CrossRef]
- Okuma, D.M.; Bernardi, D.; Horikoshi, R.J.; Bernardi, O.; Silva, A.P.; Omoto, C. Inheritance and Fitness Costs of Spodoptera frugiperda (Lepidoptera: Noctuidae) Resistance to Spinosad in Brazil. Pest Manag. Sci. 2018, 74, 1441–1448. [Google Scholar] [CrossRef]
- Painter, R. Insect Resistance in Crop Plants; University of Kansas Press: Lawrence, KS, USA, 1951. [Google Scholar]
- Smith, C.M. Plant Resistance to Arthropods: Molecular and Conventional Approaches; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Dara, S.K. The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant Defense against Herbivorous Pests: Exploiting Resistance and Tolerance Traits for Sustainable Crop Protection. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Bustos-Segura, C.; Fornoni, J.; Núñez-Farfán, J. Evolutionary Changes in Plant Tolerance against Herbivory through a Resurrection Experiment. J. Evol. Biol. 2014, 27, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Gagic, V.; Riggi, L.G.A.; Ekbom, B.; Malsher, G.; Rusch, A.; Bommarco, R. Interactive Effects of Pests Increase Seed Yield. Ecol. Evol. 2016, 6, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.K.D.; Varella, A.C.; Higley, L.G. Tolerance: The Forgotten Child of Plant Resistance. PeerJ 2017, 5, e3934. [Google Scholar] [CrossRef]
- Tiffin, P. Mechanisms of Tolerance to Herbivore Damage: What Do We Know? Evol. Ecol. 2000, 14, 523–536. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The Ecology and Evolution of Plant Tolerance to Herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Agrawal, A.A. Overcompensation of Plants in Response to Herbivory and the By-Product Benefits of Mutualism. Trends Plant Sci. 2000, 5, 309–313. [Google Scholar] [CrossRef]
- Garcia, L.C.; Eubanks, M.D. Overcompensation for Insect Herbivory: A Review and Meta-Analysis of the Evidence. Ecology 2019, 100, 1–14. [Google Scholar] [CrossRef]
- Ramula, S.; Paige, K.N.; Lennartsson, T.; Tuomi, J. Overcompensation: A 30-year Perspective. Ecology 2019, 100, e02667. [Google Scholar] [CrossRef]
- Poveda, K.; Díaz, M.F.; Ramirez, A. Can Overcompensation Increase Crop Production? Ecology 2018, 99, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Poveda, K.; Jiménez, M.I.G.; Kessler, A. The Enemy as Ally: Herbivore-Induced Increase in Crop Yield. Ecol. Appl. 2010, 20, 1787–1793. [Google Scholar] [CrossRef]
- Qu, W.; Robert, C.A.M.; Erb, M.; Hibbard, B.E.; Paven, M.; Gleede, T.; Riehl, B.; Kersting, L.; Cankaya, A.S.; Kunert, A.T.; et al. Dynamic Precision Phenotyping Reveals Mechanism of Crop Tolerance to Root Herbivory. Plant Physiol. 2016, 172, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.A.M.; Schirmer, S.; Barry, J.; Wade French, B.; Hibbard, B.E.; Gershenzon, J. Belowground Herbivore Tolerance Involves Delayed Overcompensatory Root Regrowth in Maize. Entomol. Exp. Appl. 2015, 157, 113–120. [Google Scholar] [CrossRef]
- Robert, C.A.M.; Ferrieri, R.A.; Schirmer, S.; Babst, B.A.; Schueller, M.J.; Machado, R.A.R.; Arce, C.C.M.; Hibbard, B.E.; Gershenzon, J.; Turling, T.C.J.; et al. Induced Carbon Reallocation and Compensatory Growth as Root Herbivore Tolerance Mechanisms. Plant. Cell Environ. 2014, 37, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.N.; Sardinha de Souza, B.H.; Ribeiro, Z.A.; Dos Santos, D.M.M.; Boiça, A.L. Tolerance in Maize Landraces to Diabrotica speciosa (Coleoptera: Chrysomelidae) Larvae and Its Relationship to Plant Pigments, Compatible Osmolytes, and Vigor. J. Econ. Entomol. 2021, 114, 377–386. [Google Scholar] [CrossRef]
- Dávila-Flores, A.M.; DeWitt, T.J.; Bernal, J.S. Facilitated by Nature and Agriculture: Performance of a Specialist Herbivore Improves with Host-Plant Life History Evolution, Domestication, and Breeding. Oecologia 2013, 173, 1425–1437. [Google Scholar] [CrossRef]
- Prasanna, B.M. Diversity in Global Maize Germplasm: Characterization and Utilization. J. Biosci. 2012, 37, 843–855. [Google Scholar] [CrossRef]
- Strigens, A.; Schipprack, W.; Reif, J.C.; Melchinger, A.E. Unlocking the Genetic Diversity of Maize Landraces with Doubled Haploids Opens New Avenues for Breeding. PLoS ONE 2013, 8, e57234. [Google Scholar] [CrossRef]
- Brilinger, D.; Wille, C.L.; Machado da Rosa, J.; Franco, C.R.; Carissimi Boff, M.I. Susceptibility of Brazilian Maize Landraces to the Attack of Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2020, 88, 101677. [Google Scholar] [CrossRef]
- Costa, E.N.; Fernandes, M.G.; Medeiros, P.H.; Evangelista, B.M.D. Resistance of Maize Landraces from Brazil to Fall Armyworm (Lepidoptera: Noctuidae) in the Winter and Summer Seasons. Bragantia 2020, 79, 377–386. [Google Scholar] [CrossRef]
- Costa, E.N.; Nogueira, L.; De Souza, B.H.S.; Ribeiro, Z.A.; Louvandini, H.; Zukoff, S.N.; Júnior, A.L.B. Characterization of Antibiosis to Diabrotica speciosa (Coleoptera: Chrysomelidae) in Brazilian Maize Landraces. J. Econ. Entomol. 2018, 111, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, R.T.; Pavan, B.E.; Silva, L.B.; Dourado, L.R.B.; Maggioni, K.; Baptistel, A.C. Resistance of Grain of Maize Landrace under Breeding in Southern Piauí to Attack by Sitophilus zeamais. Afr. J. Agric. Res. 2014, 9, 921–926. [Google Scholar] [CrossRef]
- Dos Santos, L.F.C.; Ruiz-Sánchez, E.; Andueza-Noh, R.H.; Garruña-Hernández, R.; Latournerie-Moreno, L.; Mijangos-Cortés, J.O. Leaf Damage by Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae) and Its Relation to Leaf Morphological Traits in Maize Landraces and Commercial Cultivars. J. Plant Dis. Prot. 2020, 127, 103–109. [Google Scholar] [CrossRef]
- Singh, G.M.; Xu, J.; Schaefer, D.; Day, R.; Wang, Z.; Zhang, F. Maize Diversity for Fall Armyworm Resistance in a Warming World. Crop Sci. 2021, 62, 1–19. [Google Scholar] [CrossRef]
- INMET—Instituto Nacional de Meteorologia Instituto Nacional de Meteorologia. Available online: https://tempo.inmet.gov.br/CondicoesRegistradas (accessed on 26 April 2021).
- AGROFIT Sistema de Agrotóxicos Fitossanitários—Ministério Da Agricultura, Pecúaria e Abastecimento. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 30 March 2021).
- Davis, F.; Ng, S.; Williams, W. Visual Rating Scales for Screening Whore-Stage Corn Resistance to Fall Armyworm. Miss. Agric. For. Exp. Stn. Tech. Bull. 1992, 186, 9. [Google Scholar]
- Meneses, A.R.; Querino, R.B.; Oliveira, C.M.; Maia, A.H.N.; Silva, P.R.R. Seasonal and Vertical Distribution of Dalbulus maidis (Hemiptera: Cicadellidae) in Brazilian Corn Fields. Fla. Entomol. 2016, 99, 750–754. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; Lopes, J.R.S.; Camargo, L.E.A.; Fungaro, M.H.P.; Nault, L.R. Genetic Diversity in Populations of Dalbulus maidis (DeLong and Wolcott) (Hemiptera: Cicadellidae) from Distant Localities in Brazil Assessed by RAPD-PCR Markers. Environ. Entomol. 2007, 36, 204–212. [Google Scholar] [CrossRef]
- Rozas, H.S.; Echeverría, H.E.; Studdert, G.A.; Andrade, F.H. No-till Maize Nitrogen Uptake and Yield: Effect of Urease Inhibitor and Application Time. Agron. J. 1999, 91, 950–955. [Google Scholar] [CrossRef]
- Abdi, H. The Bonferonni and Sidák Corrections for Multiple Comparisons. Encycl. Meas. Stat. 2007, 1, 1–9. [Google Scholar]
- SAS Institute. JMP.Pro 14 (Edn); SAS Institute: Cary, NC, USA, 2018. [Google Scholar]
- R CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Fontes-Puebla, A.A.; Bernal, J.S. Resistance and Tolerance to Root Herbivory in Maize Were Mediated by Domestication, Spread, and Breeding. Front. Plant Sci. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pearse, I.S.; Aguilar, J.; Schroder, J.; Strauss, S.Y. Macroevolutionary Constraints to Tolerance: Trade-Offs with Drought Tolerance and Phenology, but Not Resistance. Ecology 2017, 98, 2758–2772. [Google Scholar] [CrossRef]
- Brambilla, J.A.; Lange, A.; Buchelt, A.C.; Massaroto, J.A. Produtividade de Milho Safrinha No Sistema de Integração Lavoura-Pecuária, Na Região de Sorriso, Mato Grosso. Rev. Bras. Milho e Sorgo 2009, 8, 263–274. [Google Scholar] [CrossRef]
- Dourado Neto, D.; Palhares, M.; Vieira, P.A.; Manfron, P.A.; Medeiros, S.L.P.; Romano, M.R. Efeito Da População de Plantas e Do Espaçamento Sobre a Produtividade de Milho. Rev. Bras. Milho e Sorgo 2003, 2, 63–77. [Google Scholar] [CrossRef]
- Slewinski, T.L. Non-Structural Carbohydrate Partitioning in Grass Stems: A Target to Increase Yield Stability, Stress Tolerance, and Biofuel Production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef]
- Hochwender, C.G.; Marquis, R.J.; Stowe, K.A. The Potential for and Constraints on the Evolution of Compensatory Ability in Asclepias syriaca. Oecologia 2000, 122, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.N.; Cheng, W.; Crossley, D.A. Herbivore-Induced Changes in Plant Carbon Allocation: Assessment of below-Ground C Fluxes Using Carbon-14. Oecologia 1996, 107, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.A.R.; Zhou, W.; Ferrieri, A.P.; Arce, C.C.M.; Baldwin, I.T.; Xu, S.; Erb, M. Species-Specific Regulation of Herbivory-Induced Defoliation Tolerance Is Associated with Jasmonate Inducibility. Ecol. Evol. 2017, 7, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Schwachtje, J.; Minchin, P.E.H.; Jahnke, S.; Van Dongen, J.T.; Schittko, U.; Baldwin, I.T. SNF1-Related Kinases Allow Plants to Tolerate Herbivory by Allocating Carbon to Roots. Proc. Natl. Acad. Sci. USA 2006, 103, 12935–12940. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Arce, C.C.M.; Ferrieri, A.P.; Baldwin, I.T.; Erb, M. Jasmonate-dependent Depletion of Soluble Sugars Compromises Plant Resistance to Manduca sexta. New Phytol. 2015, 207, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Boalt, E.; Arvanitis, L.; Lehtilä, K.; Ehrlén, J. The Association among Herbivory Tolerance, Ploidy Level, and Herbivory Pressure in Cardamine pratensis. Evol. Ecol. 2010, 24, 1101–1113. [Google Scholar] [CrossRef]
- Kasoma, C.; Shimelis, H.; Laing, M.; Shayanowako, A.I.T.; Mathew, I. Screening of Inbred Lines of Tropical Maize for Resistance to Fall Armyworm, and for Yield and Yield-Related Traits. Crop Prot. 2020, 136, 105218. [Google Scholar] [CrossRef]
- Tayo, T.O. Growth, Development and Yield of Pigeon Pea (Cajanus cajan (L.) Millsp.) in the Lowland Tropics: 3. Effect of Early Loss of Apical Dominance. J. Agric. Sci. 1982, 98, 79–84. [Google Scholar] [CrossRef]
- Quijano-Medina, T.; Covelo, F.; Moreira, X.; Abdala-Roberts, L. Compensation to Simulated Insect Leaf Herbivory in Wild Cotton (Gossypium hirsutum): Responses to Multiple Levels of Damage and Associated Traits. Plant Biol. 2019, 21, 805–812. [Google Scholar] [CrossRef]
- Buffon, G.; Blasi, É.A.d.R.; Lamb, T.I.; Adamski, J.M.; Schwambach, J.; Ricachenevsky, F.K.; Bertolazi, A.; Silveira, V.; Lopes, M.C.B.; Sperotto, R.A. Oryza sativa Cv. Nipponbare and Oryza Barthii as Unexpected Tolerance and Susceptibility Sources against Schizotetranychus oryzae (Acari: Tetranychidae) Mite Infestation. Front. Plant Sci. 2021, 12, 613568. [Google Scholar] [CrossRef]
- Pedersen, I.F.; Christensen, J.T.; Sørensen, P.; Christensen, B.T.; Holton Rubæk, G. Early Plant Height: A Defining Factor for Yields of Silage Maize with Contrasting Phosphorus Supply. Soil Use Manag. 2022, 38, 537–548. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, X.; Buntin, G.D. Physiological, Nutritional, and Biochemical Bases of Corn Resistance to Foliage-Feeding Fall Armyworm. J. Chem. Ecol. 2009, 35, 297–306. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the Relationship between Reflectance Red Edge and Chlorophyll Content in Slash Pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An Evaluation of Noninvasive Methods to Estimate Foliar Chlorophyll Content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Capó, M.; Roig-Oliver, M.; Cardona, C.; Cursach, J.; Bartolomé, J.; Rita, J.; Baraza, E. Historic Exposure to Herbivores, Not Constitutive Traits, Explains Plant Tolerance to Herbivory in the Case of Two Medicago Species (Fabaceae). Plant Sci. 2021, 307, 110890. [Google Scholar] [CrossRef]
- Hódar, J.A.; Zamora, R.; Castro, J.; Gómez, J.M.; García, D. Biomass Allocation and Growth Responses of Scots Pine Saplings to Simulated Herbivory Depend on Plant Age and Light Availability. Plant Ecol. 2008, 197, 229–238. [Google Scholar] [CrossRef]
- Lima, P.F.S.; Teixido, A.L.; Sousa Paiva, E.A. Herbivory-Induced Overcompensation and Resource-Dependent Production of Extrafloral Nectaries in Luffa cylindrica (Cucurbitaceae). Acta Oecologica 2018, 93, 1–6. [Google Scholar] [CrossRef]
- Tito, R.; Castellani, T.T.; Fáveri, S.B.; Lopes, B.C.; Vasconcelos, H.L. From over to Undercompensation: Variable Responses to Herbivory during Ontogeny of a Neotropical Monocarpic Plant. Biotropica 2016, 48, 608–617. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Stephens, A.E.A.; Westoby, M. Effects of Insect Attack to Stems on Plant Survival, Growth, Reproduction and Photosynthesis. Oikos 2015, 124, 266–273. [Google Scholar] [CrossRef]
- Eyles, A.; Pinkard, E.A.; Davies, N.W.; Corkrey, R.; Churchill, K.; O’Grady, A.P.; Sands, P.; Mohammed, C. Whole-Plant versus Leaf-Level Regulation of Photosynthetic Responses after Partial Defoliation in Eucalyptus globulus Saplings. J. Exp. Bot. 2013, 64, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lou, Y.-R.; Tzin, V.; Jander, G. Alteration of Plant Primary Metabolism in Response to Insect Herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef]
- Retuerto, R.; Fernandez-Lema, B.; Rodriguez-Roiloa; Obeso, J.R. Increased Photosynthetic Performance in Holly Trees Infested by Scale Insects. Funct. Ecol. 2004, 18, 664–669. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How Can We Make Plants Grow Faster? A Source–Sink Perspective on Growth Rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a Compensatory Photosynthetic Response Mechanism in Tomato Leaves upon Short Time Feeding by the Chewing Insect Spodoptera exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef]
- Thomson, V.P.; Cunningham, S.A.; Ball, M.C.; Nicotra, A.B. Compensation for Herbivory by Cucumis sativus through Increased Photosynthetic Capacity and Efficiency. Oecologia 2003, 134, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Indirect Suppression of Photosynthesis on Individual Leaves by Arthropod Herbivory. Ann. Bot. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Andreadis, S.; Moustaka, J.; Panteris, E.; Tsaballa, A.; Moustakas, M. Changes in Light Energy Utilization in Photosystem II and Reactive Oxygen Species Generation in Potato Leaves by the Pinworm Tuta absoluta. Molecules 2021, 26, 2984. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Trade-Offs between Plant Growth and Defense against Insect Herbivory: An Emerging Mechanistic Synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Li, K.; Alseekh, S.; Omranian, N.; Zhao, L.; Zhou, Y.; Xiao, Y.; Jin, M.; Yang, N.; Liu, H.; et al. Genetic Determinants of the Network of Primary Metabolism and Their Relationships to Plant Performance in a Maize Recombinant Inbred Line Population. Plant Cell 2015, 27, 1839–1856. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, J.; Luo, S.; Fan, H.; Jin, Q. Costs of Jasmonic Acid Induced Defense in Aboveground and Belowground Parts of Corn (Zea mays L.). J. Chem. Ecol. 2012, 38, 984–991. [Google Scholar] [CrossRef]
- Heinrich, M.; Hettenhausen, C.; Lange, T.; Wünsche, H.; Fang, J.; Baldwin, I.T.; Wu, J. High Levels of Jasmonic Acid Antagonize the Biosynthesis of Gibberellins and Inhibit the Growth of Nicotiana attenuata Stems. Plant J. 2013, 73, 591–606. [Google Scholar] [CrossRef]
- Qi, J.; Li, J.; Han, X.; Li, R.; Wu, J.; Yu, H.; Hu, L.; Xiao, Y.; Lu, J.; Lou, Y. Jasmonic Acid Carboxyl Methyltransferase Regulates Development and Herbivory-Induced Defense Response in Rice. J. Integr. Plant Biol. 2016, 58, 564–576. [Google Scholar] [CrossRef]
- Zhang, Y.; Turner, J.G. Wound-Induced Endogenous Jasmonates Stunt Plant Growth by Inhibiting Mitosis. PLoS ONE 2008, 3, e3699. [Google Scholar] [CrossRef]
- Fontes-Puebla, A.A.; Borrego, E.J.; Kolomiets, M.V.; Bernal, J.S. Maize Biochemistry in Response to Root Herbivory Was Mediated by Domestication, Spread, and Breeding. Planta 2021, 254, 1–17. [Google Scholar] [CrossRef]
- Scofield, G.N.; Ruuska, S.A.; Aoki, N.; Lewis, D.C.; Tabe, L.M.; Jenkins, C.L.D. Starch Storage in the Stems of Wheat Plants: Localization and Temporal Changes. Ann. Bot. 2009, 103, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Korpita, T.; Gómez, S.; Orians, C.M. Cues from a Specialist Herbivore Increase Tolerance to Defoliation in Tomato. Funct. Ecol. 2014, 28, 395–401. [Google Scholar] [CrossRef]
- Weintraub, R.; Garrido, E.; Poveda, K. Age-Dependent Potato Polerance to Herbivory in Different Nutrient Evironments. Am. J. Potato Res. 2018, 95, 642–649. [Google Scholar] [CrossRef]
- Cuny, M.A.C.; Gendry, J.; Hernández-Cumplido, J.; Benrey, B. Changes in Plant Growth and Seed Production in Wild Lima Bean in Response to Herbivory Are Attenuated by Parasitoids. Oecologia 2018, 187, 447–457. [Google Scholar] [CrossRef]
- Allsup, C.M.; Paige, K.N. Belowground Fungal Associations and Water Interact to Influence the Compensatory Response of Ipomopsis aggregata. Oecologia 2016, 180, 463–474. [Google Scholar] [CrossRef]
- Vannette, R.L.; Hunter, M.D. Mycorrhizal Fungi as Mediators of Defence against Insect Pests in Agricultural Systems. Agric. For. Entomol. 2009, 11, 351–358. [Google Scholar] [CrossRef]
- Scholes, D.R.; Rasnick, E.N.; Paige, K.N. Characterization of Arabidopsis Thaliana Regrowth Patterns Suggests a Trade-off between Undamaged Fitness and Damage Tolerance. Oecologia 2017, 184, 643–652. [Google Scholar] [CrossRef]
- Villegas, J.M.; Wilson, B.E.; Way, M.O.; Gore, J.; Stout, M.J. Tolerance to Rice Water Weevil, Lissorhoptrus oryzophilus Kuschel (Coleoptera: Curculionidae), Infestations among Hybrid and Inbred Rice Cultivars in the Southern U.S. Crop Prot. 2021, 139, 105368. [Google Scholar] [CrossRef]
- Zheng, Z.; Powell, J.J.; Ye, X.; Liu, X.; Yuan, Z.; Liu, C. Overcompensation Can Be an Ideal Breeding Target. Agronomy 2021, 11, 1376. [Google Scholar] [CrossRef]
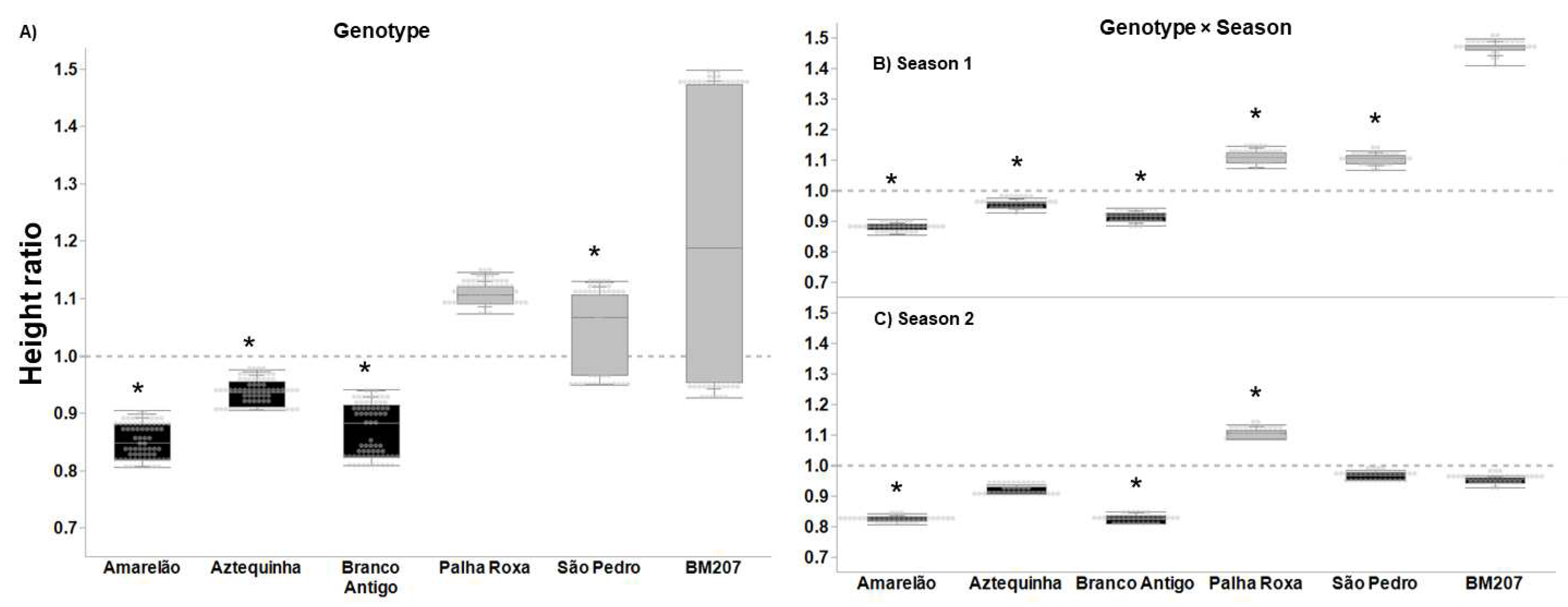
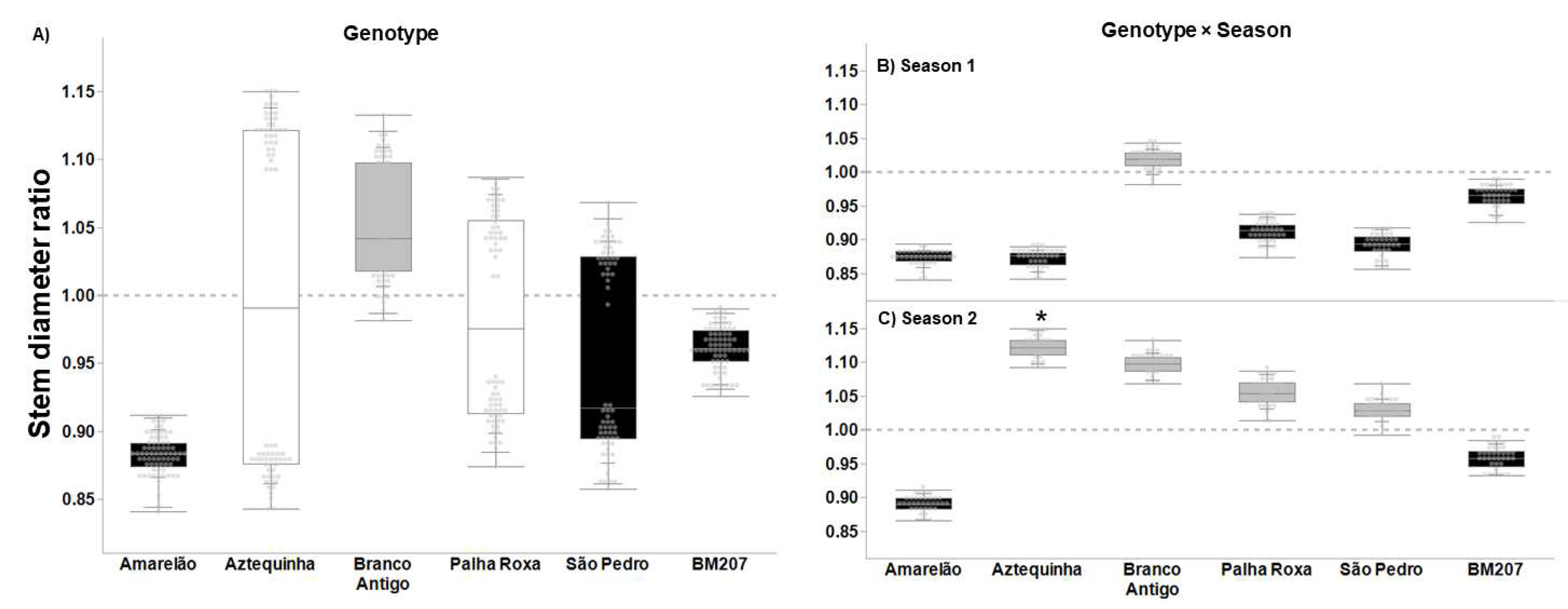
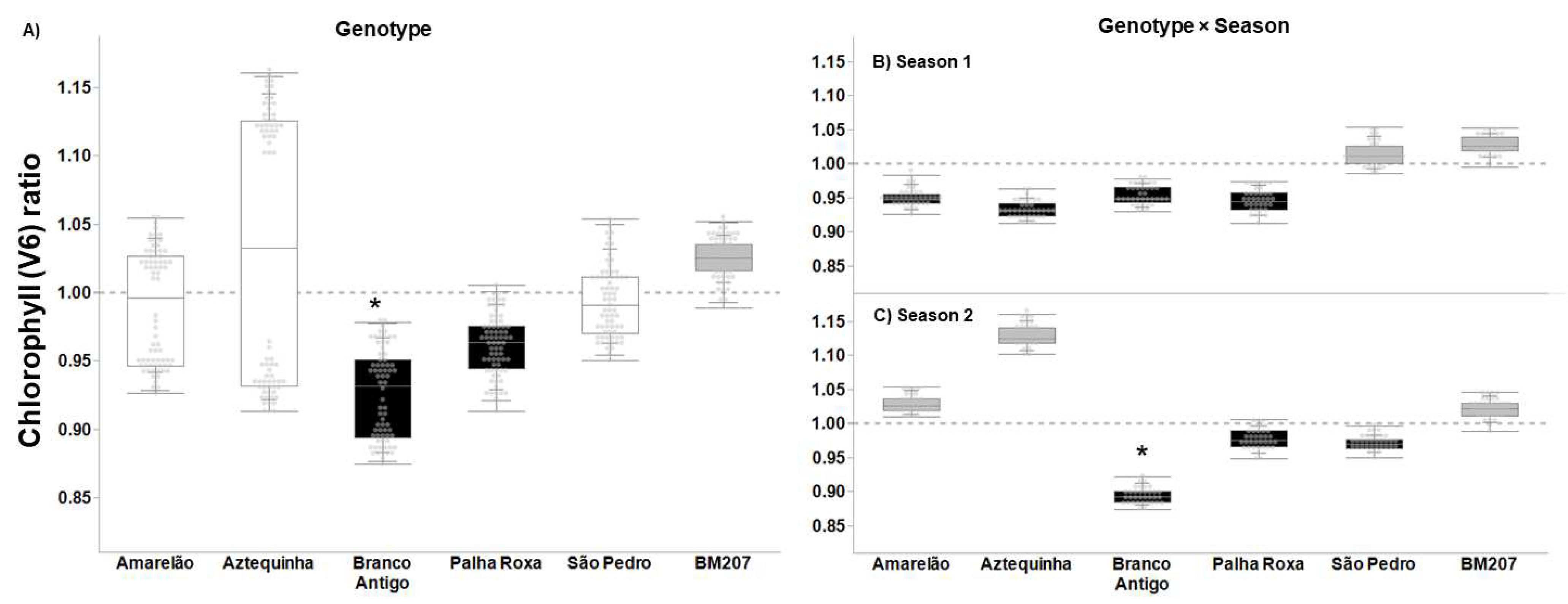
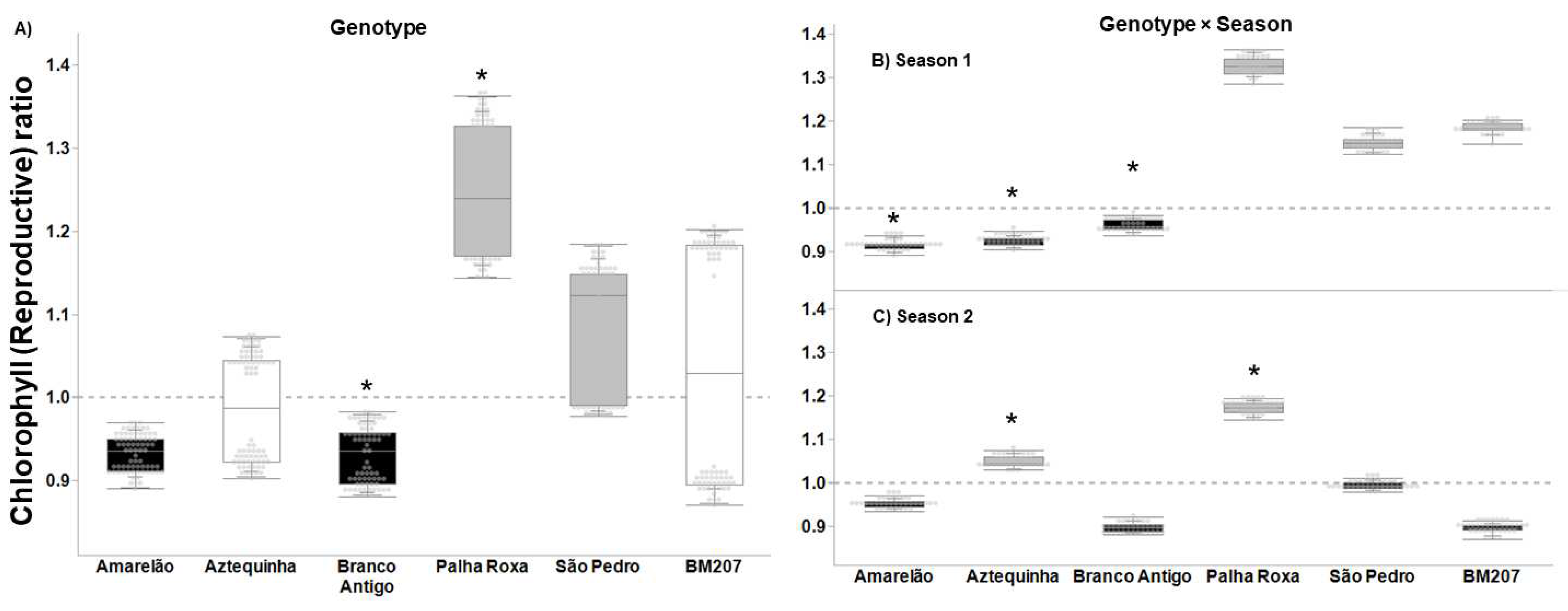

| Parameters Evaluated | DevelopmentalStage | Season/Evaluation Date | |
|---|---|---|---|
| 2017/2018 | 2018/2019 | ||
| Spodoptera frugiperda leaf injury and number of Dalbulus maidis adults | V4 | 5 January | 11 December |
| V6 | 19 January | 26 December | |
| V8 | 6 February | 11 January | |
| V12 | 20 February | 23 January | |
| Reproductive | 21 March | 12 February | |
| Chlorophyll content | V6 | 19 January | 26 December |
| Reproductive | 21 March | 12 February | |
| Plant growth | Post-reproductive | 28 April | 1 April |
| Source | Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | PlantHeight | StemDiameter | ChlorophyllV6 | Chlorophyll Reproductive | |||||
| F | p | F | p | F | p | F | p | ||
| Genotype | 5, 376 | 55.823 | <0.0001 | 4.027 | 0.001 | 3.626 | 0.003 | 16.383 | <0.0001 |
| Season | 1, 376 | 35.573 | <0.0001 | 14.186 | <0.001 | 1.707 | 0.193 | 7.622 | 0.006 |
| Genotype × Season | 5, 376 | 20.699 | <0.0001 | 2.655 | 0.023 | 4.604 | 0.001 | 7.079 | <0.0001 |
| FAW injury | 1, 376 | 0.015 | 0.902 | 1.403 | 0.237 | 1.568 | 0.212 | 0.418 | 0.519 |
| D. maidis | 1, 376 | 3.508 | 0.062 | 0.003 | 0.960 | 0.458 | 0.499 | 0.470 | 0.489 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, A.F.; Bernal, J.; Venâncio, M.G.S.; de Souza, B.H.S.; Carvalho, G.A. Comparative Tolerance Levels of Maize Landraces and a Hybrid to Natural Infestation of Fall Armyworm. Insects 2022, 13, 651. https://doi.org/10.3390/insects13070651
Lima AF, Bernal J, Venâncio MGS, de Souza BHS, Carvalho GA. Comparative Tolerance Levels of Maize Landraces and a Hybrid to Natural Infestation of Fall Armyworm. Insects. 2022; 13(7):651. https://doi.org/10.3390/insects13070651
Chicago/Turabian StyleLima, Andreísa Fabri, Julio Bernal, Maria Gabriela Silva Venâncio, Bruno Henrique Sardinha de Souza, and Geraldo Andrade Carvalho. 2022. "Comparative Tolerance Levels of Maize Landraces and a Hybrid to Natural Infestation of Fall Armyworm" Insects 13, no. 7: 651. https://doi.org/10.3390/insects13070651
APA StyleLima, A. F., Bernal, J., Venâncio, M. G. S., de Souza, B. H. S., & Carvalho, G. A. (2022). Comparative Tolerance Levels of Maize Landraces and a Hybrid to Natural Infestation of Fall Armyworm. Insects, 13(7), 651. https://doi.org/10.3390/insects13070651








