Phenotypic Plasticity of the Mimetic Swallowtail Butterfly Papilio polytes: Color Pattern Modifications and Their Implications in Mimicry Evolution
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
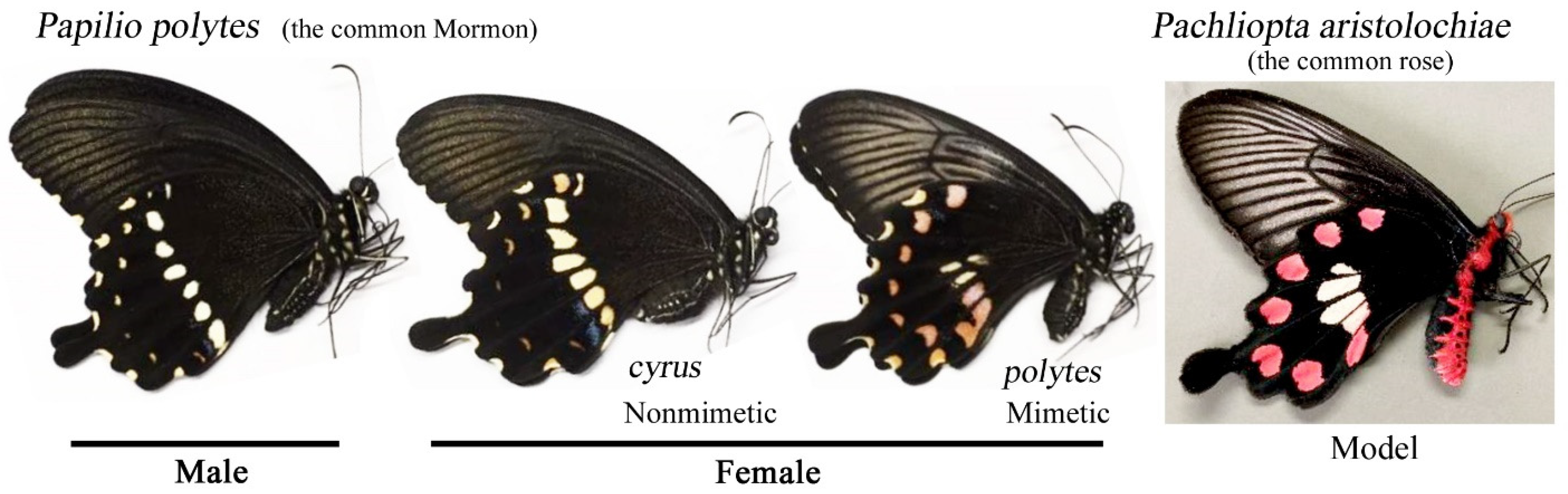
2.1. Nomenclature of Spots and Key Differences between Nonmimetic and Mimetic Forms
2.2. Butterflies and the Host Plant Leaves
2.3. Sibling Groups
2.4. Egg Collection and Larval Rearing
2.5. Cold Shock and Heat Shock Treatments
2.6. Injections of Tungstate and FB28
2.7. Evaluation of Modifications
2.8. Image Acquisition and Processing
2.9. Statistical Analyses
3. Results
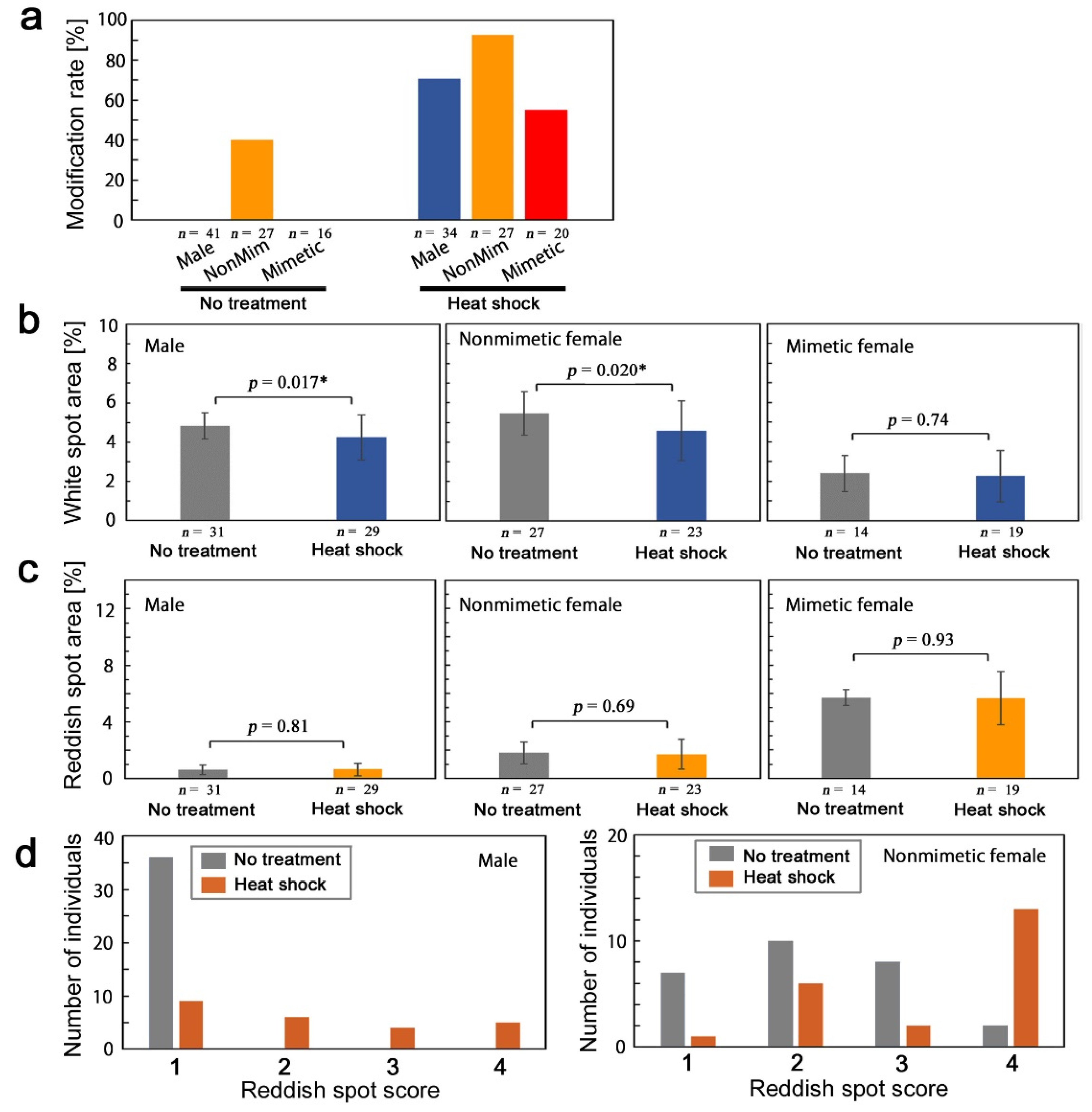
3.1. Pilot Experiments for Cold Shock and Heat Shock Conditions
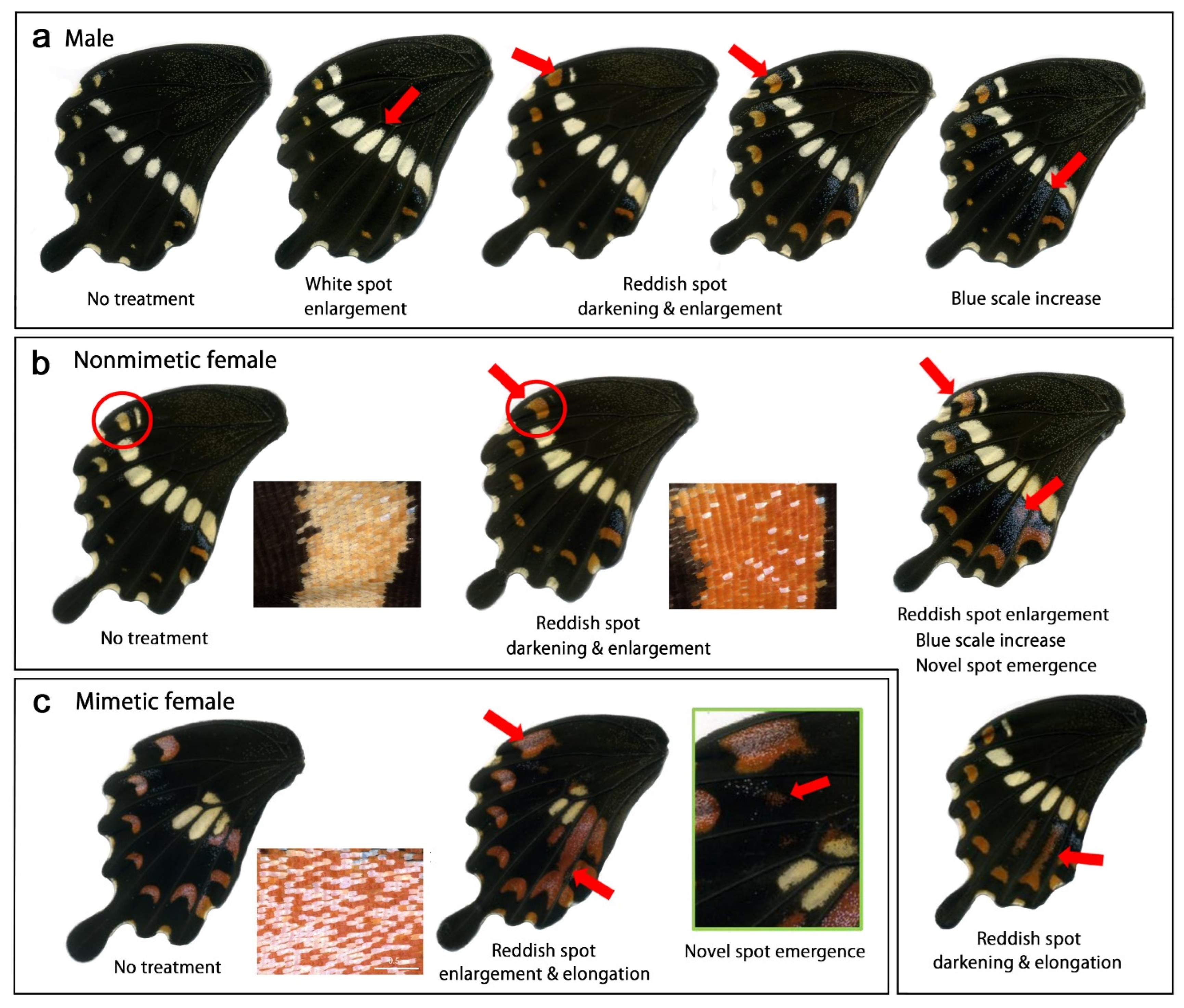
3.2. Modifications Induced by Cold Shock
3.2.1. Modification Patterns
3.2.2. Quantitative Evaluation
3.3. Modifications Induced by Heat Shock
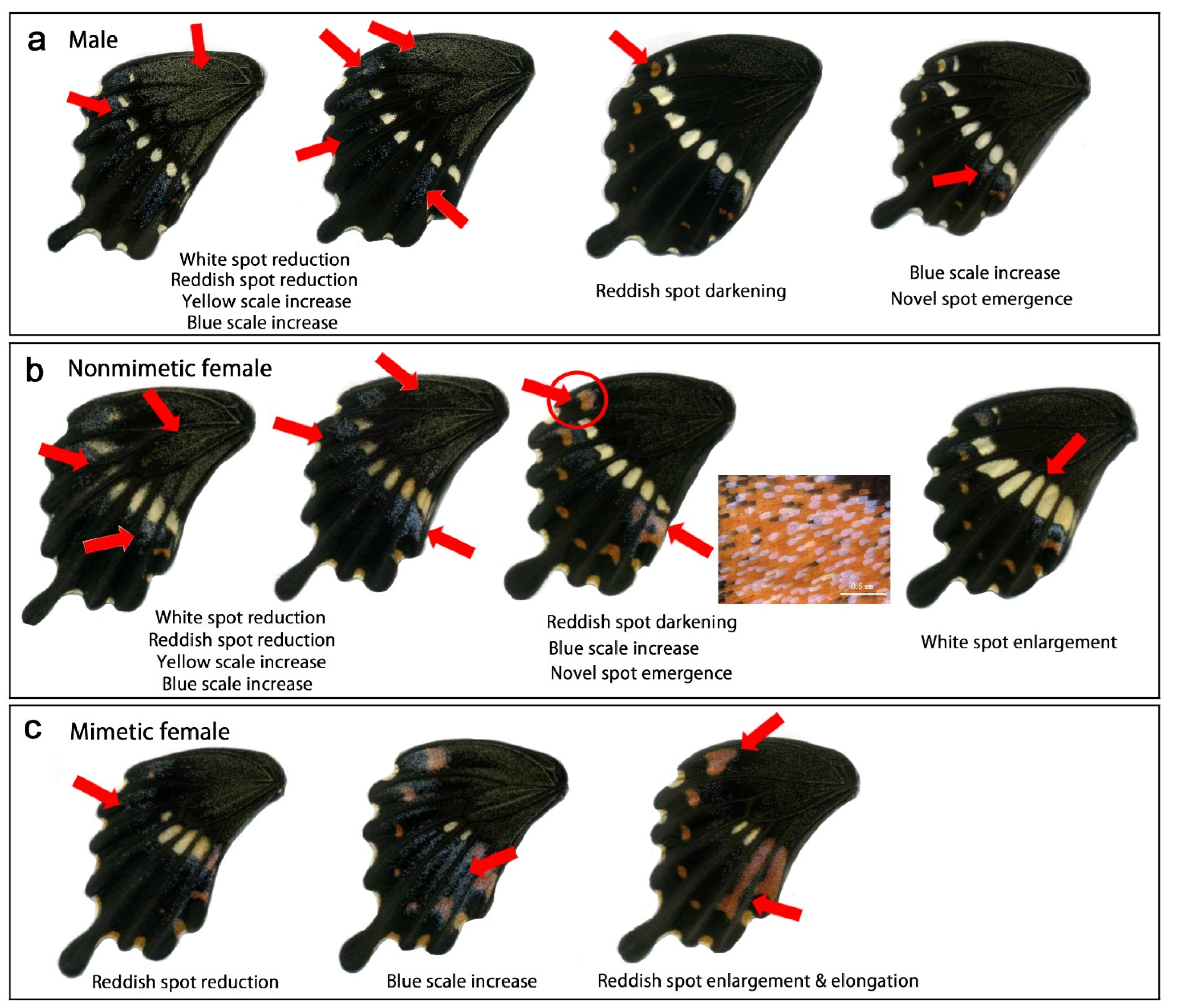
3.3.1. Modification Patterns
3.3.2. Quantitative Evaluation
3.3.3. Sibling-Dependent Response
3.4. Modification Inducers: Tungstate and FB28
3.5. Summary of Modifications
4. Discussion
4.1. Modification Induction in Papilionid Butterflies and P. polytes
4.2. Real-Time Evolution via Phenotypic Plasticity
4.3. Evolution of Mimetic and Nonmimetic Female-Limited Dimorphism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sibling Group | Mother | Treatment |
|---|---|---|
| SG1 | Nonmimetic | Heat shock and cold shock |
| SG2 | Mimetic | Cold shock |
| SG3 | Nonmimetic | Cold shock |
| SG4 | Nonmimetic | Cold shock |
| SG5 | Nonmimetic | Heat shock |
| SG6 | Mimetic | Tungstate injection (1 M, 5 or 25 μL) |
| SG7 | Nonmimetic | Tungstate injection (1 M, 1–4 μL) |
| SG8 | Nonmimetic | FB28 injection (30%, 2 μL) |
| Modifications | Male | Nonmimetic Female | Mimetic Female | |||
|---|---|---|---|---|---|---|
| NT | CS | NT | CS | NT | CS | |
| Total number of individuals | 61 | 46 | 39 | 32 | 21 | 16 |
| White spot reduction | 0 | 0 | 0 | 0 | 0 | 0 |
| White spot enlargement | 0 | 5 | 0 | 5 | 0 | 2 |
| Reddish spot reduction | 0 | 0 | 0 | 0 | 0 | 0 |
| Reddish spot enlargement | 0 | 25 | 0 | 8 | 0 | 3 |
| Reddish spot darkening | 2 | 29 | 7 | 28 | 0 | 0 |
| Reddish spot elongation | 0 | 0 | 0 | 1 | 0 | 0 |
| Novel spot emergence | 0 | 0 | 0 | 4 | 0 | 1 |
| Increase in blue scales | 0 | 2 | 0 | 4 | 0 | 0 |
| Increase in yellow scales (forewing) | 0 | 0 | 0 | 0 | 0 | 0 |
| Increase in yellow scales (hindwing) | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of modified individuals | 2 | 40 | 7 | 31 | 0 | 6 |
| Modification rate (%) | 3.3% | 87.0% | 17.9% | 96.9% | 0% | 37.5% |
| Modifications | Male | Nonmimetic Female | Mimetic Female | |||
|---|---|---|---|---|---|---|
| NT | HS | NT | HS | NT | HS | |
| Total number of individuals | 41 | 34 | 27 | 27 | 16 | 20 |
| White spot reduction | 0 | 7 | 0 | 6 | 0 | 1 |
| White spot enlargement | 0 | 1 | 0 | 1 | 0 | 2 |
| Reddish spot reduction | 0 | 3 | 0 | 1 | 0 | 6 |
| Reddish spot enlargement | 0 | 2 | 0 | 1 | 0 | 4 |
| Reddish spot darkening | 0 | 15 | 10 | 15 | 0 | 0 |
| Reddish spot elongation | 0 | 0 | 0 | 0 | 0 | 0 |
| Novel spot emergence | 0 | 2 | 1 | 11 | 0 | 0 |
| Increase in blue scales | 0 | 2 | 0 | 5 | 0 | 3 |
| Increase in yellow scales (forewing) | 0 | 3 | 0 | 4 | 0 | 0 |
| Increase in yellow scales (hindwing) | 0 | 5 | 0 | 1 | 0 | 2 |
| Number of modified individuals | 0 | 24 | 11 | 25 | 0 | 11 |
| Modification rate (%) | 0% | 70.6% | 40.1% | 92.6% | 0% | 55.0% |
| Modifications | Male | Nonmimetic Female | ||||||
|---|---|---|---|---|---|---|---|---|
| SG1 | SG5 | SG1 | SG5 | |||||
| NT | HS | NT | HS | NT | HS | NT | HS | |
| Total number of individuals | 10 | 10 | 31 | 24 | 12 | 12 | 15 | 15 |
| White spot reduction | 0 | 4 | 0 | 3 | 0 | 3 | 0 | 3 |
| White spot enlargement | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Reddish spot reduction | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 |
| Reddish spot enlargement | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| Reddish spot darkening | 0 | 3 | 0 | 12 | 3 | 0 | 7 | 7 |
| Reddish spot elongation | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 |
| Novel spot emergence | 0 | 0 | 0 | 2 | 0 | 3 | 1 | 8 |
| Increase in blue scales | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 2 |
| Increase in yellow scales (forewing) | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 3 |
| Increase in yellow scales (hindwing) | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 1 |
| Number of modified individuals | 0 | 8 | 0 | 16 | 3 | 11 | 8 | 14 |
| Modification rate (%) | 0% | 80.0% | 0% | 66.7% | 25.0% | 91.7% | 53.3% | 93.3% |
References
- Waddington, C.H. Canalization of development and the inheritance of acquired characters. Nature 1942, 150, 563–565. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Developmental Plasticity and Evolution; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Nijhout, H.F.; Davidowitz, G. Developmental perspectives on phenotypic variation, canalization, and fluctuating asymmetry. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: New York, NY, USA, 2003; pp. 3–13. [Google Scholar]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F.; Epel, D. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution; Sinauer: Sunderland, MA, USA, 2009. [Google Scholar]
- Pfennig, D.W.; Wund, M.A.; Snell-Rood, E.C.; Cruickshank, T.; Schlichting, C.D.; Moczek, A.P. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 2010, 25, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Whitman, D.W.; Agrawal, A.A. What is phenotypic plasticity and why it is important. In Phenotypic Plasticity of Insects: Mechanisms and Consequences; Whitman, D., Ananthakrishnan, T.N., Eds.; CRC Press: Enfield, NH, USA, 2009; pp. 1–63. [Google Scholar]
- Smith-Gill, S.J. Developmental plasticity: Developmental conversion versus phenotypic modulation. Am. Zool. 1983, 23, 47–55. [Google Scholar] [CrossRef]
- Nijhout, H.F. The Development and Evolution of Butterfly Wing Patterns; Smithsonian Institution Press: Washington, DC, USA, 1991; pp. 119–131. [Google Scholar]
- Van der Burg, K.R.L.; Reed, R.D. Seasonal plasticity: How do butterfly wing pattern traits evolve environmental responsiveness? Curr. Opin. Genet. Dev. 2021, 69, 82–87. [Google Scholar] [CrossRef]
- Daniels, E.V.; Murad, R.; Mortazavi, A.; Reed, R.D. Extensive transcriptional response associated with seasonal plasticity of butterfly wing patterns. Mol. Ecol. 2014, 23, 6123–6134. [Google Scholar] [CrossRef]
- Van der Burg, K.R.L.; Lewis, J.J.; Brack, B.J.; Fandino, R.A.; Mazo-Vargas, A.; Reed, R.D. Genomic architecture of a genetically assimilated seasonal color pattern. Science 2020, 370, 721–725. [Google Scholar] [CrossRef]
- Allen, C.E.; Zwaan, B.J.; Brakefield, P.M. Evolution of sexual dimorphism in the Lepidoptera. Annu. Rev. Entomol. 2011, 56, 445–464. [Google Scholar] [CrossRef]
- Oliver, J.C.; Monteiro, A. On the origins of sexual dimorphism in butterflies. Proc. Biol. Sci. 2011, 278, 1981–1988. [Google Scholar] [CrossRef]
- Clarke, C.A.; Sheppard, P.M. The genetics of the mimetic butterfly Papilio polytes L. Trans. Linn. Soc. Lond. 1972, 23, 495–566. [Google Scholar]
- Igarashi, S. Papilionidae and Their Early Stages; Kodansha: Tokyo, Japan, 1979. (In Japanese) [Google Scholar]
- Shirôzu, T. The Standard of Butterflies in Japan; Gakken: Tokyo, Japan, 2006. (In Japanese) [Google Scholar]
- Otaki, J.M.; Ogasawara, T.; Yamamoto, H. Tungstate-induced color-pattern modifications of butterfly wings are independent of stress response and ecdysteriod effect. Zool. Sci. 2005, 22, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Reversed type of color-pattern modifications of butterfly wings: A physiological mechanism of wing-wide color-pattern determination. J. Insect Physiol. 2007, 53, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Taira, W.; Otaki, J.M. Color-pattern evolution in response to environmental stress in butterflies. Front. Genet. 2012, 3, 15. [Google Scholar] [CrossRef]
- Shapiro, A.M. Canalization of the phenotype of Nymphalis antiopa (Lepidoptera: Nymphalidae) from subarctic and montane climates. J. Res. Lepid. 1980, 19, 82–87. [Google Scholar] [CrossRef]
- Shapiro, A.M. Phenotypic plasticity in temperate and subarctic Nymphalis antiopa (Nymphalidae): Evidence for adaptive canalization. J. Lepid. Soc. 1981, 35, 124–131. [Google Scholar]
- Nijhout, H.F. Colour pattern modification by coldshock in Lepidoptera. J. Embryol. Exp. Morphol. 1984, 81, 287–305. [Google Scholar] [CrossRef]
- Otaki, J.M. Color-pattern modifications of butterfly wings induced by transfusion and oxyanions. J. Insect Physiol. 1998, 44, 1181–1190. [Google Scholar] [CrossRef]
- Umebachi, Y.; Osanai, M. Perturbation of the wing color pattern of a swallowtail butterfly, Papilio xuthus, induced by acid carboxypeptidase. Zool. Sci. 2003, 20, 325–331. [Google Scholar] [CrossRef][Green Version]
- Serfas, M.S.; Carroll, S.B. Pharmacologic approaches to butterfly wing patterning: Sulfated polysaccharides mimic or antagonize cold shock and alter the interpretation of gradients of positional information. Dev. Biol. 2005, 287, 416–424. [Google Scholar] [CrossRef]
- Otaki, J.M.; Yamamoto, H. Species-specific color-pattern modifications of butterfly wings. Dev. Growth Differ. 2004, 46, 1–14. [Google Scholar] [CrossRef]
- Otaki, J.M. Physiologically induced color-pattern changes in butterfly wings: Mechanistic and evolutionary implications. J. Insect Physiol. 2008, 54, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Sourakov, A. Giving eyespots a shiner: Pharmacologic manipulation of the Io moth wing pattern. F1000Research 2017, 6, 1319. [Google Scholar] [CrossRef]
- Sourakov, A. Emperors, admirals and giants, zebras, tigers and woolly bears: Casting a broader net in exploring heparin effects on Lepidoptara wing patterns. F1000Research 2018, 7, 1842. [Google Scholar] [CrossRef] [PubMed]
- Connahs, H.; Rhen, T.; Simmons, R.B. Physiological perturbation reveals modularity of eyespot development in the painted lady butterfly, Vanessa cardui. PLoS ONE 2016, 11, e0161745. [Google Scholar] [CrossRef]
- Piszter, G.; Kertész, K.; Horváth, Z.E.; Bálint, Z.; Biró, L.P. Reproducible phenotype alteration due to prolonged cooling of the pupae of Polyommatus icarus butterflies. PLoS ONE 2019, 14, e0225388. [Google Scholar] [CrossRef] [PubMed]
- Kertész, K.; Piszter, G.; Horváth, Z.E.; Bálint, Z.; Biró, L.P. Changes in structural and pigmentary colours in response to cold stress in Polyommatus icarus butterflies. Sci. Rep. 2017, 7, 1118. [Google Scholar] [CrossRef]
- Mahdi, S.H.; Gima, S.; Tomita, Y.; Yamasaki, H.; Otaki, J.M. Physiological characterization of the cold-shock-induced humoral factor for wing color-pattern changes in butterflies. J. Insect Physiol. 2010, 56, 1022–1031. [Google Scholar] [CrossRef]
- Dhungel, B.; Otaki, J.M. Local pharmacological effects of tungstate on the color-pattern determination of butterfly wings: A possible relationship between the eyespot and parafocal element. Zool. Sci. 2009, 26, 758–764. [Google Scholar] [CrossRef]
- Otaki, J.M. Butterfly eyespot color pattern formation requires physical contact of the pupal wing epithelium with extracellular materials for morphogenic signal propagation. BMC Dev. Biol. 2020, 20, 6. [Google Scholar] [CrossRef]
- Mahdi, S.H.A.; Yamasaki, H.; Otaki, J.M. Heat-shock-induced color-pattern changes of the blue pansy butterfly Junonia orithya: Physiological and evolutionary implications. J. Therm. Biol. 2011, 36, 312–321. [Google Scholar] [CrossRef]
- Otaki, J.M.; Yamamoto, H. Color-pattern modifications and speciation in butterflies of the genus Vanessa and its related genera Cynthia and Bassaris. Zool. Sci. 2004, 21, 967–976. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otaki, J.M. Phenotypic plasticity of wing color patterns revealed by temperature and chemical applications in a nymphalid butterfly Vanessa indica. J. Therm. Biol. 2008, 33, 128–139. [Google Scholar] [CrossRef]
- Otaki, J.M. Physiological side-effect model for diversification of non-functional or neutral traits: A possible evolutionary history of Vanessa butterflies (Lepidoptera, Nymphalidae). Trans. Lepid. Soc. Jpn. 2008, 59, 87–102. [Google Scholar] [CrossRef]
- Otaki, J.M. Stress-induced color-pattern modifications and evolution of the painted lady butterflies Vanessa cardui and Vanessa kershawi. Zool. Sci. 2007, 24, 811–819. [Google Scholar] [CrossRef]
- Otaki, J.M. Color-pattern modifications and speciaion in lycaenid butterflies. Trans. Lepid. Soc. Jpn. 2003, 54, 197–205. [Google Scholar] [CrossRef]
- Otaki, J.M.; Hiyama, A.; Iwata, M.; Kudo, T. Phenotypic plasticity in the range-margin population of the lycaenid butterfly Zizeeria maha. BMC Evol. Biol. 2010, 10, 252. [Google Scholar] [CrossRef]
- Ohsaki, N. Preferential predation of female butterflies and the evolution of Batesian mimicry. Nature 1995, 378, 173–175. [Google Scholar] [CrossRef]
- Ohsaki, N. A common mechanism explaining the evolution of female-limited and both-sex Batesian mimicry in butterflies. J. Anim. Ecol. 2005, 74, 728–734. [Google Scholar] [CrossRef]
- Kunte, K. Mimetic butterflies support Wallace’s model of sexual dimorphism. Proc. Biol. Sci. 2008, 275, 1617–1624. [Google Scholar] [CrossRef]
- Westerman, E.L.; Letchinger, R.; Tenger-Trolander, A.; Massardo, D.; Palmer, D.; Kronforst, M.R. Does male preference play a role in maintaining female limited polymorphism in a Batesian mimetic butterfly? Behav. Processes 2018, 150, 47–58. [Google Scholar] [CrossRef]
- Westerman, E.L.; Antonson, N.; Kreutzmann, S.; Peterson, A.; Pineda, S.; Kronforst, M.R.; Olson-Manning, C.F. Behaviour before beauty: Signal weighting during mate selection in the butterfly Papilio polytes. Ethology 2019, 125, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Tatsuta, H.; Tsuji, K. Mimicry genes reduce pre-adult survival rate in Papilio polytes: A possible new mechanism for maintaining female-limited polymorphism in Batesian mimicry. J. Evol. Biol. 2020, 33, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Kunte, K.; Zhang, W.; Tenger-Trolander, A.; Palmer, D.H.; Martin, A.; Reed, R.D.; Mullen, S.P.; Kronforst, M.R. doublesex is a mimicry supergene. Nature 2014, 507, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Iijima, T.; Kajitani, R.; Yamaguchi, J.; Ando, T.; Suzuki, Y.; Sugano, S.; Fujiyama, A.; Kosugi, S.; Hirakawa, H.; et al. A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat. Genet. 2015, 47, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Iijima, T.; Yoda, S.; Fujiwara, H. The mimetic wing pattern of Papilio polytes butterflies is regulated by a doublesex-orchestrated gene network. Commun. Biol. 2019, 2, 257. [Google Scholar] [CrossRef]
- Komata, S.; Kitamura, T.; Fujiwara, H. Batesian mimicry has evolved with deleterious effects of the pleiotropic gene doublesex. Sci. Rep. 2020, 10, 21333. [Google Scholar] [CrossRef]
- Deshmukh, R.; Lakhe, D.; Kunte, K. Tissue-specific developmental regulation and isoform usage underlie the role of doublesex in sex differentiation and mimicry in Papilio swallowtails. R. Soc. Open Sci. 2020, 7, 200792. [Google Scholar] [CrossRef]
- Nadeau, N.; Pardo-Diaz, C.; Whibley, A.; Supple, M.A.; Saenko, S.V.; Wallbank, R.W.R.; Wu, G.C.; Maroja, L.; Ferguson, L.; Hanly, J.J.; et al. The gene cortex controls mimicry and crypsis in butterflies and moths. Nature 2016, 534, 106–110. [Google Scholar] [CrossRef]
- Otaki, J.M. FB28 Injection into Junonia Orithya; University of the Ryukyus: Okinawa, Japan, 2021; unpublished data. [Google Scholar]
- Nishikawa, H.; Iga, M.; Yamaguchi, J.; Saito, K.; Kataoka, H.; Suzuki, Y.; Sugano, S.; Fujiwara, H. Molecular basis of wing coloration in a Batesian mimic butterfly, Papilio polytes. Sci. Rep. 2013, 3, 3184. [Google Scholar] [CrossRef]
- Otaki, J.M. Color-pattern analysis of eyespots in butterfly wings: A critical examination of morphogen gradient models. Zool. Sci. 2011, 28, 403–413. [Google Scholar] [CrossRef]
- Otaki, J.M. Generation of butterfly wing eyespot patterns: A model for morphological determination of eyespot and parafocal element. Zool. Sci. 2011, 28, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Structural analysis of eyespots: Dynamics of morphogenic signals that govern elemental positions in butterfly wings. BMC Syst. Biol. 2012, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Perlman, D.L.; Perlman, M.P. An investigation into the effects of coldshock on the eastern tiger swallowtail butterfly Pterourus (Papilio) glaucus (C. Linnaeus 1758) (Lepidoptera: Papilionidae). Holarctic Lepid. 2019, 13, 1–43. [Google Scholar]
- Woestmann, L.; Saastamoinen, M. The importance of trans-generational effects in Lepidoptera. Curr. Zool. 2016, 62, 489–499. [Google Scholar] [CrossRef]
- Rutherford, S.L.; Lindquist, S. Hsp90 as a capacitor for morphological evolution. Nature 1998, 396, 336–342. [Google Scholar] [CrossRef]
- Nijhout, H.F. Elements of butterfly wing patterns. J. Exp. Zool. 2001, 291, 213–225. [Google Scholar] [CrossRef]
- Otaki, J.M. Color pattern analysis of nymphalid butterfly wings: Revision of the nymphalid groundplan. Zool. Sci. 2012, 29, 568–576. [Google Scholar] [CrossRef]
- Otaki, J.M. Morphological and spatial diversity of the discal spot on the hindwings of nymphalid butterflies: Revision of the nymphalid groundplan. Insects 2020, 11, 654. [Google Scholar] [CrossRef]
- Otaki, J.M. The fractal geometry of the nymphalid groundplan: Self-similar configuration of color pattern symmetry systems in butterfly wings. Insects 2021, 12, 39. [Google Scholar] [CrossRef]
- Uesugi, K. The adaptive significance of Batesian mimicry in the swallowtail butterfly, Papilio polytes (Insecta, Papiliondae): Associative learning in a predator. Ethology 1996, 102, 762–775. [Google Scholar] [CrossRef]
- Uesugi, K. Temporal change in records of the mimetic butterfly Papilio polytes with establishment of its model Pachliopa aristolochiae in the Ryukyu Islands. Jpn. J. Entomol. 1991, 59, 183–198. [Google Scholar]
- Katoh, M.; Tatsuta, H.; Tsuji, K. Rapid evolution of a Batesian mimicry trait in a butterfly responding to arrival of a new model. Sci. Rep. 2017, 7, 6369. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.H.; Tan, Y.Q.; Finkbeiner, S.D.; Briscoe, A.D.; Monteiro, A.; Kronforst, M.R. Experimental field tests of Batesian mimicry in the swallowtail butterfly Papilio polytes. Ecol. Evol. 2018, 8, 7657–7666. [Google Scholar] [CrossRef] [PubMed]
- Tsurui-Sato, K.; Sato, Y.; Kato, E.; Katoh, M.; Kimura, R.; Tatsuta, H.; Tsuji, K. Evidence for frequency-dependent selection maintaining polymorphism in the Batesian mimic Papilio polytes in multiple islands in the Ryukyus, Japan. Ecol. Evol. 2019, 9, 5991–6002. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsurui-Sato, K.; Katoh, M.; Kimura, R.; Tatsuta, H.; Tsuji, K. Population genetic structure and evolution of Batesian mimicry in Papilio polytes from the Ryukyu Islands, Japan, analyzed by genotyping-by-sequencing. Ecol. Evol. 2020, 11, 872–886. [Google Scholar] [CrossRef]
- Sekimura, T.; Fujihashi, Y.; Takeuchi, Y. A model for population dynamics of the mimetic butterfly Papilio polytes in the Sakishima Islands, Japan. J. Theor. Biol. 2014, 361, 133–140. [Google Scholar] [CrossRef]
- Katoh, M.; Tatsuta, H.; Tsuji, K. Ultraviolet exposure has an epigenetic effect on a Batesian mimetic trait in the butterfly Papilio polytes. Sci. Rep. 2018, 8, 13416. [Google Scholar] [CrossRef]
- Tuomaala, M.; Kaitala, A.; Rutowski, R.L. Females show greater changes in wing colour with latitude than males in the green-veined white butterfly, Pieris napi (Lepidoptera: Pieridae). Biol. J. Linn. Soc. 2012, 107, 899–909. [Google Scholar] [CrossRef]




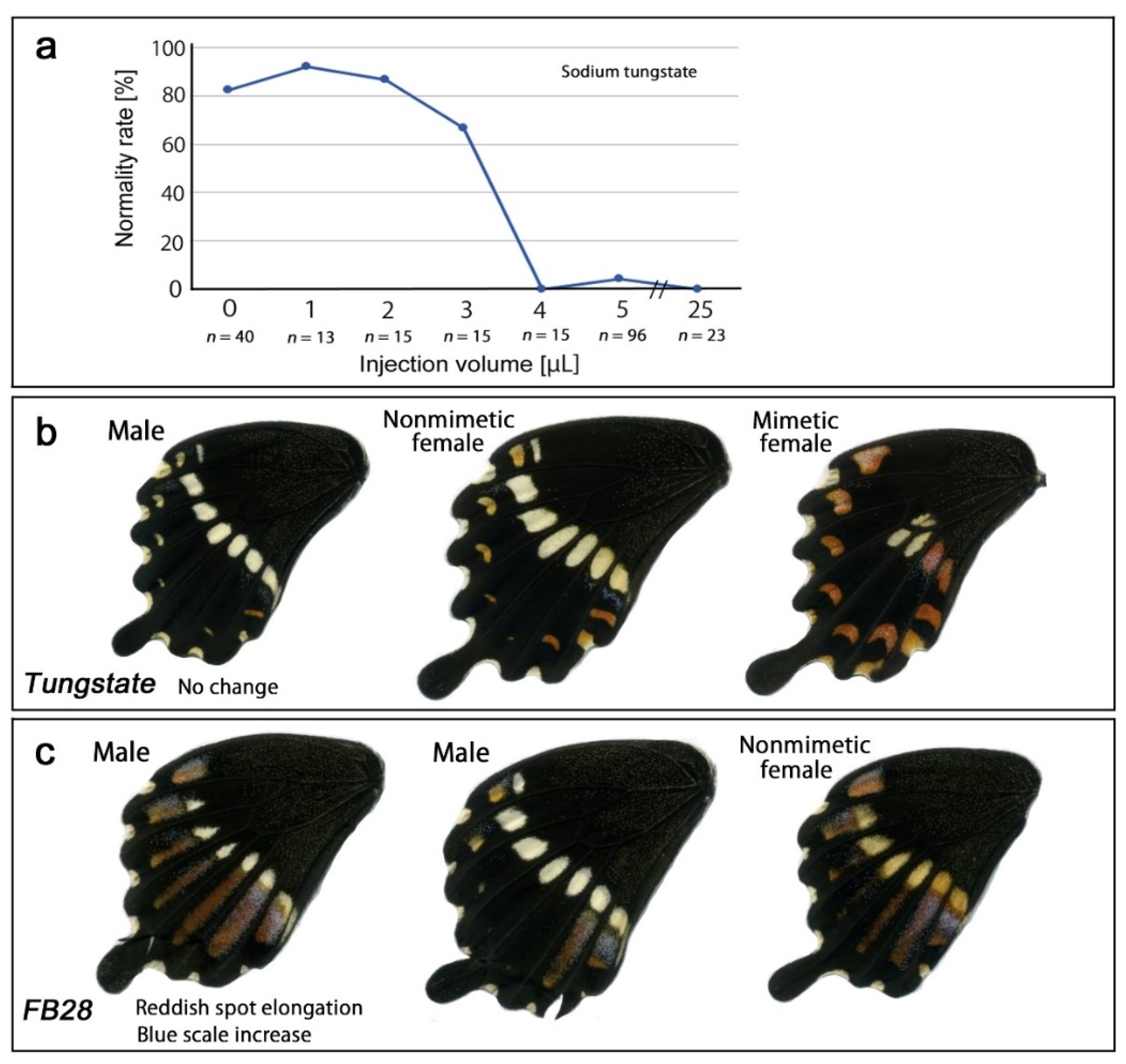

| Treatment Mode | White Spot Area | Reddish Spot Area | Reddish Spot Darkness | Novel Spot |
|---|---|---|---|---|
| Cold shock | Enlarged in males | Enlarged, Elongated | Darkened | Emerged only in females |
| Heat shock | Reduced | Reduced (+Enlarged), Elongated | Darkened | Emerged in both sexes |
| Sodium tungstate | No change | No change | No change | No change |
| FB28 | Not applicable | Elongated | Not applicable | No change |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimajiri, T.; Otaki, J.M. Phenotypic Plasticity of the Mimetic Swallowtail Butterfly Papilio polytes: Color Pattern Modifications and Their Implications in Mimicry Evolution. Insects 2022, 13, 649. https://doi.org/10.3390/insects13070649
Shimajiri T, Otaki JM. Phenotypic Plasticity of the Mimetic Swallowtail Butterfly Papilio polytes: Color Pattern Modifications and Their Implications in Mimicry Evolution. Insects. 2022; 13(7):649. https://doi.org/10.3390/insects13070649
Chicago/Turabian StyleShimajiri, Tomoyuki, and Joji M. Otaki. 2022. "Phenotypic Plasticity of the Mimetic Swallowtail Butterfly Papilio polytes: Color Pattern Modifications and Their Implications in Mimicry Evolution" Insects 13, no. 7: 649. https://doi.org/10.3390/insects13070649
APA StyleShimajiri, T., & Otaki, J. M. (2022). Phenotypic Plasticity of the Mimetic Swallowtail Butterfly Papilio polytes: Color Pattern Modifications and Their Implications in Mimicry Evolution. Insects, 13(7), 649. https://doi.org/10.3390/insects13070649







