Transcriptome Analysis Reveals Early Hemocyte Responses upon In Vivo Stimulation with LPS in the Stick Insect Bacillus rossius (Rossi, 1788)
Abstract
Simple Summary
Abstract
1. Introduction
2. Results and Discussion
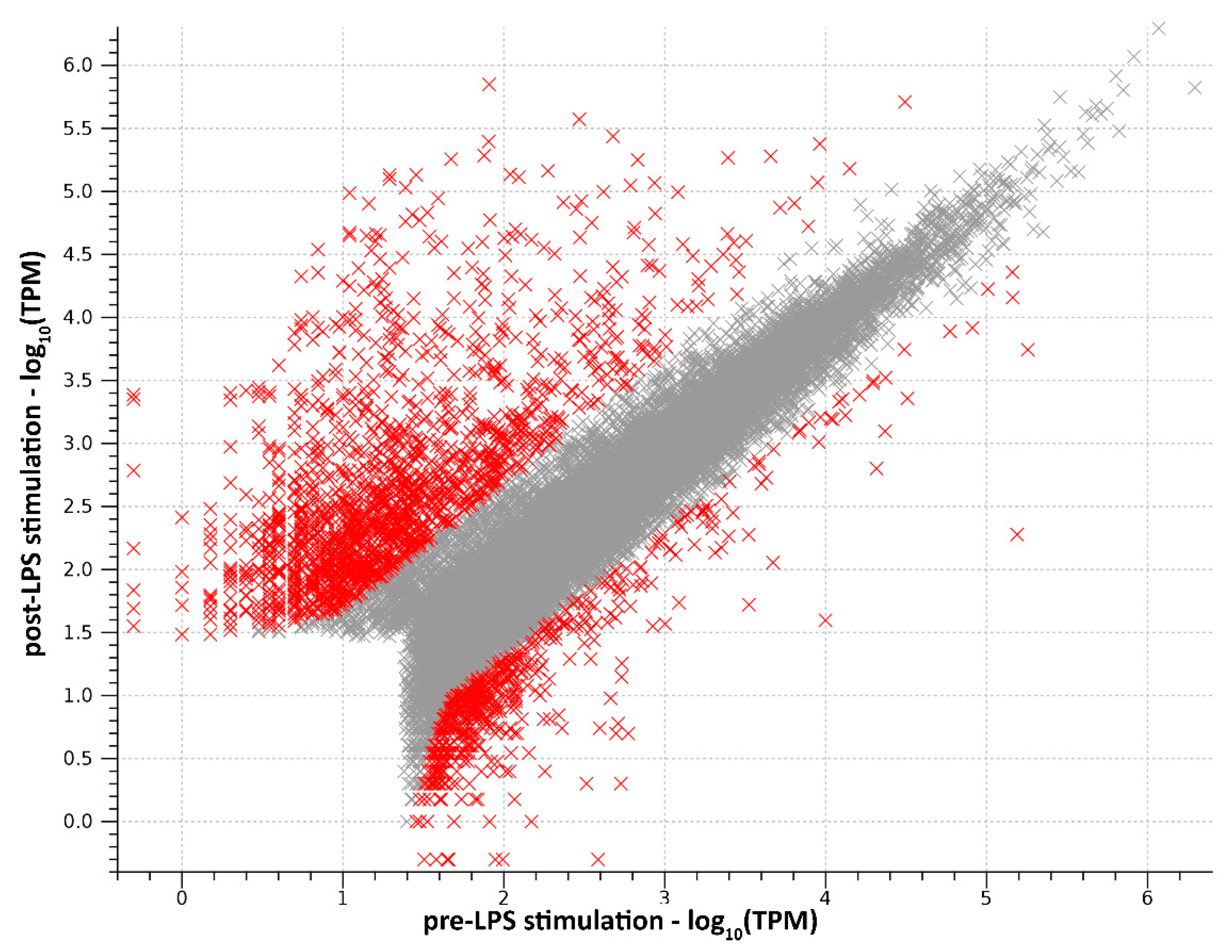
2.1. RNA-Seq Data Processing and Transcriptome Assembly
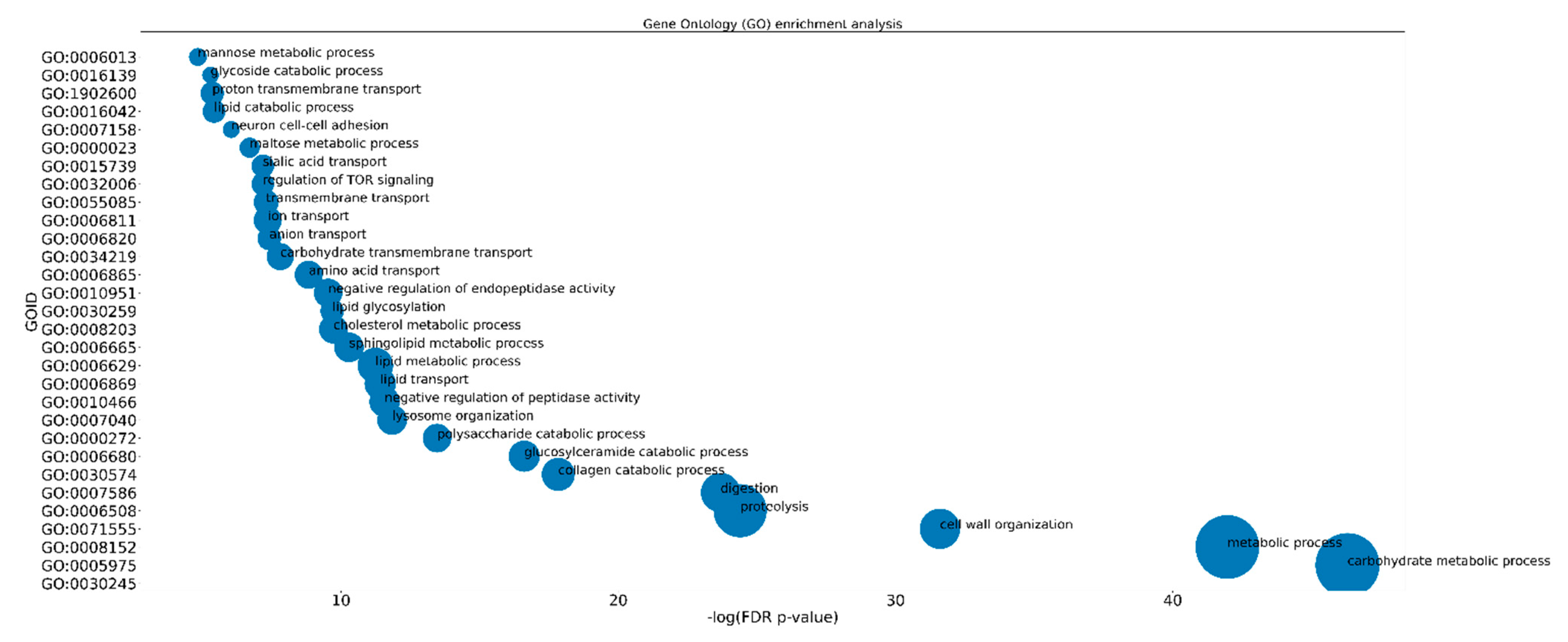
2.2. Functional Analysis of DEGs
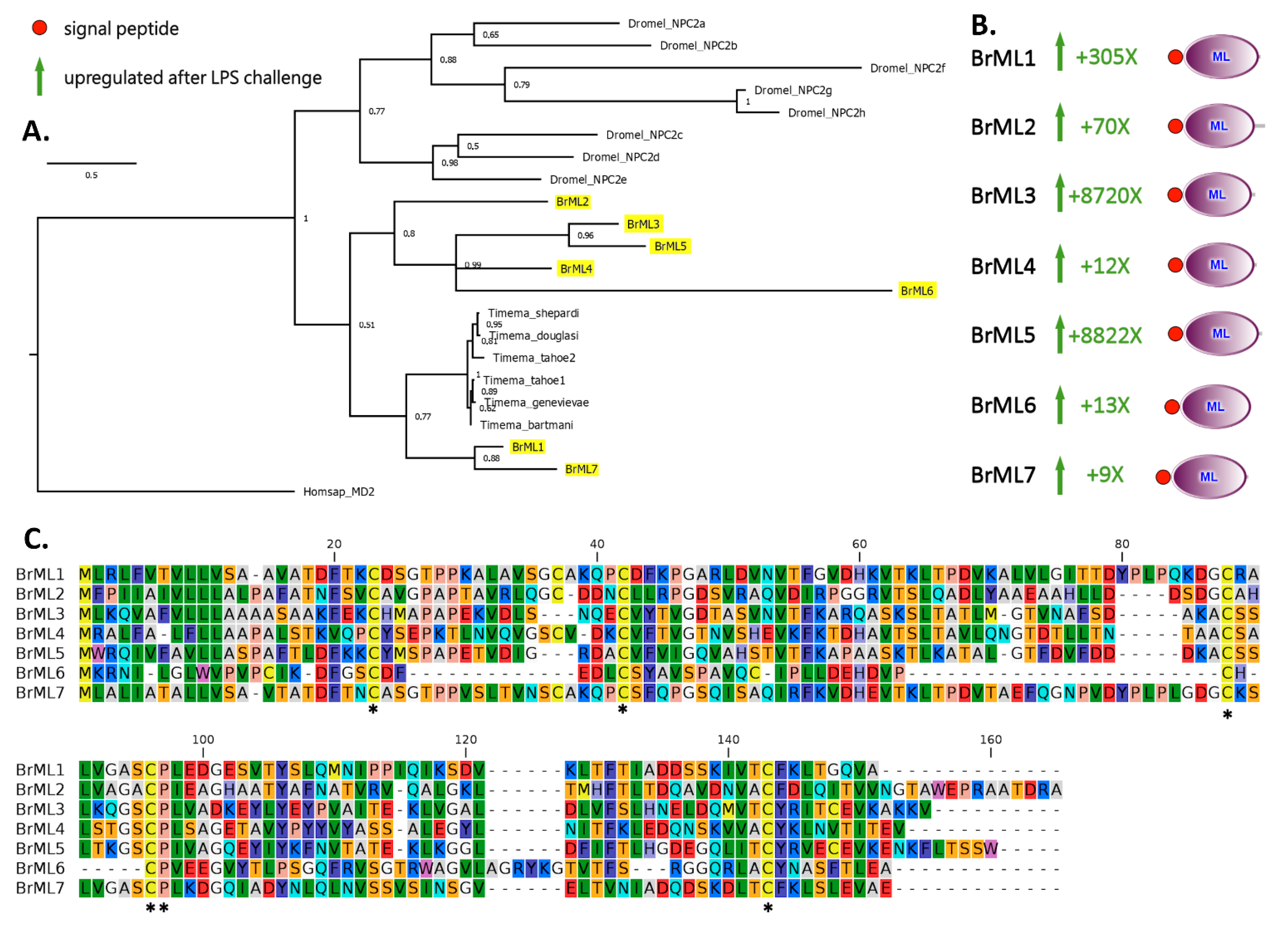
2.3. A Subgroup of C-Type Lectins and ML Domain-Containing Proteins Were Strongly Upregulated within Two Hours of LPS Stimulation
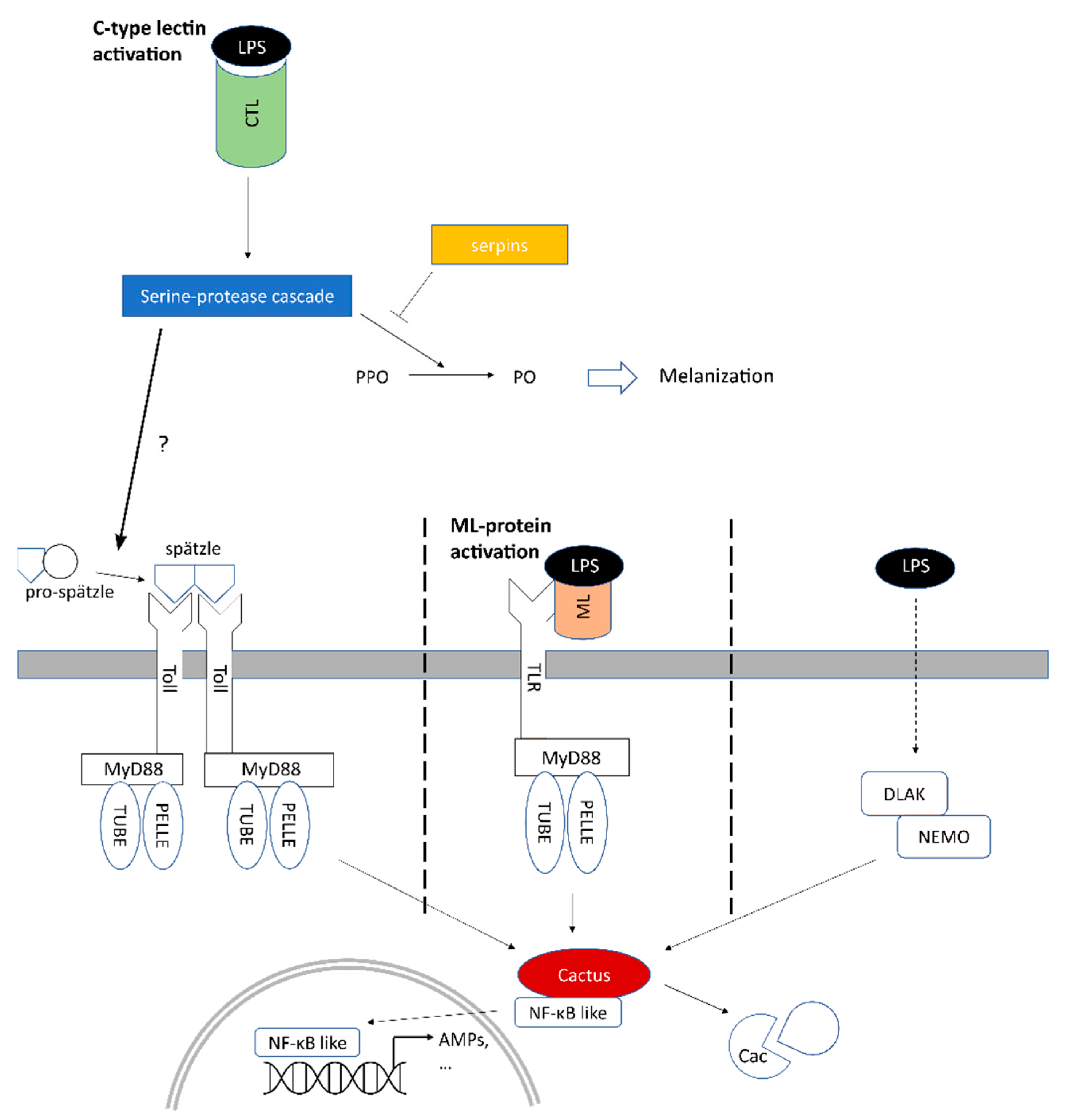
2.4. Several Serine Proteases Are Strongly Overexpressed and May Trigger Melanization
2.5. Involvement of Toll and Imd Signaling Pathways in the Early Response to LPS
2.6. Upregulation of Other Immune Effectors and Processes
3. Materials and Methods
3.1. Insect Rearing, Experimental Stimulation, and Hemocyte Collection
3.2. RNA Extraction and Transcriptome Sequencing
3.3. RNAseq Data Processing—De Novo Assembly and Annotation
3.4. DEG and Functional Enrichment Analysis
3.5. Protein Prediction and Identification
3.6. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du Pasquier, L. The immune system of invertebrates and vertebrates. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 1–15. [Google Scholar] [CrossRef]
- Strand, M.R. Insect hemocytes and their role in immunity. In Insect Immunology; Beckage, N.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 25–47. ISBN 9780123739766. [Google Scholar]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Theopold, U.; Li, D.; Fabbri, M.; Scherfer, C.; Schmidt, O. The coagulation of insect hemolymph. Cell. Mol. Life Sci. 2002, 59, 363–372. [Google Scholar] [CrossRef]
- Charles, H.M.; Killian, K.A. Response of the insect immune system to three different immune challenges. J. Insect Physiol. 2015, 81, 97–108. [Google Scholar] [CrossRef]
- Wu, C.; Crowhurst, R.N.; Dennis, A.B.; Twort, V.G.; Liu, S.; Newcomb, R.D.; Ross, H.A.; Buckley, T.R. De Novo Transcriptome Analysis of the Common New Zealand Stick Insect Clitarchus hookeri (Phasmatodea) Reveals Genes Involved in Olfaction, Digestion and Sexual Reproduction. PLoS ONE 2016, 11, e0157783. [Google Scholar] [CrossRef]
- Scapigliati, G.; Fausto, A.M.; Mazzini, M. Morphological and cytoskeletal characterization of hemocytes in stick insects (Phasmatodea). Bolletino di Zool. 1993, 60, 25–32. [Google Scholar] [CrossRef][Green Version]
- Scapigliati, G.; Mazzini, M. In vivo and in vitro phagocytosis by hemocytes of the stick insect Bacillus rossius. Bolletino di Zool. 1994, 61, 115–120. [Google Scholar] [CrossRef][Green Version]
- Scapigliati, G.; Pecci, M.; Piermattei, A.; Mazzini, M. Characterization of a monoclonal antibody against a 180 kDa hemocyte polypeptide involved in cellular defence reactions of the stick insect: Bacillus rossius. J. Insect Physiol. 1997, 43, 345–354. [Google Scholar] [CrossRef]
- De Figueroa, J.M.T.; Buonocore, F.; Mazzini, M.; Scapigliati, G. Cytofluorimetric analysis of Bacillus rossius haemocytes (Phasmatodea, Bacillidae). Ital. J. Zool. 2001, 68, 9–14. [Google Scholar] [CrossRef]
- Ranf, S. Immune Sensing of Lipopolysaccharide in Plants and Animals: Same but Different. PLoS Pathog. 2016, 12, e1005596. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ryu, J.H.; Han, S.J.; Choi, K.H.; Nam, K.B.; Jang, I.H.; Lemaitre, B.; Brey, P.T.; Lee, W.J. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and β-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J. Biol. Chem. 2000, 275, 32721–32727. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-Z.; Zhong, X.; Yu, X.-Q. Drosophila melanogaster NPC2 proteins bind bacterial cell wall components and may function in immune signal pathways. Insect Biochem. Mol. Biol. 2012, 42, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Ning, M.; Li, J.; Zhang, P.; Wang, L.; Xu, S.; Zhong, Y.; Wang, Z.; Song, Q.; Li, B. A C-type lectin with dual-CRD from Tribolium castaneum is induced in response to bacterial challenge. Pest Manag. Sci. 2020, 76, 3965–3974. [Google Scholar] [CrossRef]
- Liao, J.-X.; Yin, Z.-X.; Huang, X.-D.; Weng, S.-P.; Yu, X.-Q.; He, J.-G. Cloning and characterization of a shrimp ML superfamily protein. Fish Shellfish Immunol. 2011, 30, 713–719. [Google Scholar] [CrossRef]
- Jiao, T.; Yang, T.T.; Wang, D.; Gao, Z.Q.; Wang, J.L.; Tang, B.P.; Liu, Q.N.; Zhang, D.Z.; Dai, L.S. Characterization and expression analysis of immune-related genes in the red swamp crayfish, Procambarus clarkii in response to lipopolysaccharide challenge. Fish Shellfish Immunol. 2019, 95, 140–150. [Google Scholar] [CrossRef]
- He, S.; Johnston, P.R.; Mcmahon, D.P. Analyzing Immunity in Non-model Insects Using De Novo Transcriptomics. In Immunity in Insects; Sandrelli, F., Tettamanti, G., Eds.; Springer Protocols Handbooks; Humana: New York, NY, USA, 2020; pp. 35–51. ISBN 9781071602591. [Google Scholar]
- Gasmi, L.; Jakubowska, A.K.; Ferré, J.; Ogliastro, M.; Herrero, S. Characterization of two groups of Spodoptera exigua Hübner (Lepidoptera: Noctuidae) C-type lectins and insights into their role in defense against the densovirus JcDV. Arch. Insect Biochem. Physiol. 2018, 97, e21432. [Google Scholar] [CrossRef]
- Schnitger, A.K.D.; Yassine, H.; Kafatos, F.C.; Osta, M.A. Two C-type lectins cooperate to defend Anopheles gambiae against Gram-negative bacteria. J. Biol. Chem. 2009, 284, 17616–17624. [Google Scholar] [CrossRef]
- Drickamer, K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature 1992, 360, 183–186. [Google Scholar] [CrossRef]
- Bi, J.; Feng, F.; Li, J.; Mao, J.; Ning, M.; Song, X.; Xie, J.; Tang, J.; Li, B. A C-type lectin with a single carbohydrate-recognition domain involved in the innate immune response of Tribolium castaneum. Insect Mol. Biol. 2019, 28, 649–661. [Google Scholar] [CrossRef]
- Jomori, T.; Natori, S. Molecular cloning of cDNA for lipopolysaccharide-binding protein from the hemolymph of the American cockroach, Periplaneta americana: Similarity of the protein with animal lectins and its acute phase expression. J. Biol. Chem. 1991, 266, 13318–13323. [Google Scholar] [CrossRef]
- Shi, X.Z.; Kang, C.J.; Wang, S.J.; Zhong, X.; Beerntsen, B.T.; Yu, X.Q. Functions of Armigeres subalbatus C-type lectins in innate immunity. Insect Biochem. Mol. Biol. 2014, 52, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Miyazawa, S.; Kitami, M.; Tabunoki, H.; Ueda, K.; Sato, R. Characterization of a Novel C-Type Lectin, Bombyx mori Multibinding Protein, from the B. mori Hemolymph: Mechanism of Wide-Range Microorganism Recognition and Role in Immunity. J. Immunol. 2006, 177, 4594–4604. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Ling, E.; Yu, X.Q. Drosophila C-type lectins enhance cellular encapsulation. Mol. Immunol. 2007, 44, 2541–2548. [Google Scholar] [CrossRef]
- Gasmi, L.; Ferré, J.; Herrero, S. High Bacterial Agglutination Activity in a Single-CRD C-Type Lectin from Spodoptera exigua (Lepidoptera: Noctuidae). Biosensors 2017, 7, 12. [Google Scholar] [CrossRef]
- Liu, F.-F.; Liu, Z.; Li, H.; Zhang, W.-T.; Wang, Q.; Zhang, B.-X.; Sun, Y.-X.; Rao, X.-J. CTL10 has multiple functions in the innate immune responses of the silkworm, Bombyx mori. Dev. Comp. Immunol. 2022, 127, 104309. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Liu, Y.; Zhao, X.F.; Wang, J.X. Characterization of a C-type lectin from the cotton bollworm, Helicoverpa armigera. Dev. Comp. Immunol. 2009, 33, 772–779. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, Q.; Tang, L.; Chen, L.; Liu, X.S.; Wang, Y.F. Involvement of a pattern recognition receptor C-type lectin 7 in enhancing cellular encapsulation and melanization due to its carboxyl-terminal CRD domain in the cotton bollworm, Helicoverpa armigera. Dev. Comp. Immunol. 2014, 44, 21–29. [Google Scholar] [CrossRef]
- Xialu, W.; Jinghai, Z.; Ying, C.; Youlei, M.; Wenjun, Z.; Guoyuan, D.; Wei, L.; Mingyi, Z.; Chunfu, W.; Rong, Z. A novel pattern recognition protein of the Chinese oak silkmoth, Antheraea pernyi, is involved in the pro-PO activating system. BMB Rep. 2013, 46, 358–363. [Google Scholar] [CrossRef]
- Yu, X.Q.; Gan, H.; Kanost, M.R. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem. Mol. Biol. 1999, 29, 585–597. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Lin, Y.-P.; Liu, M.; Liang, X.-Y.; Ji, Y.-N.; Tang, B.-Z.; Hou, Y.-M. Functional conservation and division of two single-carbohydrate-recognition domain C-type lectins from the nipa palm hispid beetle Octodonta nipae (Maulik). Dev. Comp. Immunol. 2019, 100, 103416. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; You, M.; Rao, X.-J.; Yu, X.-Q. Insect C-type lectins in innate immunity. Dev. Comp. Immunol. 2018, 83, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, F.; Zhu, K.; Zhu, M.; Li, Q.; Hu, Q.; Zhang, J.; Zhang, R.; Yu, X.-Q. Comparative genomic analysis of C-type lectin-domain genes in seven holometabolous insect species. Insect Biochem. Mol. Biol. 2020, 126, 103451. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.J.; Shahzad, T.; Liu, S.; Wu, P.; He, Y.T.; Sun, W.J.; Fan, X.Y.; Yang, Y.F.; Shi, Q.; Yu, X.Q. Identification of C-type lectin-domain proteins (CTLDPs) in silkworm Bombyx mori. Dev. Comp. Immunol. 2015, 53, 328–338. [Google Scholar] [CrossRef]
- Rao, X.J.; Cao, X.; He, Y.; Hu, Y.; Zhang, X.; Chen, Y.R.; Blissard, G.; Kanost, M.R.; Yu, X.Q.; Jiang, H. Structural features, evolutionary relationships, and transcriptional regulation of C-type lectin-domain proteins in Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 75–85. [Google Scholar] [CrossRef]
- Christophides, G.K.; Zdobnov, E.; Barillas-Mury, C.; Birney, E.; Blandin, S.; Blass, C.; Brey, P.T.; Collins, F.H.; Danielli, A.; Dimopoulos, G.; et al. Immunity-Related Genes and Gene Families in Anopheles gambiae. Science (80-) 2002, 298, 159–165. [Google Scholar] [CrossRef]
- Zou, Z.; Evans, J.D.; Lu, Z.; Zhao, P.; Williams, M.; Sumathipala, N.; Hetru, C.; Hultmark, D.; Jiang, H. Comparative genomic analysis of the Tribolium immune system. Genome Biol. 2007, 8, R177. [Google Scholar] [CrossRef]
- Inohara, N.; Nuez, G. ML—A conserved domain involved in innate immunity and lipid metabolism. Trends Biochem. Sci. 2002, 27, 219–221. [Google Scholar] [CrossRef]
- Ishida, Y.; Tsuchiya, W.; Fujii, T.; Fujimoto, Z.; Miyazawa, M.; Ishibashi, J.; Matsuyama, S.; Ishikawa, Y.; Yamazaki, T. Niemann-Pick type C2 protein mediating chemical communication in the worker ant. Proc. Natl. Acad. Sci. USA 2014, 111, 3847–3852. [Google Scholar] [CrossRef]
- Shimazu, R.; Akashi, S.; Ogata, H.; Nagai, Y.; Fukudome, K.; Miyake, K.; Kimoto, M. MD-2, a molecule that confers lipopolysaccharide responsiveness on toll- like receptor 4. J. Exp. Med. 1999, 189, 1777–1782. [Google Scholar] [CrossRef]
- Visintin, A.; Iliev, D.B.; Monks, B.G.; Halmen, K.A.; Golenbock, D.T. MD-2. Immunobiology 2006, 211, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.I.; Akashi, S.; Yamada, T.; Tanimura, N.; Kobayashi, M.; Konno, K.; Matsumoto, F.; Fukase, K.; Kusumoto, S.; Nagai, Y.; et al. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int. Immunol. 2004, 16, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.-Q.; Ling, E.; Rao, X.-J.; Yu, X.-Q. A novel ML protein from Manduca sexta may function as a key accessory protein for lipopolysaccharide signaling. Mol. Immunol. 2008, 45, 2772–2781. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Jiang, H.; Kanost, M.R.; Wang, Y. Serine proteases and their homologs in the Drosophila melanogaster genome: An initial analysis of sequence conservation and phylogenetic relationships. Gene 2003, 304, 117–131. [Google Scholar] [CrossRef]
- Cao, X.; Gulati, M.; Jiang, H. Serine protease-related proteins in the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2017, 88, 48–62. [Google Scholar] [CrossRef]
- Kanost, M.R.; Gorman, M.J. Phenoloxidases in Insect Immunity. In Insect Immunology; Beckage, N.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123739766. [Google Scholar]
- Ling, E.; Yu, X.Q. Prophenoloxidase binds to the surface of hemocytes and is involved in hemocyte melanization in Manduca sexta. Insect Biochem. Mol. Biol. 2005, 35, 1356–1366. [Google Scholar] [CrossRef]
- Marmaras, V.J.; Charalambidis, N.D.; Zervas, C.G. Immune Response in Insects: The Role of Phenoloxidase in Defense Reactions in Relation to Melanization and Sclerotization. Arch. Insect Biochem. Physiol. 1996, 31, 119–133. [Google Scholar] [CrossRef]
- Asada, N.; Yokoyama, G.; Kawamoto, N.; Norioka, S.; Hatta, T. Prophenol oxidase A3 in Drosophila melanogaster: Activation and the PCR-based cDNA sequence. Biochem. Genet. 2003, 41, 151–163. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Deng, J.; Jiang, H. The structure of a prophenoloxidase (PPO) from Anopheles gambiae provides new insights into the mechanism of PPO activation. BMC Biol. 2016, 14, 2. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Ma, C.; Kanost, M.R. Subunit composition of pro-phenol oxidase from Manduca sexta: Molecular cloning of subunit ProPO-P1. Insect Biochem. Mol. Biol. 1997, 27, 835–850. [Google Scholar] [CrossRef]
- Kim, M.S.; Baek, M.J.; Lee, M.H.; Park, J.W.; Lee, S.Y.; Söderhäll, K.; Lee, B.L. A New Easter-type Serine Protease Cleaves a Masquerade-like Protein during Prophenoloxidase Activation in Holotrichia diomphalia Larvae. J. Biol. Chem. 2002, 277, 39999–40004. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Thamilarasi, K.; Venkatesh, G.R.; Srinivas, P.; Bhatnagar, R.K. Immune cascade of Spodoptera litura: Cloning, expression, and characterization of inducible prophenol oxidase. Biochem. Biophys. Res. Commun. 2005, 337, 394–400. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Budd, A.; Kanost, M.R.; Michel, K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cell. Mol. Life Sci. 2011, 68, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Kambris, Z.; Lemaitre, B.; Hashimoto, C. Two proteases defining a melanization cascade in the immune system of Drosophila. J. Biol. Chem. 2006, 281, 28097–28104. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cho, M.Y.; Hyun, J.H.; Lee, K.M.; Homma, K.I.; Natori, S.; Kawabata, S.I.; Iwanaga, S.; Lee, B.L. Molecular cloning of cDNA for pro-phenol-oxidase-activating factor i, a serine protease is induced by lipopolysaccharide or 1,3-β-glucan in coleopteran insect, Holotrichia diomphalia larvae. Eur. J. Biochem. 1998, 257, 615–621. [Google Scholar] [CrossRef]
- Lee, K.Y.; Zhang, R.; Kim, M.S.; Park, J.W.; Park, H.Y.; Kawabata, S.I.; Lee, B.L. A zymogen form of masquerade-like serine proteinase homologue is cleaved during pro-phenoloxidase activation by Ca2+ in coleopteran and Tenebrio molitor larvae. Eur. J. Biochem. 2002, 269, 4375–4383. [Google Scholar] [CrossRef]
- Yu, X.Q.; Jiang, H.; Wang, Y.; Kanost, M.R. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2003, 33, 197–208. [Google Scholar] [CrossRef]
- Lecroisey, A.; Boulard, C.; Keil, B. Chemical and Enzymatic Characterization of the Collagenase from the Insect Hypodearma lineatum. Eur. J. Biochem. 1979, 101, 385–393. [Google Scholar] [CrossRef]
- Lecroisey, A.; Gilles, A.M.; De Wolf, A.; Keil, B. Complete amino acid sequence of the collagenase from the insect Hypoderma lineatum. J. Biol. Chem. 1987, 262, 7546–7551. [Google Scholar] [CrossRef]
- Perona, J.J.; Tsu, C.A.; Craik, C.S.; Fletterick, R.J. Crystal structure of an ecotin-collagenase complex suggests a model for recognition and cleavage of the collagen triple helix. Biochemistry 1997, 36, 5381–5392. [Google Scholar] [CrossRef] [PubMed]
- Van Wormhoudt, A.; Le Chevalier, P.; Sellos, D. Purification, biochemical characterization and N-terminal sequence of a serine-protease with chymotrypsic and collagenolytic activities in a tropical shrimp, penaeus vannamei (crustacea, decapoda). Comp. Biochem. Physiol.—Part B Biochem. 1992, 103, 675–680. [Google Scholar] [CrossRef]
- Altincicek, B.; Vilcinskas, A. Metamorphosis and collagen-IV-fragments stimulate innate immune response in the greater wax moth, Galleria mellonella. Dev. Comp. Immunol. 2006, 30, 1108–1118. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Tian, Y.; Abro, N.A.; Nong, X.; Zhang, Z.; Wang, G. Molecular identification and immunity functional characterization of lmserpin1 in locusta migratoria manilensis. Insects 2021, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Spellman, P.T.; Tzou, P.; Rubin, G.M.; Lemaitre, B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002, 21, 2568–2579. [Google Scholar] [CrossRef]
- Kaneko, T.; Goldman, W.E.; Mellroth, P.; Steiner, H.; Fukase, K.; Kusumoto, S.; Harley, W.; Fox, A.; Golenbock, D.; Silverman, N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 2004, 20, 637–649. [Google Scholar] [CrossRef]
- Leulier, F.; Parquet, C.; Pili-Floury, S.; Ryu, J.-H.; Caroff, M.; Lee, W.-J.; Mengin-Lecreulx, D.; Lemaitre, B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat. Immunol. 2003, 4, 478–484. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Sun, J.-J.; Xu, S.; He, Z.-H.; Shi, X.-Z.; Zhao, X.-F.; Wang, J.-X. Activation of Toll Pathway Is Different between Kuruma Shrimp and Drosophila. Front. Immunol. 2017, 8, 1151. [Google Scholar] [CrossRef]
- Browne, N.; Heelan, M.; Kavanagh, K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Dudzic, J.P.; Hanson, M.A.; Iatsenko, I.; Kondo, S.; Lemaitre, B. More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila. Cell Rep. 2019, 27, 1050–1061.e3. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R. Innate immunity against moulds: Lessons learned from invertebrate models. Immunol. Investig. 2011, 40, 676–691. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S. Peptidoglycan recognition proteins in Drosophila immunity. Dev. Comp. Immunol. 2014, 42, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weber, A.N.R.; Atilano, M.L.; Filipe, S.R.; Gay, N.J.; Ligoxygakis, P. Sensing of Gram-positive bacteria in Drosophila: GNBP1 is needed to process and present peptidoglycan to PGRP-SA. EMBO J. 2006, 25, 5005–5014. [Google Scholar] [CrossRef]
- Nishide, Y.; Kageyama, D.; Yokoi, K.; Jouraku, A.; Tanaka, H.; Futahashi, R.; Fukatsu, T. Functional crosstalk across IMD and Toll pathways: Insight into the evolution of incomplete immune cascades. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182207. [Google Scholar] [CrossRef]
- Makarova, O.; Rodriguez-Rojas, A.; Eravci, M.; Weise, C.; Dobson, A.; Johnston, P.; Rolff, J. Antimicrobial defence and persistent infection in insects revisited. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150296. [Google Scholar] [CrossRef]
- Tauszig, S.; Jouanguy, E.; Hoffmann, J.A.; Imler, J.L. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl. Acad. Sci. USA 2000, 97, 10520–10525. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spatzle/Toll/Cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Geisler, R.; Bergmann, A.; Hiromi, Y.; Nüsslein-Volhard, C. cactus, a gene involved in dorsoventral pattern formation of Drosophila, is related to the IκB gene family of vertebrates. Cell 1992, 71, 613–621. [Google Scholar] [CrossRef]
- Drier, E.A.; Huang, L.H.; Steward, R. Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation. Genes Dev. 1999, 13, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Han, S.J.; Ryu, J.H.; Choi, K.H.; Hong, Y.S.; Chung, Y.H.; Perrot, S.; Raibaud, A.; Brey, P.T.; Lee, W.J. Lipopolysaccharide-activated kinase, an essential component for the induction of the antimicrobial peptide genes in Drosophila melanogaster cells. J. Biol. Chem. 2000, 275, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Silverman, N.; Zhou, R.; Stöven, S.; Pandey, N.; Hultmark, D.; Maniatis, T. A Drosophila IκB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000, 14, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Shelomi, M.; Jacobs, C.; Vilcinskas, A.; Vogel, H. The unique antimicrobial peptide repertoire of stick insects. Dev. Comp. Immunol. 2020, 103, 103471. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Jobichen, C.; Ong, T.C.; Gao, Y.F.; Tiong, Y.S.; Wong, K.N.; Chew, F.T.; Sivaraman, J.; Mok, Y.K. Crystal Structure of Der f 7, a Dust Mite Allergen from Dermatophagoides farinae. PLoS ONE 2012, 7, e44850. [Google Scholar] [CrossRef]
- Melén, E.; Pomés, A.; Vailes, L.D.; Arruda, L.K.; Chapman, M.D. Molecular cloning of per a 1 and definition of the cross-reactive Group 1 cockroach allergens. J. Allergy Clin. Immunol. 1999, 103, 859–864. [Google Scholar] [CrossRef]
- Mueller, G.A.; Pedersen, L.C.; Lih, F.B.; Glesner, J.; Moon, A.F.; Chapman, M.D.; Tomer, K.B.; London, R.E.; Pomés, A. The novel structure of the cockroach allergen Bla g 1 has implications for allergenicity and exposure assessment. J. Allergy Clin. Immunol. 2013, 132, 1420–1426.e9. [Google Scholar] [CrossRef]
- Pomés, A.; Melén, E.; Vailes, L.D.; Retief, J.D.; Arruda, L.K.; Chapman, M.D. Novel allergen structures with tandem amino acid repeats derived from German and American cockroach. J. Biol. Chem. 1998, 273, 30801–30807. [Google Scholar] [CrossRef]
- Fischer, H.M.; Wheat, C.W.; Heckel, D.G.; Vogel, H. Evolutionary origins of a novel host plant detoxification gene in butterflies. Mol. Biol. Evol. 2008, 25, 809–820. [Google Scholar] [CrossRef]
- Nolan, T.; Petris, E.; Müller, H.M.; Cronin, A.; Catteruccia, F.; Crisanti, A. Analysis of two novel midgut-specific promoters driving transgene expression in Anopheles stephensi mosquitoes. PLoS ONE 2011, 6, e16471. [Google Scholar] [CrossRef]
- Foo, A.C.Y.; Thompson, P.M.; Chen, S.H.; Jadi, R.; Lupo, B.; DeRose, E.F.; Arora, S.; Placentra, V.C.; Premkumar, L.; Perera, L.; et al. The mosquito protein AEG12 displays both cytolytic and antiviral properties via a common lipid transfer mechanism. Proc. Natl. Acad. Sci. USA 2021, 118, e2019251118. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, S.; Meiri, N.; Yi, C.-L.; Musco, S.; Ma, W.; Goldberg, J.; Alkon, D.L. Late memory-related genes in the hippocampus revealed by RNA fingerprinting. Proc. Natl. Acad. Sci. USA 1997, 94, 9669–9673. [Google Scholar] [CrossRef] [PubMed]
- Alkon, D.L.; Nelson, T.J.; Zhao, W.; Cavallaro, S. Time domains of neuronal Ca2+ signaling and associative memory: Steps through a calexcitin, ryanodine receptor, K+ channel cascade. Trends Neurosci. 1998, 21, 529–537. [Google Scholar] [CrossRef]
- Miao, Y.T.; Deng, Y.; Jia, H.K.; Liu, Y.D.; Hou, M.L. Proteomic analysis of watery saliva secreted by white-backed planthopper, sogatella furcifera. PLoS ONE 2018, 13, e0193831. [Google Scholar] [CrossRef]
- Stendahl, O.; Krause, K.H.; Krischer, J.; Jerström, P.; Theler, J.M.; Clark, R.A.; Carpentier, J.L.; Lew, D.P. Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science (80-) 1994, 265, 1439–1441. [Google Scholar] [CrossRef]
- Garin, J.; Diez, R.; Kieffer, S.; Dermine, J.F.; Duclos, S.; Gagnon, E.; Sadoul, R.; Rondeau, C.; Desjardins, M. The phagosome proteome: Insight into phagosome functions. J. Cell Biol. 2001, 152, 165–180. [Google Scholar] [CrossRef]
- Müller-Taubenberger, A. Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. EMBO J. 2001, 20, 6772–6782. [Google Scholar] [CrossRef]
- Huang, Y.; Hui, K.; Jin, M.; Yin, S.; Wang, W.; Ren, Q. Two endoplasmic reticulum proteins (calnexin and calreticulin) are involved in innate immunity in Chinese mitten crab (Eriocheir sinensis). Sci. Rep. 2016, 6, 27578. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The Cell Biology of Phagocytosis. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Moreira, R.; Romero, A.; Rey-Campos, M.; Pereiro, P.; Rosani, U.; Novoa, B.; Figueras, A. Stimulation of Mytilus galloprovincialis Hemocytes With Different Immune Challenges Induces Differential Transcriptomic, miRNomic, and Functional Responses. Front. Immunol. 2020, 11, 606102. [Google Scholar] [CrossRef]
- Ramond, E.; Dudzic, J.P.; Lemaitre, B. Comparative RNA-Seq analyses of Drosophila plasmatocytes reveal gene specific signatures in response to clean injury and septic injury. PLoS ONE 2020, 15, e0235294. [Google Scholar] [CrossRef] [PubMed]
- Martínez, B.A.; Hoyle, R.G.; Yeudall, S.; Granade, M.E.; Harris, T.E.; Castle, J.D.; Leitinger, N.; Bland, M.L. Innate immune signaling in Drosophila shifts anabolic lipid metabolism from triglyceride storage to phospholipid synthesis to support immune function. PLOS Genet. 2020, 16, e1009192. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Luan, H.H.; Medzhitov, R. An evolutionary perspective on immunometabolism. Science (80-) 2019, 363, eaar3932. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simão, F.A.; Zdobnov, E.M. BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]

| Total TRINITY transcripts | 23,173 |
| Percent GC | 41.41 |
| Contig N50 | 3862 |
| Median contig length | 1572 |
| Average contig | 2319.10 |
| Total assembled bases | 53,740,459 |
| Overall BUSCO score | C: 82.5% [S: 81.0%, D: 1.5%], F: 4.0%, M: 13.5%, n: 1367 |
| Transcript ID | Annotation | Gene Expression Level (TPM) | Fold Change | |
|---|---|---|---|---|
| Pre-LPS | Post-LPS | |||
| TRINITY_DN276173_c2_g1_i1 | BrML3 | 4.03 | 25,414.18 | 8720.33 |
| TRINITY_DN8207_c0_g3_i1 | Trypsin-like serine protease | 11.47 | 12,709.61 | 1267.28 |
| TRINITY_DN1061_c0_g3_i1 | Trypsin-like serine protease | 2.74 | 9178.71 | 3834.95 |
| TRINITY_DN109048_c0_g2_i1 | BrCTL4 | 4.04 | 9050.65 | 2545.93 |
| TRINITY_DN5181_c8_g1_i1 | Short secretory low complexity protein | 14.32 | 7221.79 | 573.01 |
| TRINITY_DN111798_c0_g1_i6 | BrIARP1 | 9.12 | 5861.88 | 772.57 |
| TRINITY_DN4089_c1_g1_i1 | Trypsin-like serine protease | 0.71 | 4683.20 | 4062.45 |
| TRINITY_DN46586_c0_g2_i1 | BrML5 | 0.32 | 4603.06 | 8821.77 |
| TRINITY_DN1395_c0_g1_i10 | Trypsin-like serine protease | 66.67 | 4426.62 | 74.75 |
| TRINITY_DN8856_c6_g1_i1 | Lipoprotein lipase | 3.98 | 4133.16 | 1243.97 |
| TRINITY_DN4136_c0_g1_i8 | Putative chitin-binding protein | 36.08 | 4041.63 | 135.11 |
| TRINITY_DN28212_c0_g2_i1 | Trypsin-like serine protease | 4.27 | 3707.48 | 1046.21 |
| TRINITY_DN51244_c0_g1_i1 | BrCTL1 | 16.60 | 3487.76 | 256.77 |
| TRINITY_DN1757_c0_g1_i1 | Lipoprotein lipase | 0.82 | 3188.92 | 4361.47 |
| TRINITY_DN317100_c0_g1_i1 | Putative chitin-binding protein | 8.84 | 2448.76 | 348.49 |
| TRINITY_DN1615_c0_g1_i3 | BrIARP2 | 17.81 | 2295.07 | 160.16 |
| TRINITY_DN52225_c0_g2_i1 | BrIARP4 | 9.51 | 2139.52 | 262.01 |
| TRINITY_DN16_c1_g4_i1 | Trypsin-like serine protease | 0.31 | 1967.38 | 5513.76 |
| TRINITY_DN994_c0_g2_i2 | Endoglucanase | 0.27 | 1958.19 | 6906.46 |
| TRINITY_DN57351_c0_g1_i1 | Lipoprotein lipase | 28.59 | 1914.74 | 81.72 |
| TRINITY_DN10752_c0_g1_i1 | Fatty acid binding protein | 177.40 | 1855.10 | 12.65 |
| TRINITY_DN1099_c0_g3_i1 | Uncharacterized protein | 1.14 | 1848.74 | 1965.27 |
| TRINITY_DN187_c0_g1_i1 | Lipase | 8.10 | 1800.15 | 276.31 |
| TRINITY_DN1611_c0_g1_i1 | Carboxylesterase | 0.26 | 1790.37 | 6423.79 |
| TRINITY_DN67078_c0_g1_i1 | Lysosomal acid glucosylceramidase | 0.92 | 1712.40 | 2258.41 |
| TRINITY_DN213_c0_g1_i1 | BrIARP3 | 14.21 | 1660.98 | 145.14 |
| TRINITY_DN25071_c0_g1_i3 | Zinc carboxypeptidase | 26.19 | 1612.73 | 76.54 |
| TRINITY_DN1061_c0_g1_i2 | Trypsin-like serine protease | 8.25 | 1610.60 | 239.02 |
| TRINITY_DN950_c0_g6_i1 | Zinc carboxypeptidase | 27.03 | 1592.18 | 73.63 |
| TRINITY_DN12045_c0_g3_i2 | Calnexcitin-1 | 8.76 | 1334.26 | 181.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidoli, C.; Miccoli, A.; Buonocore, F.; Fausto, A.M.; Gerdol, M.; Picchietti, S.; Scapigliati, G. Transcriptome Analysis Reveals Early Hemocyte Responses upon In Vivo Stimulation with LPS in the Stick Insect Bacillus rossius (Rossi, 1788). Insects 2022, 13, 645. https://doi.org/10.3390/insects13070645
Bidoli C, Miccoli A, Buonocore F, Fausto AM, Gerdol M, Picchietti S, Scapigliati G. Transcriptome Analysis Reveals Early Hemocyte Responses upon In Vivo Stimulation with LPS in the Stick Insect Bacillus rossius (Rossi, 1788). Insects. 2022; 13(7):645. https://doi.org/10.3390/insects13070645
Chicago/Turabian StyleBidoli, Carlotta, Andrea Miccoli, Francesco Buonocore, Anna Maria Fausto, Marco Gerdol, Simona Picchietti, and Giuseppe Scapigliati. 2022. "Transcriptome Analysis Reveals Early Hemocyte Responses upon In Vivo Stimulation with LPS in the Stick Insect Bacillus rossius (Rossi, 1788)" Insects 13, no. 7: 645. https://doi.org/10.3390/insects13070645
APA StyleBidoli, C., Miccoli, A., Buonocore, F., Fausto, A. M., Gerdol, M., Picchietti, S., & Scapigliati, G. (2022). Transcriptome Analysis Reveals Early Hemocyte Responses upon In Vivo Stimulation with LPS in the Stick Insect Bacillus rossius (Rossi, 1788). Insects, 13(7), 645. https://doi.org/10.3390/insects13070645








