A Highly Troglomorphic New Genus of Sminthuridae (Collembola, Symphypleona) from the Brazilian Semiarid Region †
Abstract
:Simple Summary
Abstract
1. Introduction
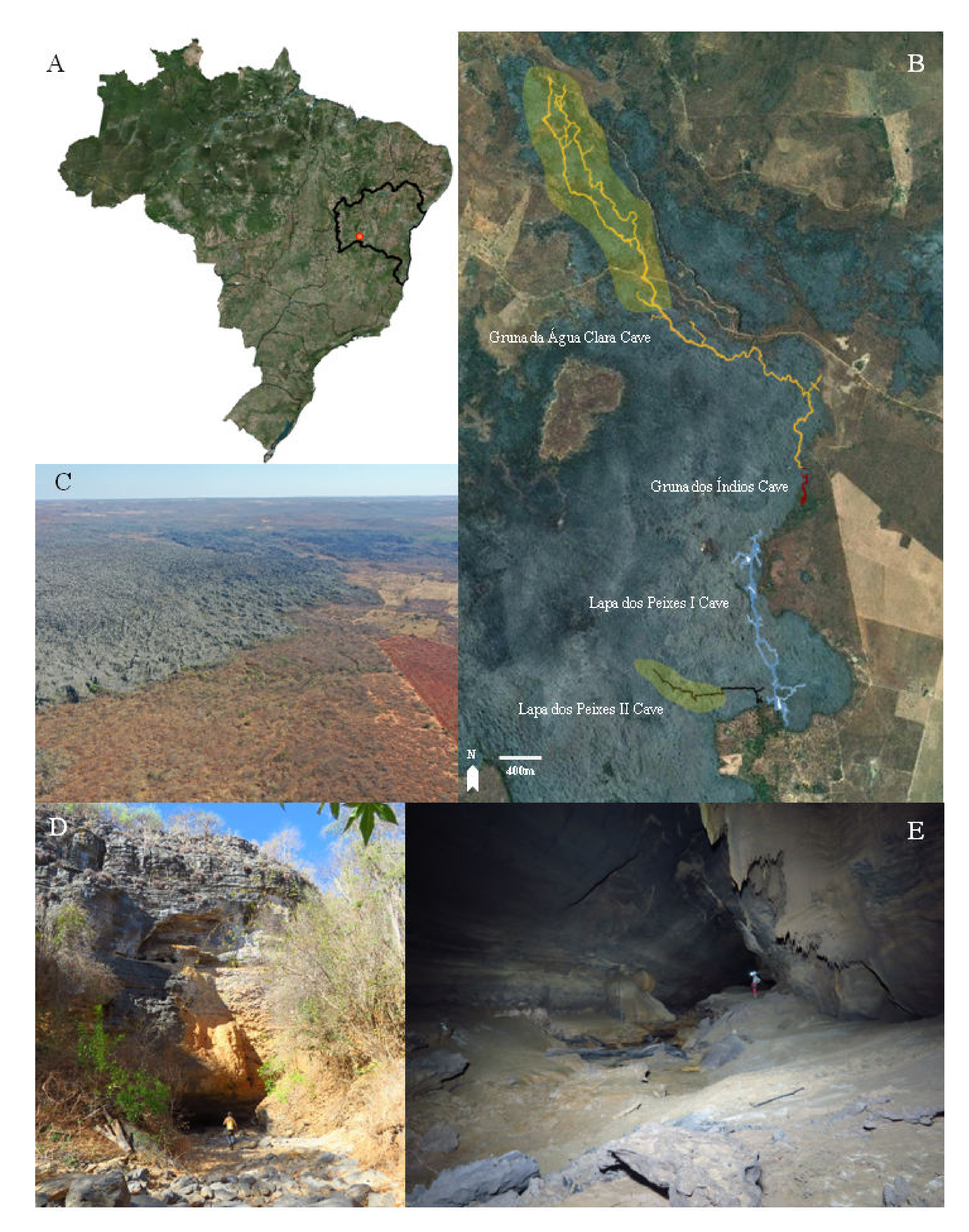
2. Materials and Methods
3. Results
3.1. Taxonomic Summary and Subfamily Diagnosis
- Suborder Appendiciphora Bretfeld, 1986 [41]
- Superfamily Sminthuroidea Bretfeld, 1994 [42]
- Family Sminthuridae Lubbock, 1862 [43]
| 1. | Ungues with cavity and/or a filament-like tunica, tibiotarsal distal whorl with six or fewer chaetae…………………………………………………………………………………………………………………………………… | |
| ……………………………………………Songhaicinae Sánchez-García and Engel, 2016 [28] sensu Bernard and Wynne, 2017 [27] | ||
| -. | Ungues without cavity or a filament-like tunica, tibiotarsal distal whorl with 7–9 chaetae…………………………………… | 2 |
| 2. | Antennae shorter than the body, males’ Abd. VI with a pair of spines similar to the subanal appendages of the females, dens ventrally with 0–6 chaetae ……………………………………………………………………………………………………… | |
| ………………………………………………………………………………Sphyrothecinae Betsch, 1980 [25] sensu Bretfeld, 1999 [26] | ||
| -. | Antennae subequal or longer than the body, males’ Abd. VI without subanal appendages-like spines, dens ventrally with 9–15 chaetae……………………………………………………………………………………………………………………………… | |
| ………………………………………………………………………………Sminthurinae Lubbock, 1862 [43] sensu Bretfeld, 1999 [26] | ||
3.2. Troglobentosminthurus gen. nov. Bellini, Medeiros and Souza
| 1. | Eyes and ungual inner teeth absent…………………………………………………………………………………………………………………………………………… | |
| ………………………………………………………………………………………………………Galeriella Ćurčić and Lučić, 2007 in [53] | ||
| -. | Eyes and ungual inner teeth present…………………………………………………………………………………………………… | 2 |
| 2 | Clypeus and Ant. III with sexually dimorphic chaetae on males, parafurcal area with a pair of neosminthuroid chaetae…… | |
| ………………………………………………………………………………………………………Keratosminthurus Zeppelini, 2020 in [4] | ||
| -. | Clypeus and Ant. III without sexually dimorphic chaetae, parafurcal area lacking neosminthuroid chaetae………………… | 3 |
| 3. | Specimens pale, eyes 5 + 5, head frontal area without spines, pretarsi posterior chaeta absent, dens ventrally with nine chaeta……………………………………………………………………………………………………………………………………………… | |
| ……………………………………………………………………………………………………………Troglobentosminthurus gen. nov. | ||
| -. | Specimens pigmented, eyes 8 + 8, head frontal area with spines, pretarsi posterior chaeta present, dens ventrally with 13 chaetae………………………………………………………………………………………………………………………………………… | |
| ………………………………………………………………………………………………………………………………………………………4 | ||
| 4. | Males with dimorphic short candle-shaped spines on the dorsal abdomen, capitate tenent-hairs present on tibiotarsi II–III…………………………………………………………………………………………………………………………………… | |
| …………………………………………………………………………………………………………………Richardsitas Betsch, 1975 [52] | ||
| -. | Specimens without sexually dimorphic spines on the dorsal abdomen, tenent-hairs acuminate………………………………… | |
| …………………………………………………………………………………………………………………Temeritas Richards, 1963 in [51] | ||
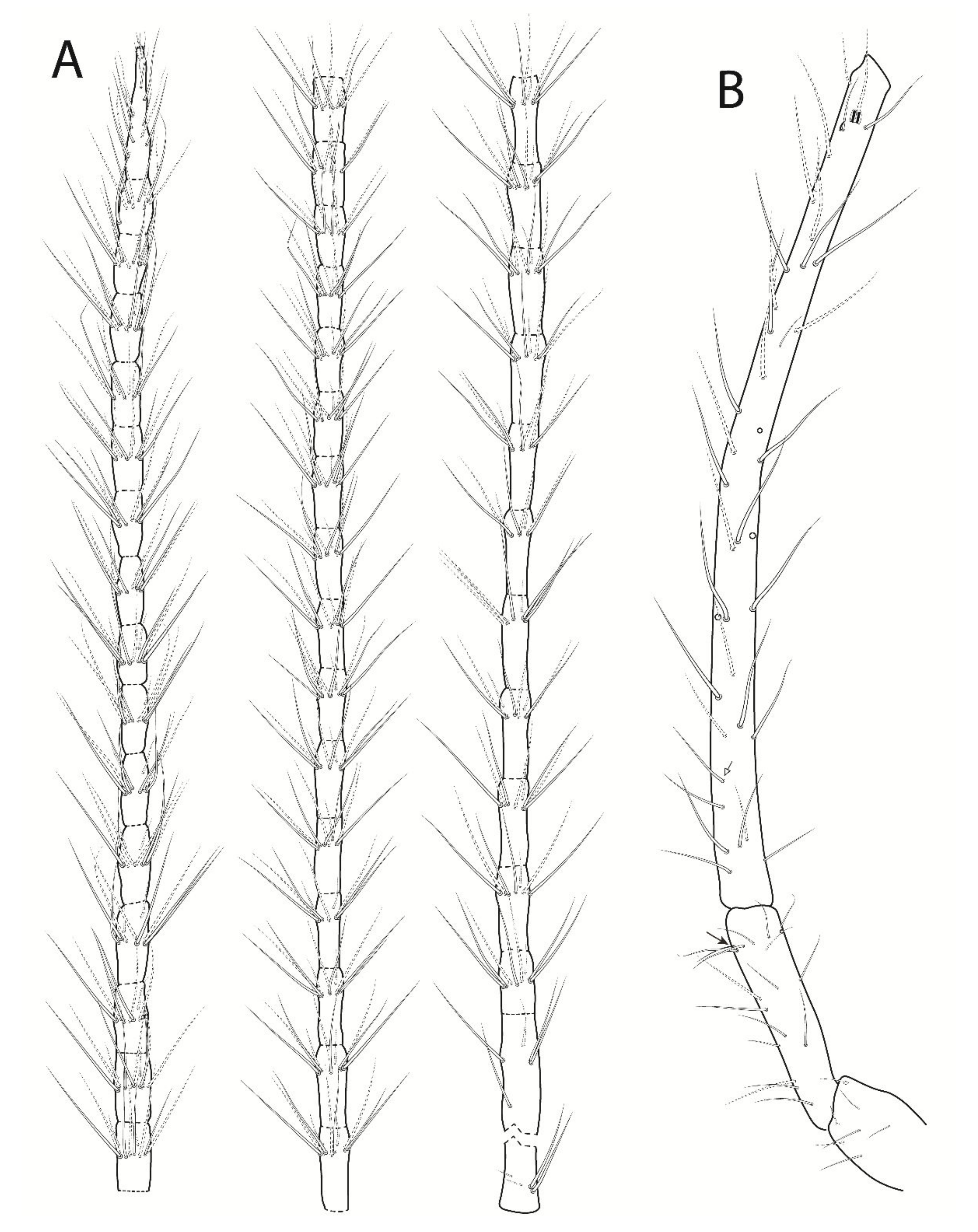
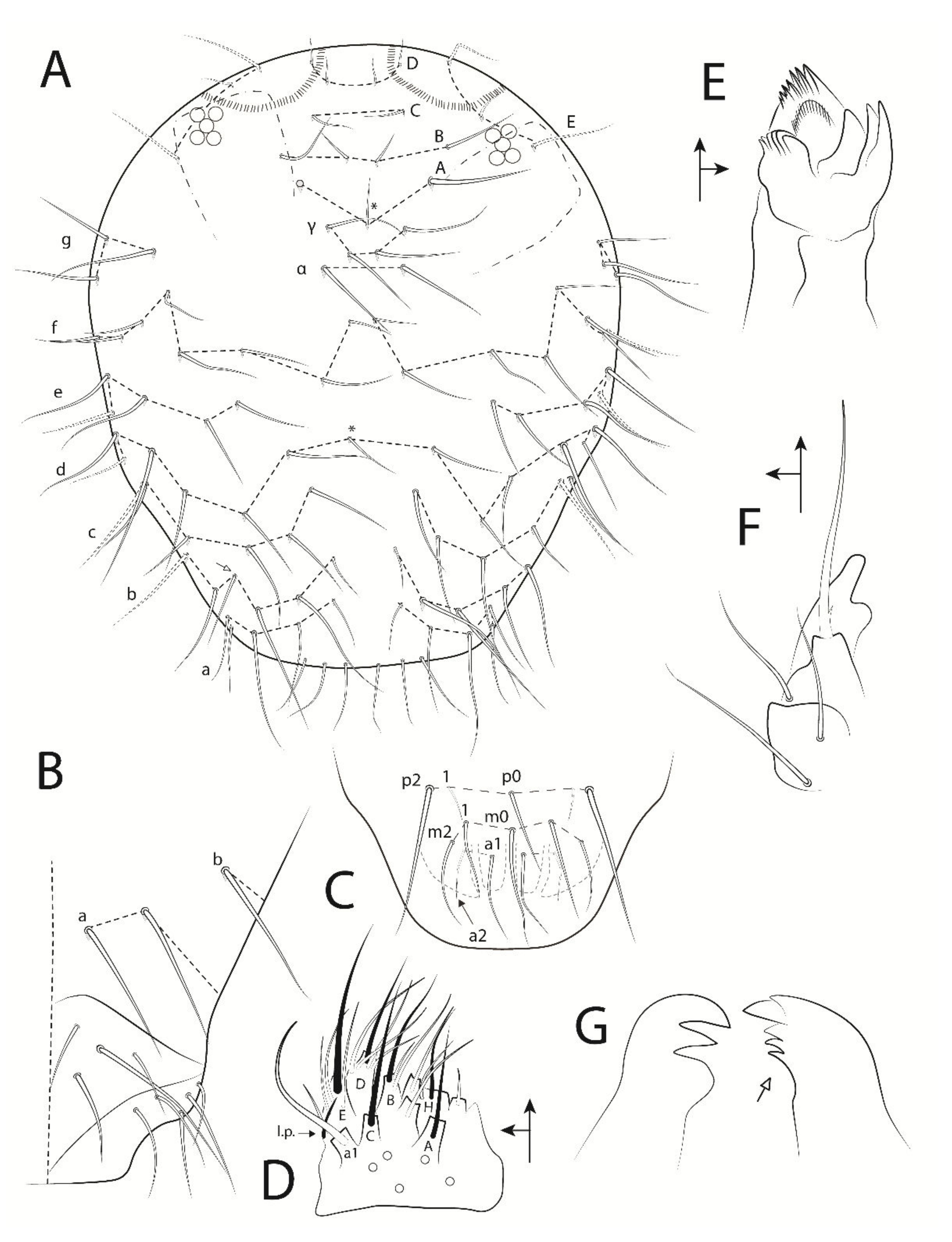
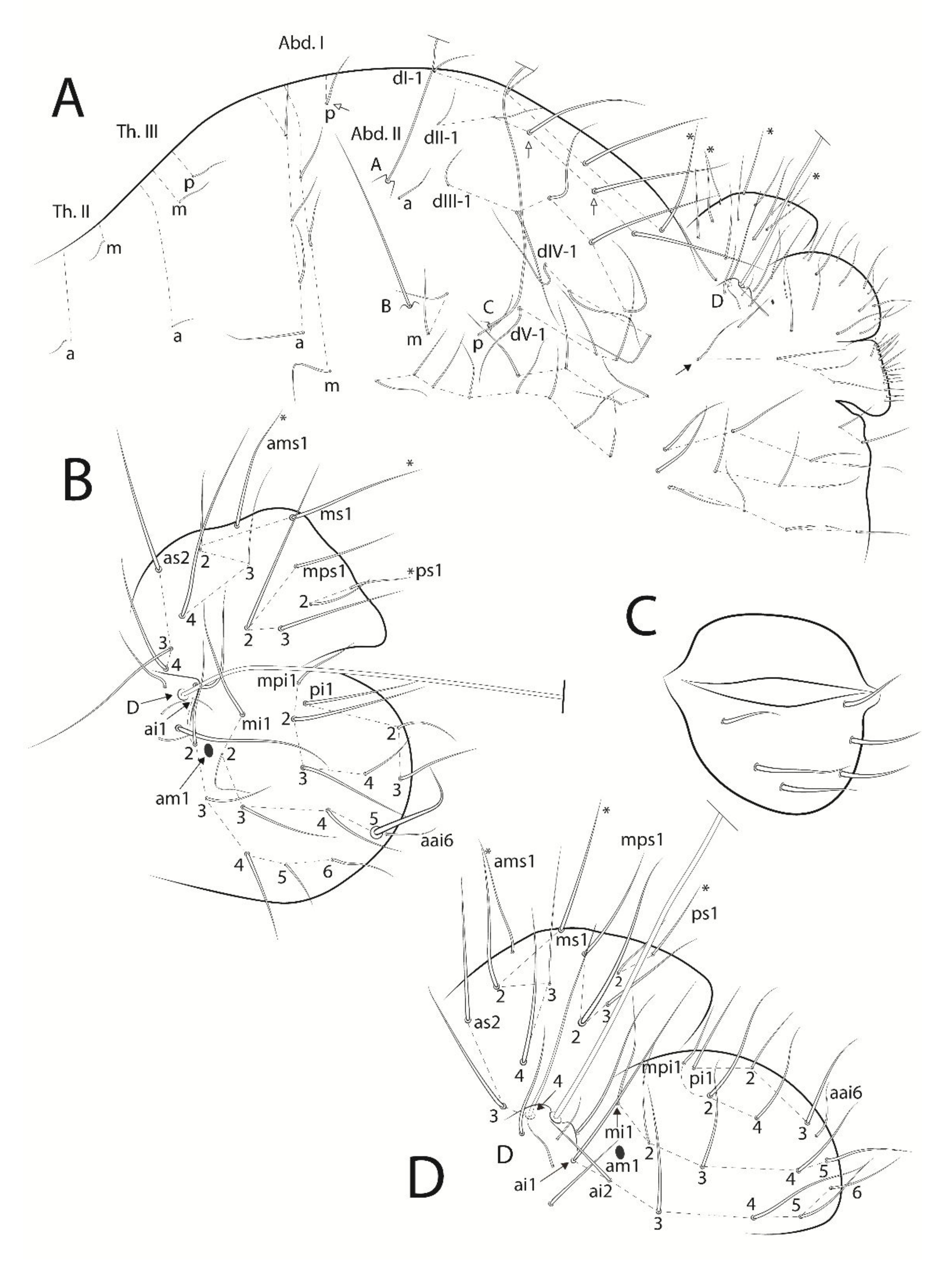
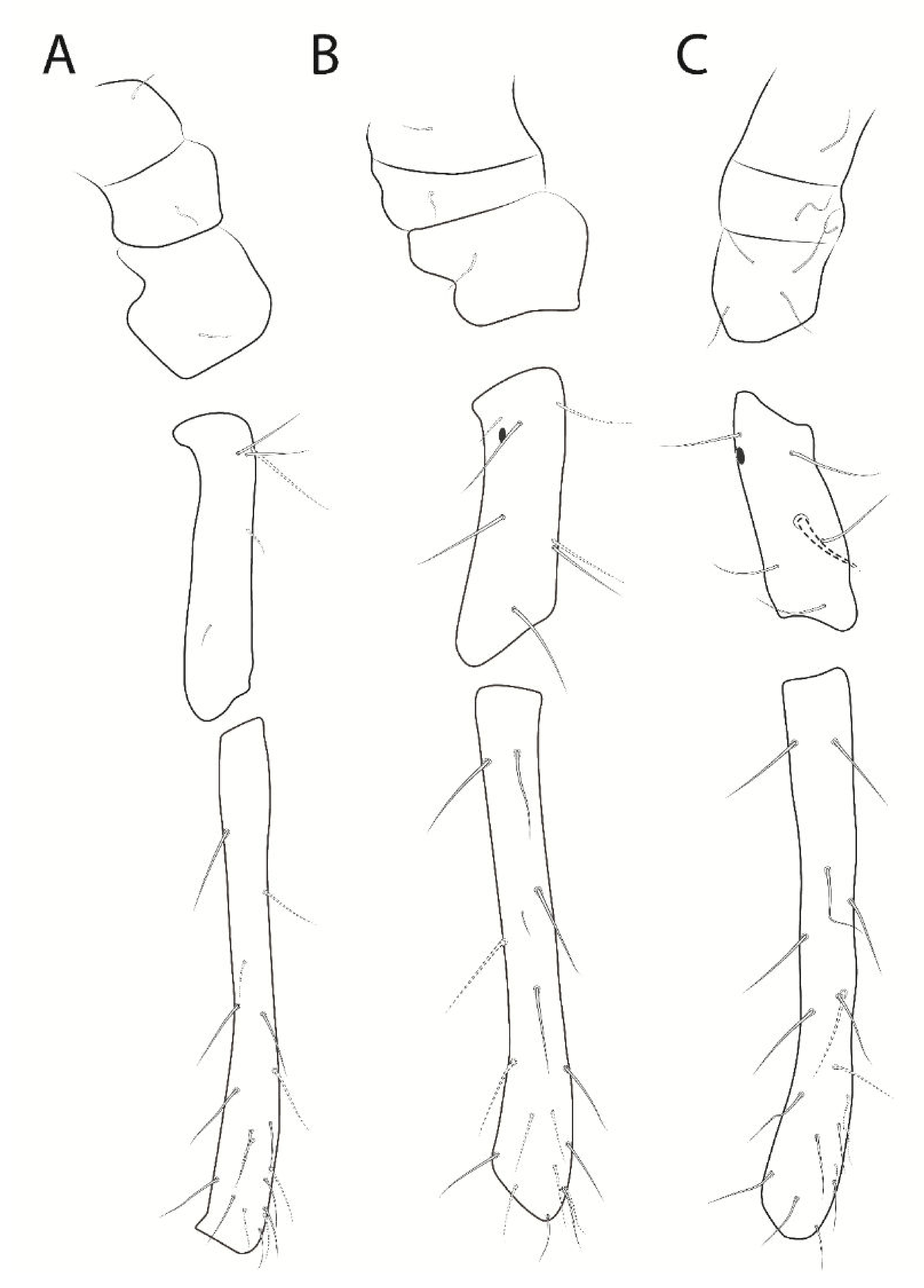
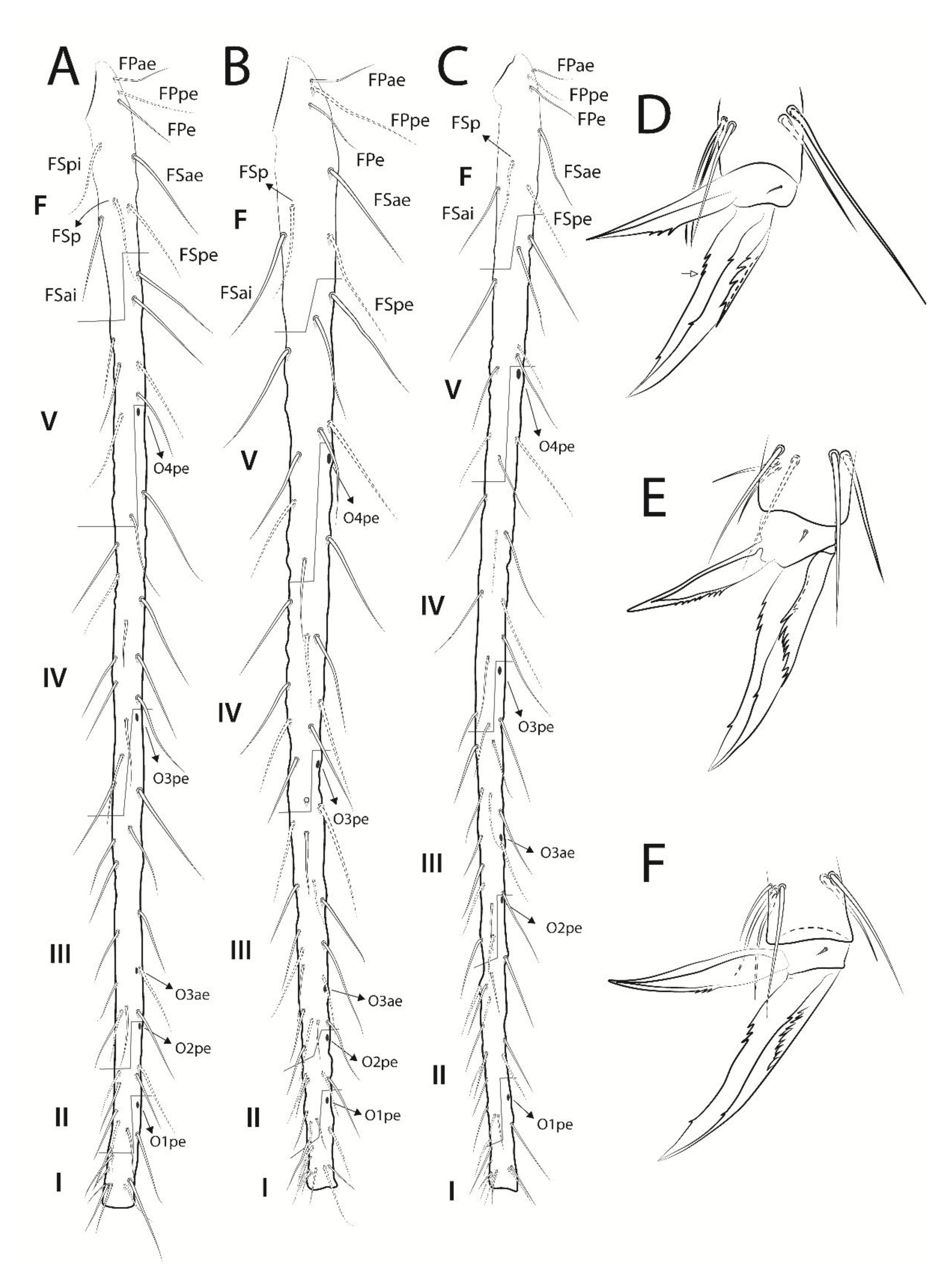
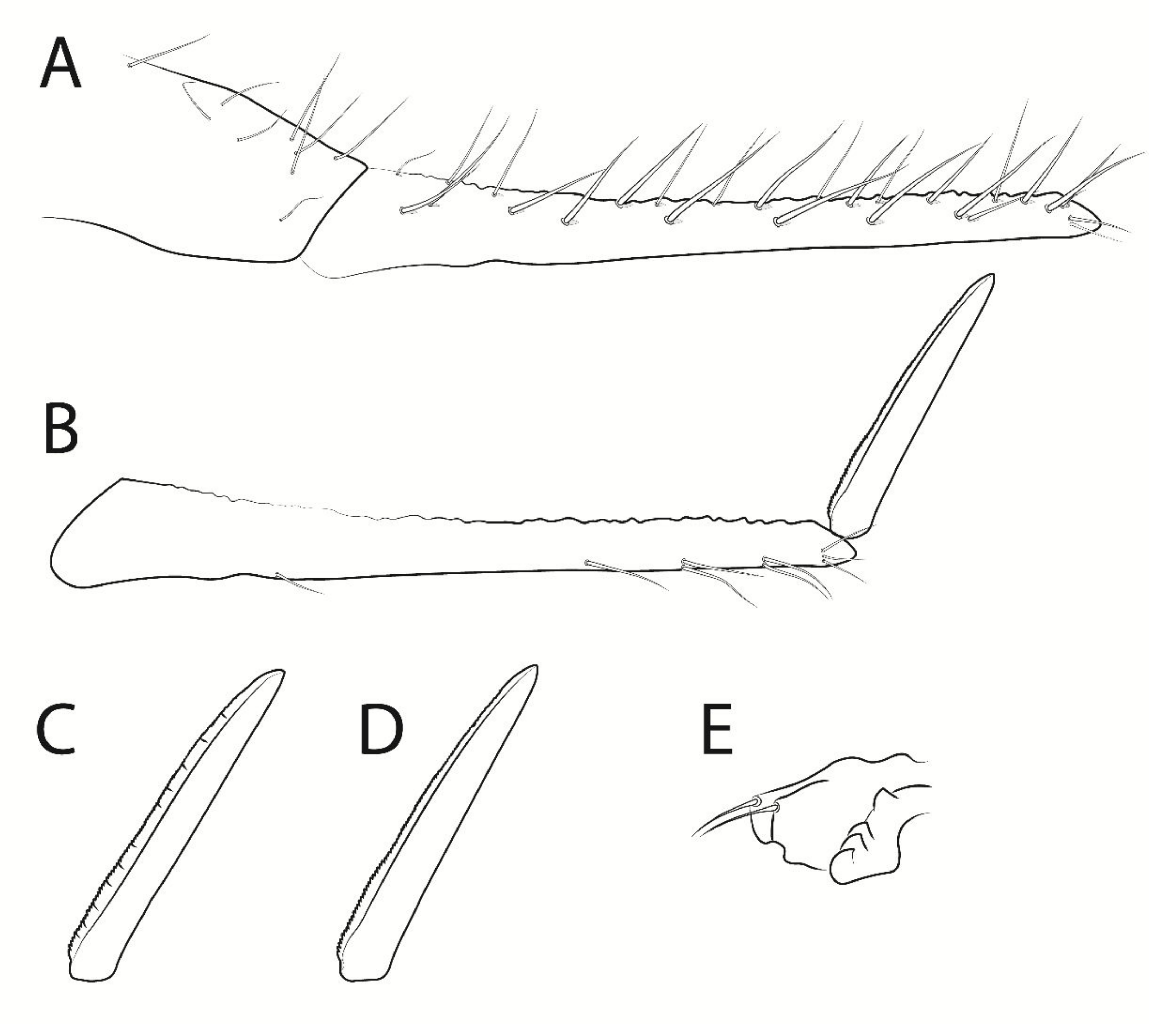
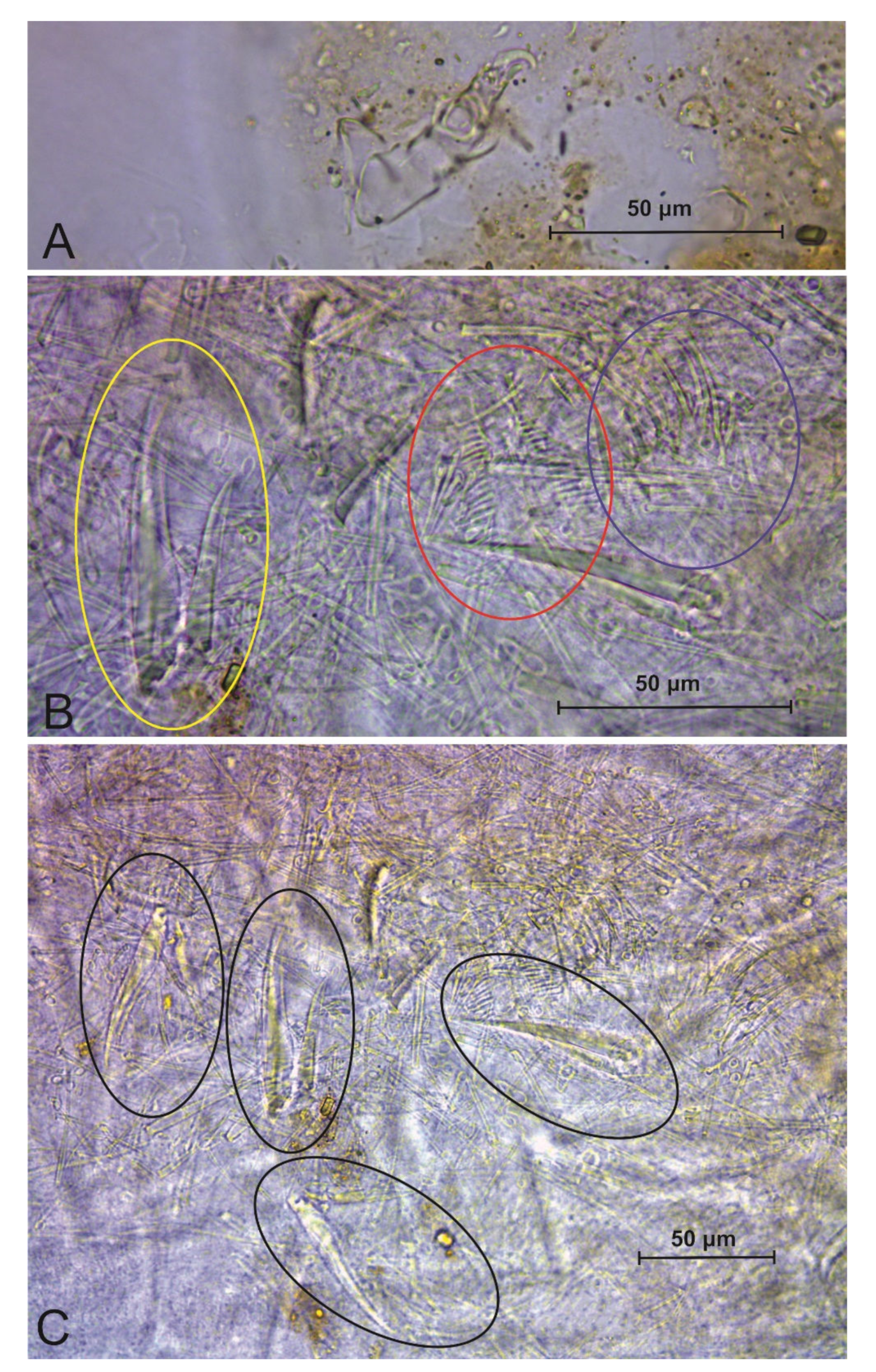
3.3. Troglobentosminthurus luridus gen. nov. sp. nov. Souza, Medeiros and Bellini (Figures 1–10 and Table 2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culik, M.P.; Zeppelini-Filho, D. Diversity and distribution of Collembola (Arthropoda: Hexapoda) of Brazil. Biodivers. Conserv. 2013, 12, 1119–1143. [Google Scholar] [CrossRef]
- Zeppelini, D.; Queiroz, G.C.; Bellini, B.C. Symphypleona in Catálogo Taxonômico da Fauna do Brasil. PNUD. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/470 (accessed on 6 June 2022).
- Bretfeld, G. Known and new genera and species of Symphypleona (Insecta, Collembola) obtained by canopy fogging in Central Amazonia, Brazil. Amazoniana 2002, 17, 109–137. [Google Scholar]
- Zeppelini, D.; Brito, R.A.; Zampaulo, R.; Lima, E.C. A new highly dimorphic genus of Sminthuridae (Collembola: Symphypleona) from Brazil. Zootaxa 2020, 4729, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.D.; Palacios-Vargas, J.G.; Bellini, B.C. A new genus and a new species of Sminthuridae (Collembola: Symphypleona) from Atlantic Forest of Brazil. Zootaxa 2015, 3990, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Bonet, F. Nuevos generous y species de hipogastrúridos de México (Collembola). Rev. Soc. Mex. Hist. Nat. 1945, 6, 13–45. [Google Scholar]
- Nicolet, H. Recherches pour servir á l’Histoire des Podurelles. Nouv. Mém. Soc. Helvet. Sci. Nat. 1842, 6, 1–88. [Google Scholar]
- Palacios-Vargas, J.G.; Wilson, J.M. Troglobius coprophagus, a new genus and species of cave Collembola from Madagascar, with notes on its ecology. Int. J. Speleol. 1990, 19, 67–73. [Google Scholar] [CrossRef]
- Mills, H.B. Collembola from Yucatan Caves. Carnegie Inst. Wash. Publ. 1938, 491, 183–190. [Google Scholar]
- Schäffer, C. Apterygoten. Hamb. Magalh. Samm. 1897, 8, 1–48. [Google Scholar]
- Yosii, R. Höhlencollembola Japans II. Jpn. J. Environ. Entomol. Zool. 1956, 11, 609–627. [Google Scholar]
- Börner, C. Das System der Collembolen nebst Beschreibung neuer Collembolen des Hamburger Naturhistorischen Museums. Mitt. Aus Dem Nat. Mus. Hambg. 1906, 23, 147–188. [Google Scholar]
- Bonet, F.; Tellez, C. Un Nuevo Genero de Esminturidos (Collembola). Rev. Soc. Mex. Hist. Nat. 1947, 8, 193–203. [Google Scholar]
- Palacios-Vargas, J.G.; Gnaspini-Netto, P. A new Brazilian species of Acherontides (Collembola: Hypogastruridae), with notes on its Ecology. J. Kansas Entomol. Soc. 1992, 65, 443–447. [Google Scholar]
- Oliveira, J.V.L.C.; Brito, N.P.; Zeppelini, D. Two New Cyphoderus Nicolet (Collembola: Paronellidae) of the “bidenticulati-group” with Dental Plurichaetosis from Brazil. Neotrop. Entomol. 2021, 50, 579–592. [Google Scholar] [CrossRef]
- Zeppelini, D.; Silva, D.D.; Palacios-Vargas, J.G. A new species of Troglobius (Collembola, Paronellidae, Cyphoderinae) from a Brazilian iron cave. Subterr. Biol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, R. Paronellid Collembola from Caves of Central and South America collected by P. Strinati. Rev. Suisse Zool. 1988, 95, 449–459. [Google Scholar]
- Cipola, N.G.; Oliveira, J.V.L.C.; Bellini, B.C.; Ferreira, A.S.; Lima, E.C.A.; Brito, R.A.; Stievano, L.C.; Souza, P.G.C.; Zeppelini, D. Review of Eyeless Pseudosinella Schäffer (Collembola, Entomobryidae, and Lepidocyrtinae) from Brazilian Caves. Insects 2020, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Brito, R.A.; Lima, E.C.A.; Zeppelini, D. Three new species of Collembola (Arthropoda: Hexapoda) from Brazil. Zootaxa 2019, 4700, 401–430. [Google Scholar] [CrossRef]
- Zeppelini-Filho, D.; Palacios-Vargas, J.G. A new troglomorphic species of Arrhopalites (Collembola: Arrhopalitidae) from Brazil. J. N. Y. Entomol. Soc. 1999, 107, 78–81. [Google Scholar]
- Zeppelini, D.; Brito, R.A. Two new species of Pararrhopalites (Collembola: Symphypleona: Sminthuridae) in Brazil. Fla. Entomol. 2014, 97, 1733–1744. [Google Scholar] [CrossRef]
- Bento, D.M.; Ferreira, R.L.; Prous, X.; Souza-Silva, M.; Bellini, B.C.; Vasconcellos, A. Seasonal variations in cave invertebrate communities in the semiarid Caatinga, Brazil. J. Cave Karst Stud. 2016, 78, 61–71. [Google Scholar] [CrossRef]
- Bento, D.M.; Souza-Silva, M.; Vasconcellos, A.; Bellini, B.C.; Prous, X.; Ferreira, R.L. Subterranean “oasis” in the Brazilian semiarid region: Neglected sources of biodiversity. Biodivers. Conserv. 2021, 30, 3837–3857. [Google Scholar] [CrossRef]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World. Available online: http://www.collembola.org (accessed on 6 June 2022).
- Betsch, J.M. Éléments pour une monographie des Collemboles Symplyplêones (Hexapodes, Aptérygotes). Mém. Mus. Natl. Hist. Nat. Sér. A, Zool. 1980, 116, 1–227. [Google Scholar]
- Bretfeld, G. Synopses on Palaeartic Collembola, Volume 2. Symphypleona. Abh. Ber. Naturkundemus. Gorlitz 1999, 71, 1–318. [Google Scholar]
- Bernard, C.E.; Wynne, J.J. Disparrhopalites naasaveqw n. sp. from caves at Wupatki National Monument, Arizona, synonymy of Dietersminthurus Palacios-Vargas, Cuéllar & Vázquez, 1998 with Disparrhopalites Stach, 1956 and composition of Songhaicinae (Collembola: Sminthuridae). Zootaxa 2017, 4319, 77–90. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Engel, M.S. Long-term stasis in a diverse fauna of Early Cretaceous springtails (Collembola: Symphypleona). J. Syst. Palaeontol. 2016, 15, 513–537. [Google Scholar] [CrossRef]
- Medeiros, G.S.; Greenslade, P.; Bellini, B.C. The Rare Richardsitas Betsch (Collembola, Symphypleona, Sminthuridae): A New Species from Australia with Comments on the Genus and on the Sminthurinae. Insects 2020, 11, 519. [Google Scholar] [CrossRef]
- Arlé, R.; Mendonça, C. Estudo preliminar das espécies de Dicranocentrus Schött, 1893, ocorrentes no Parque Nacional da Tijuca, Rio de Janeiro (Collembola). Rev. Brasil. Biol. 1982, 42, 41–49. [Google Scholar]
- Jordana, R.; Arbea, J.I.; Simón, C.; Luciáñez, M.J. Fauna Iberica Volume 8: Collembola, Poduromorpha, 1st ed.; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 1997; pp. 1–807. [Google Scholar]
- Fjellberg, A. The Labial Palp in Collembola. Zool. Anz. 1999, 237, 309–330. [Google Scholar]
- Cipola, N.G.; Morais, J.W.; Bellini, B.C. A new species of Seira (Collembola: Entomobryidae: Seirini) from Northern Brazil, with the addition of new chaetotaxic characters. Zoologia 2014, 31, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Betsch, J.M.; Waller, A. Chaetotaxic nomenclature of the head, thorax and abdomen in Symphypleona (Insecta, Collembola). Acta Zool. Fenn. 1994, 195, 5–12. [Google Scholar]
- Vargovitsh, R.S. New cave Arrhopalitidae (Collembola: Symphypleona) from the Crimea (Ukraine). Zootaxa 2009, 2047, 1–47. [Google Scholar] [CrossRef]
- Vargovitsh, R.S. New troglomorphic Arrhopalitidae (Collembola: Symphypleona) from the Western Caucasus. Zootaxa 2012, 3174, 1–21. [Google Scholar] [CrossRef]
- Vargovitsh, R.S. Cavernicolous Arrhopalites abchasicus sp. nov. (Collembola: Symphypleona: Arrhopalitidae) from the West Caucasus with a key to the World species of the genus. Zootaxa 2013, 3666, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Betsch, J.M. An ontogenetically focused chaetotaxial scheme in Symphypleona (collembolan): The 6th abdominal segment. Pedobiologia 1997, 41, 13–18. [Google Scholar]
- Nayrolles, P. Chetotaxie tibiotarsale des collemboles symphyplèones. Trav. Lab. Ecobiol. Arthr. édaph. Toulouse 1988, 5, 1–19. [Google Scholar]
- Börner, C. Neue Collembolenformen und zur Nomenclatur der Collembola Lubbock. Zool. Anz. 1901, 24, 696–712. [Google Scholar]
- Bretfeld, G. Phylogenetic systematics of the higher taxa of Symphypleona Borner, 1901 (Insecta, Entognatha, Collembola). Proc. 2nd Intern. Sem. Apterygota 1986, 1, 302–311. [Google Scholar]
- Bretfeld, G. Sturmius epiphytus n. gen. n. spec. from Colombia, a taxon of the Symphypleona (Insecta, Colembola) with an unexpected character combination. Description and position in non-Linnean and Linnean classifications of the Symphypleona. Zoolog. Syst. Evol. Res. 1994, 32, 264–281. [Google Scholar] [CrossRef]
- Lubbock, J. Notes on the Thysanura. Part, I. Trans. Linn. Soc. Lond. 1862, 23, 429–448. [Google Scholar] [CrossRef]
- Zeppelini, D.; Silva, D.D. A new Stenognathriopes (Collembola, Symphypleona, Bourletiellidae) from Brazilian coast. Zootaxa 2012, 3540, 51–58. [Google Scholar] [CrossRef]
- Yosii, R. On some Collembola of Japan and adjacent countries. Contrib. Biol. Lab. Kyoto Univ. 1965, 19, 1–71. [Google Scholar]
- Lawrence, P.N. South Pacific Symphypleona. Pac. Insects 1968, 10, 325–340. [Google Scholar]
- Ellis, W.N. Some Collembola from Ibiza with descriptions of three new species, and a note on Hypogastrura serrata (Agren, 1904). Bul. Zool. Mus. Univ. Amsterdam 1974, 3, 125–141. [Google Scholar]
- Snider, R.J. New species of Sminthuridae from North America (Collembola: Symphypleona). Gt. Lakes Entomol. 1978, 11, 217–241. [Google Scholar]
- Betsch, J.M.; Weiner, W.M. Sphyrotheca Börner, 1906 and Szeptyckitheca gen. n. (Collembola, Symphypleona) from North Korea. Acta Zool. Cracov. 2009, 52, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Zeppelini, D.; Lopes, B.C.H.; Lima, E.C.A. A new species of Szeptyckitheca (Collembola, Symphypleona, Sphyrothecinae) from Brazil. Neotrop. Entomol. 2018, 48, 269–276. [Google Scholar] [CrossRef]
- Delamare-Deboutteville, C.; Massoud, Z. Collemboles symphypléones. In Biologie de L’Amérique Australe, 1st ed.; Delamare-Deboutteville, C., Rapoport, E., Eds.; Éditions du CNRS: Paris, France, 1963; Volume 2, pp. 169–289. [Google Scholar]
- Betsch, J.M. Étude des Collemboles de Madagascar IV—Deux nouveaux genres de Symphypléones à dimorphisme sexuel important: Parabourletiella et Richardsitas. Rev. Ecol. Biol. Sol. 1975, 12, 477–485. [Google Scholar]
- Ćurčić, B.P.M.; Lučić, L.R.; Tomić, V.T.; Makarov, S.E.; Karaman, I.M. Galeriella liciniana, a new cave genus and species of springtails (Collembola, Sminthuridae) from Herzegovina. Arch. Biol. Sci. 2007, 59, 63–64. [Google Scholar] [CrossRef]
- Medeiros, G.S.; Bellini, B.C. Two new species of Temeritas Richards (Collembola, Symphypleona, Sminthuridae) from Neotropical Region, with coments on the genus. Zootaxa 2019, 4586, 536–552. [Google Scholar] [CrossRef]
- Lukić, M. Chapter 35–Collembola. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: Cambridge, UK, 2019; Volume 1, pp. 308–319. [Google Scholar]
- Latreille, P.A. Histoire naturelle, générale et particulière des Crustacés et des Insectes III. F. Dufart Paris 1802, 15, 69–83. [Google Scholar]
- Stach, J. The Apterygotan Fauna of Poland in Relation to the World-Fauna of This Group of Insects. Family: Sminthuridae, 1st ed.; Polska Akademia Nauk, PWN: Krakow, Poland, 1956; pp. 1–287. [Google Scholar]
- Betsch, J.M. Étude des Collemboles de Madagascar V—Sur deux Symphypléones de la forét séche en secteur bioclimatique subaride. Bull. Soc. Entomol. Fr. 1977, 82, 119–125. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes-Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Cole, M.M. Cerrado, Caatinga and Pantanal: The Distribution and Origin of the Savanna Vegetation of Brazil. Geog. J. 1960, 126, 168–179. [Google Scholar] [CrossRef]
- Souza-Silva, M.; Cerqueira, R.F.V.; Pellegrini, T.G.; Ferreira, R.L. Habitat selection of cave-restricted fauna in a new hotspot of subterranean biodiversity in Neotropics. Biodivers. Conserv. 2021, 30, 4223–4250. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.; New, T.R. The seven impediments in invertebrate conservation and how to overcome them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.L.; Bernard, E.; Cruz-Júnior, F.W.; Piló, L.B.; Calux, A.; Souza-Silva, M.; Barlow, J.; Pompeu, P.S.; Cardoso, P.; Mammola, S.; et al. Brazilian cave heritage under siege. Science 2022, 375, 1238–1239. [Google Scholar] [CrossRef]
- Xiong, Y.; Gao, Y.; Yin, W.Y.; Luan, Y.X. Molecular phylogeny of Collembola inferred from ribosomal RNA genes. Mol. Phylogenet. Evol. 2008, 49, 728–735. [Google Scholar] [CrossRef]
- Schneider, C.; Cruaud, C.; D’Haese, C.A.D. Unexpected diversity in Neelipleona revealed by molecular phylogeny approach (Hexapoda, Collembola). Soil Org. 2011, 83, 383–398. [Google Scholar]
- Yu, D.; Zhang, F.; Stevens, M.I.; Yan, Q.; Liu, M.; Hu, F. New insight into the systematics of Tomoceridae (Hexapoda, Collembola) by integrating molecular and morphological evidence. Zool. Scr. 2015, 45, 286–299. [Google Scholar] [CrossRef]
- Leo, C.; Carapelli, A.; Cicconardi, F.; Frati, F.; Nardi, F. Mitochondrial genome diversity in Collembola: Phylogeny, dating and gene order. Diversity 2019, 11, 169. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Yu, D.; Xie, Z.; Dong, J.; Ding, Y.; Yao, H.; Greenslade, P. Phylomitogenomic analyses on collembolan higher taxa with enhanced taxon sampling and discussion on method selection. PLoS ONE 2020, 15, e0230827. [Google Scholar] [CrossRef] [PubMed]
- Cucini, C.; Fanciulli, P.P.; Frati, F.; Convey, P.; Nardi, F.; Carapelli, A. Re-evaluating the internal phylogenetic relationships of Collembola by means of Mitogenome Data. Genes 2021, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, C.A. Morphological appraisal of Collembola phylogeny with special emphasis on Poduromorpha and a test of the aquatic origin hypothesis. Zool. Scr. 2003, 32, 563–586. [Google Scholar] [CrossRef]


| Subfamilies/ Features | Antennae/Body Ratio | Ant. IV Subsegments | Distal Whorl of Chaetae on Tibiotarsi | Pretarsal Chaetae | Ungues with Cavity and/or a Filament-Like Tunica | Neosminthuroid Chaetae on the Parafurcal Area | Males’ Abd. VI Ventrally with a Pair of Spines | Dens Ventral Chaetae | Mucronal Apex |
|---|---|---|---|---|---|---|---|---|---|
| Sminthurinae | =/(>) | 9–46 | 8–(9) | 1–(2) | − | +/(−) | − | 9–15 | (sym)/asym |
| Songhaicinae | variable | 10–26 | 6 or less | 1–(2) | + | +/(−) | − | 5–13 | sym |
| Sphyrothecinae | < | 0–12 * | 7–9 | 2 | − | + | + | 0–6 | asym |
| Genera/ Features | Ant. IV Subsegments | Eyes | Head Sexual Dimorphism | Antennae Sexual Dimorphism | Head Frontal Area Spines | Abdominal Dorso-Anterior Spines | Abdominal Dorso-Posterior Spines | Large Abdomen Sexual Dimorphism | Neosminthuroid Chaetae on Large Abdomen | Tibiotarsi distal Whorl Chaetae | Pretarsi Posterior Chaeta | Capitate Tenent-Hairs on Tibiotarsi II–III | Ungues Tunica | Ungues Pseudonychia | Ungues Inner teeth | Unguiculi Apical filament | Unguiculi Inner Teeth | Dens Ventral Chaetae | Mucronal Chaeta | Mucro Sexual Dimorphism |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Galeriella | 32 | 0 + 0 | −? | − | −? | −? | −? | −? | −? | ? | ? | − | − | − | − | − | − | ? | − | −? |
| Keratosminthurus | 18–20 | 8 + 8 | + | + | − | − | − | − | + | 9 | + | − | +/− | - | 1 | + | +/− | 12–13 | − | − |
| Richardsitas | 28–30 | 8 + 8 | − | − | + | + | + | + | − | 9 | + | + | − | + | 1–2 | + | + | 13 | +/− | − |
| Temeritas | 18–46 | 8 + 8 | − | − | + | +/− | − | +/− | − | 9 | + | − | +/− | +/− | 1–2 | + | +/− | 13 | +/− | − |
| Troglobentosminthurus gen. nov. | 44 | 5 + 5 | − | − | − | − | − | + | − | 8–9 | − | − | − | + | 3–4 | − | + | 9 | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, P.G.C.; Medeiros, G.d.S.; Ferreira, R.L.; Souza-Silva, M.; Bellini, B.C. A Highly Troglomorphic New Genus of Sminthuridae (Collembola, Symphypleona) from the Brazilian Semiarid Region. Insects 2022, 13, 650. https://doi.org/10.3390/insects13070650
de Souza PGC, Medeiros GdS, Ferreira RL, Souza-Silva M, Bellini BC. A Highly Troglomorphic New Genus of Sminthuridae (Collembola, Symphypleona) from the Brazilian Semiarid Region. Insects. 2022; 13(7):650. https://doi.org/10.3390/insects13070650
Chicago/Turabian Stylede Souza, Paolla Gabryelle Cavalcante, Gleyce da Silva Medeiros, Rodrigo Lopes Ferreira, Marconi Souza-Silva, and Bruno Cavalcante Bellini. 2022. "A Highly Troglomorphic New Genus of Sminthuridae (Collembola, Symphypleona) from the Brazilian Semiarid Region" Insects 13, no. 7: 650. https://doi.org/10.3390/insects13070650
APA Stylede Souza, P. G. C., Medeiros, G. d. S., Ferreira, R. L., Souza-Silva, M., & Bellini, B. C. (2022). A Highly Troglomorphic New Genus of Sminthuridae (Collembola, Symphypleona) from the Brazilian Semiarid Region. Insects, 13(7), 650. https://doi.org/10.3390/insects13070650










