Simple Summary
Field margin plants are important in providing resources for natural enemies (NEs) and improving biological control of crop pests. However, the use of field margin plants for biological control particularly of important common bean pests is understudied in smallholder farming systems of sub-Saharan Africa (SSA). We evaluated the potential of field margin plants with respect to intercropping systems in common bean fields to enhance the population of NEs of common bean pests. We observed a high assemblage of important NEs of common bean pests for some insect taxa with minimal impact of intercropping on NEs. Field margin plants could be managed to provide a wide range of resources to NEs and therefore biological control of common bean pests.
Abstract
Field margins support important ecosystem services including natural pest regulation. We investigated the influence of field margins on the spatial and temporal distribution of natural enemies (NEs) of bean pests in smallholder farming systems. We sampled NEs from high and low plant diversity bean fields using sweep netting and coloured sticky traps, comparing monocropped and intercropped farms. NEs collected from within crops included predatory bugs, lacewings, predatory flies, parasitic flies, parasitic wasps, lady beetles, and a range of other predatory beetles; with the most dominant group being parasitic wasps. Overall, high plant diversity fields had a higher number of NEs than low-diversity fields, regardless of sampling methods. The field margin had a significantly higher number of lacewings, parasitic wasps, predatory bugs, syrphid flies, and other predatory beetles relative to the crop, but beneficial insects were collected throughout the fields. However, we observed marginally higher populations of NEs in intercropping than in monocropping although the effect was not significant in both low and high plant diversity fields. We recommend smallholder farmers protect the field margins for the added benefit of natural pest regulation in their fields.
1. Introduction
Common bean (Phaseolus vulgaris L.) is one of the most important legume crops in sub-Saharan Africa (SSA) for the provision of proteins, vitamins, energy, and macronutrients [1,2] and due to its ability to fix nitrogen which contributes to soil fertility [2]. However, common bean production is constrained by insect pests [3]. One of the most serious is the black bean aphid Aphis fabae Scopoli which causes yield losses in the common bean of up to 90% in East Africa [4,5,6,7]. A. fabae also transmit plant diseases including bean common mosaic necrosis virus (BCMNV), bean common mosaic virus (BCMV) and cucumber mosaic virus (CMV) [8]. While many smallholder farmers do not apply any control measures because of the high cost and lack of knowledge [9,10], others apply synthetic pesticides indiscriminately [11]. This approach is not sustainable and there are potential direct and indirect effects of chemical pesticides on human health and beneficial insects, including natural enemies of pests (NE) that are biological control agents of insect pests via predation and parasitism [12,13,14]. The adoption of more sustainable farming practices will benefit both the environment and human health. One alternative is conservation biological control which can regulate arthropod NE populations through multitrophic interactions and a balance between pests and their NEs [15]. Conserving locally adapted NEs is cost-effective and relatively simple and is an important consideration in pest management decisions [16] and strategies such as engineering agroecosystems to provide extra resources that would be limited in field crops [17]. A key component of landscapes that support NE populations are non-crop habitats rich in plant biodiversity, such as field margins that offer nectar, pollen, shelter and alternative hosts to NE communities, and thus provide support to enhance their populations and enhance sustainable agricultural benefits [18,19,20,21,22,23,24]. The presence of non-crop habitats surrounding or within arable land has been associated with increasing arthropod NEs of pests by providing floral resources, thus sustaining their populations [25,26]. For instance, important NEs such as parasitoids use nectar to fulfil their nutrition requirements at some stages of their development while spiders, lady beetles, rove beetles, syrphid flies, true bugs and lacewings use non-crop habitats to provide them with refuge, alternative hosts, pupation and overwintering sites [25,26,27,28,29,30,31,32,33,34,35,36,37]. It has been found that populations of NEs in field crops decline as the distance from the field margin increases and this demonstrates the essential function of field margins in maintaining NEs [38]. Non-crop habitats, such as field margins are important during crop senescence as NEs move from field crops to other resources [39]. However, floral resources provide different benefits to specific taxa of NEs [40], and thus NEs would respond differently to the proportions of non-crop habitats. For instance, the abundance of carabids was observed to decrease with the increase of the proportion or the presence of non-crop habitats; however, populations of spiders were not affected by the proportion or presence of non-crop habitat [41,42]. Thus, semi-natural habitats at a landscape scale offer benefits to NEs [43,44], while establishing non-crop habitats such as field margins will benefit NEs at a local scale [45], to enhance the ecosystem service of biological control of pests in agricultural fields. Existing knowledge about how non-crop host plants support NE communities is insufficient in many cropping systems including beans but it is essential when planning conservation biological control interventions [15,46]. The provision of alternative habitat and plant resources to support increased NE populations is an approach to pest management that will likely be economically and environmentally sustainable for smallholder farmers in SSA because of the availability of plants at local scales [22,47]. The importance of agroecosystem diversity and abundance, particularly in the field margins, for arthropod NE communities in smallholder bean farming systems of SSA is poorly understood. In addition, some studies have shown that intercropping enhances NEs of pests more than monocropping systems in legumes [48,49] although this is also understudied in smallholder common bean growing systems of SSA. In this study we took the framework of the following assumptions around how field margins are expected to benefit the crop and tested how they affect NE populations:
- Plant-rich field margins influence the number of NEs in bean fields
- An increase in NEs assemblage in field margins influence their numbers within the crop.
- Intercropping in bean fields is associated with high populations of NEs compared to monocropping.
2. Materials and Methods
2.1. Sampling Natural Enemies from the Fields
This field trial was carried out in Kwa Sadala Village in the Hai District, Kilimanjaro Region, Tanzania (3°10′0″ S, 37°10′0″ E). Thirty-two sites ≥0.20 ha with either high (n = 16) or low (n = 16) plant diversity were selected based on the observed number of plant species in non-crop vegetation around each farm [50]. To quantify the diversity of the plant species in our field sites, the Shannon index (H′) [51] was used according to the formula below to calculate low diversity fields (H′ = 1.2) and high diversity fields (H′ = 2.3).
Shannon Index (H′) = H = −∑(pi(ln pi)).
- pi—Proportion (n/N) of individuals of particular species in a whole community,
- n—individuals of a particular species,
- N—total number of individuals found,
- ∑—Sum symbol,
- ln = natural logarithm to base e.
The field margins were at least ≥2.5 m wide. The surrounding composition was similar in all fields (the arable fields, dominated by several flowering weed species) with similar management practices without chemical spray. Fields were located at least 50 m apart.
A further parameter was the inclusion of cropping practice where half of the farmer fields at each level (low and high plant diversity fields) practiced monocropping of common beans (Phaseolus vulgaris), whereas the other half of farmer fields intercropped beans with maize (Zea mays L.). Sweep netting was carried out, one replicate per site per visit, using a standard canvas hand sweep net to sample insects. Each sweep replicate consisted of three parallel transects in which the net was swept back and forth ten times: transect 1 along the margin, at least 0.5 m from the crop; transect 2 in the crop edge, 5 m from the margin, and transect 3 in the centre of the crop, >15 m away from the margin. The insects collected by sweep netting were transferred to 95 to 99% ethanol for preservation. This was repeated six times over the growing season, one time at seedling, two times at vegetative and flowering/pod formation, and one time at physiological maturity before pod drying. Yellow sticky traps that had glue on both sides measured 25 × 10 cm (Real IPM, Nairobi, Kenya) were placed in the field margins monthly from May to August, corresponding to the growth stages of the crop. Every two sticky cards in each of the field margins for thirty-two sites were attached at the height of approximately 1 m from the ground to a wooden cane with a string wire. The sticky cards were collected after 48 h [52]. Cards were brought to the laboratory, for isolation of NEs captured. The cards were examined under a dissecting microscope to record NEs [53] and then the insects were removed from the traps using soft and thin forceps [52]. The insects were preserved in 95 to 99% ethanol.
Insects collected were categorized into taxonomic groups: parasitic wasps (Hymenoptera: Ichneumonidae and Braconidae) including Aphidius spp.; predatory bugs (Hemiptera: Reduviidae and known predatory Pentatomidae); lady beetles (Coleoptera: Coccinellidae) including Cheilomenes lunata; lacewings (Neuroptera: Chrysopidae) including Chrysoperla congrua; parasitic flies (specifically Diptera: Tachinidae); hoverflies (Diptera: known aphidophagous (Syrphinae) Syrphidae only, and excluding Eristalini species with aquatic larvae); predatory flies (specifically Diptera: Dolichopodidae and Asilidae with predatory adults); and all other predatory beetles (Coleoptera: known predatory Carabidae, Lycidae and Staphylinidae). Specimens were identified to the highest level of resolution possible but focused on characterising them by life history and functional groups. We categorised the Pentatomidae, Carabidae and Syrphidae into “known predators” and analysed only these data.
2.2. Estimation of Aphid Severity
The severity of A. fabae infestation was estimated using a visual rating of 1–6, where: 1 = no aphids; 2 = 1–100 aphids; 3 = 101–300 aphids; 4 = 301–600; 5 = 601–1000 and 6>1000 aphids as used previously [54] from ten randomly selected bean plants in each field weekly throughout the crop development stages.
2.3. Statistical Analysis
We used the generalized linear models (GLM) procedure assuming Poisson distribution with log link function to compare the number of NEs (dependent variable) among high and low-diversity fields, location in the fields, months and cropping systems (explanatory variables) [55]. The model with the best fit was selected using the Akaike Information Criterion (AIC) and Bayesian Information Criteria (BIC) tests [56], whereas the model with the lowest AIC and BIC values was selected. The Shapiro-Wilk test was used to check for normality (SPSS Version 22.0). We estimated the overdispersion parameter by Pearson chi-square divided by degrees of freedom and estimated by maximum likelihood [44]. Pairwise comparisons were done with the Holm multiple comparisons test in the ‘emmeans’ package in (RStudio Version 1.2.1335) [57].
3. Results
Spatial and Temporal Distribution of Natural Enemies in Bean Fields
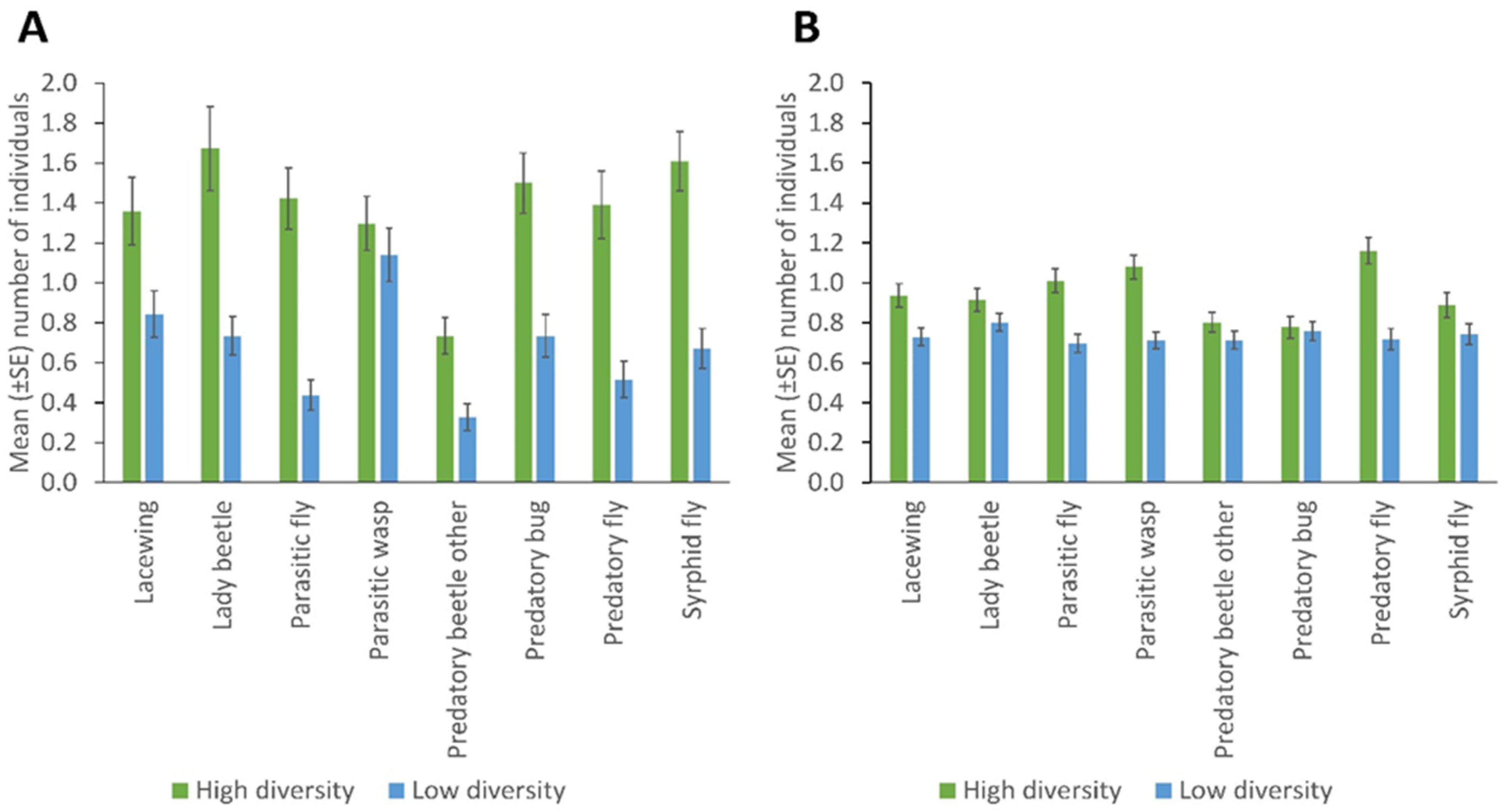
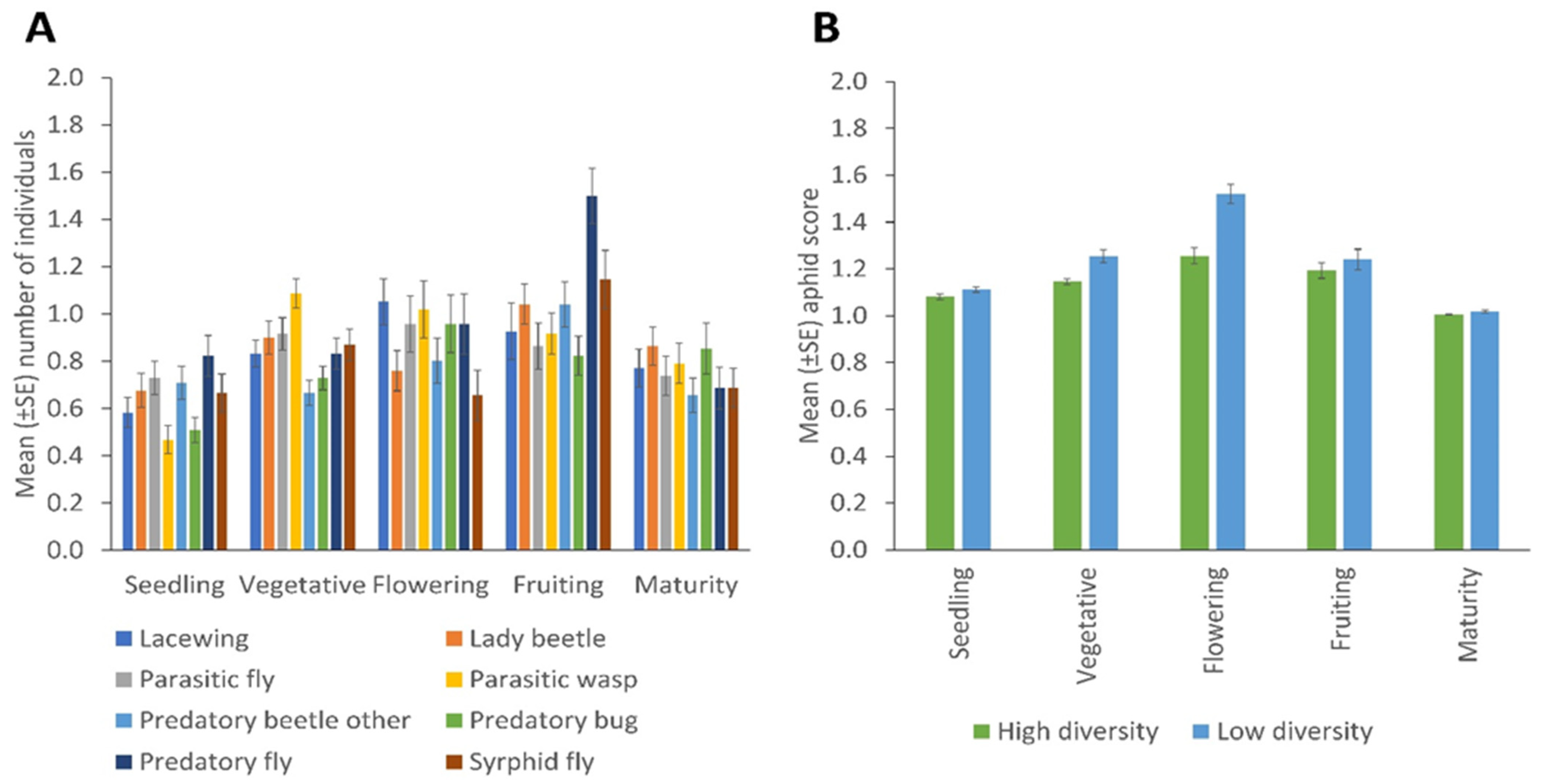
With the sticky trapping, the most abundant taxa were parasitic wasps (Ichneumonidae and Braconidae), with the Braconidae (particularly Aphidius colemani) being the dominant family) while with the sweep netting the most abundant taxa were the predatory flies (Dolichopodidae and Asilidae) with the Dolichopodidae being the dominant family in the study. The hymenopteran taxonomic data were obtained from a parallel study using mitochondrial cytochrome oxidase I barcoding of insects collected from sentinel plants, showing the common hymenopteran groups present in the study area [58]. The high-diversity fields had a significantly higher number of lady beetles, predatory flies, hoverflies, predatory bugs, parasitic flies, other predatory beetles (p = 0.001), and lacewings (p = 0.005), caught through sticky trapping used to monitor the field margins for NEs, than the fields with low diversity. No significant differences were observed in the number of parasitic wasps between high and low-diversity fields (Figure 1A; Supplementary Table S1).
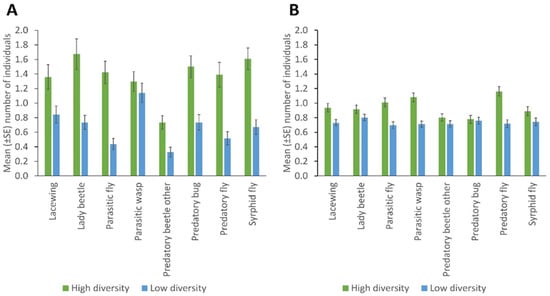
Figure 1.
Natural enemies’ abundance in fields with high and low plant diversity margins, collected by (A) sticky trapping and (B) sweep netting (Error bars = s.e.m.)
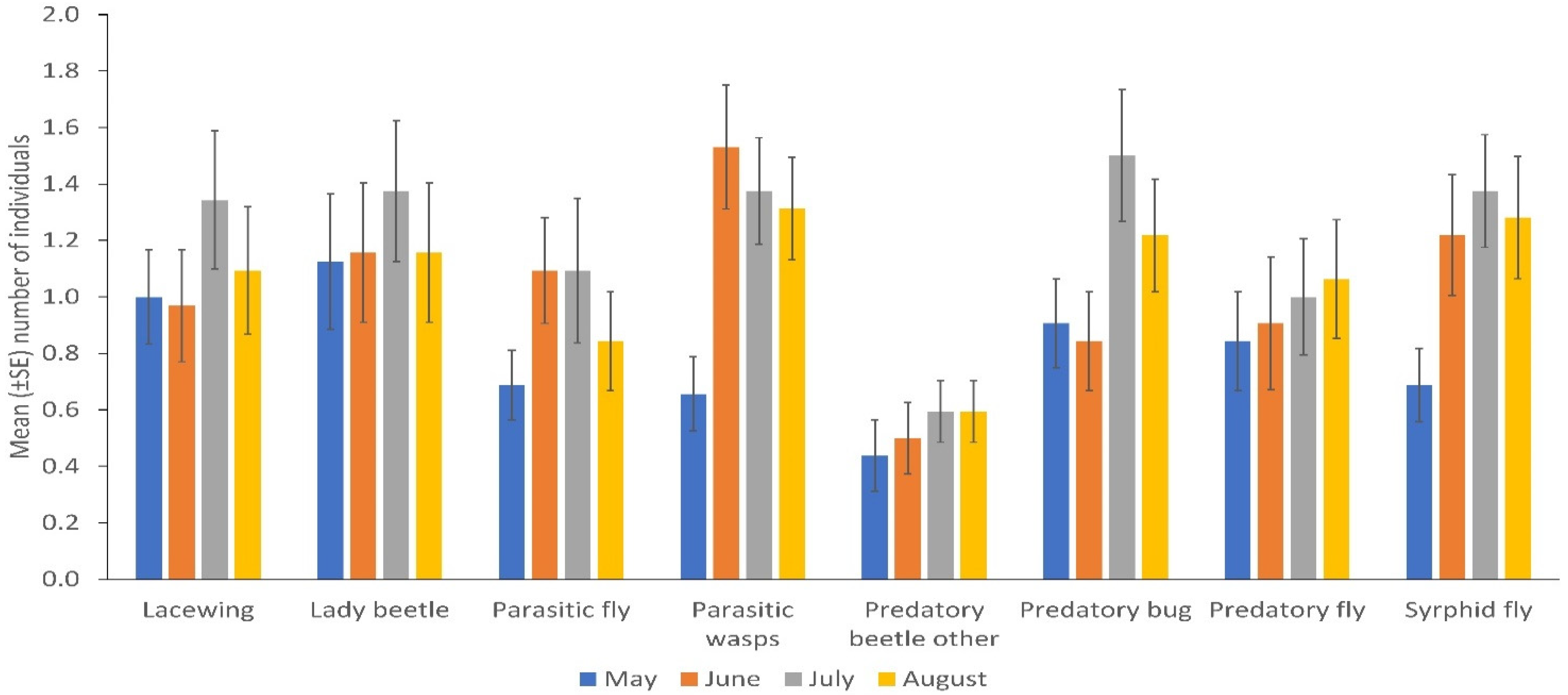
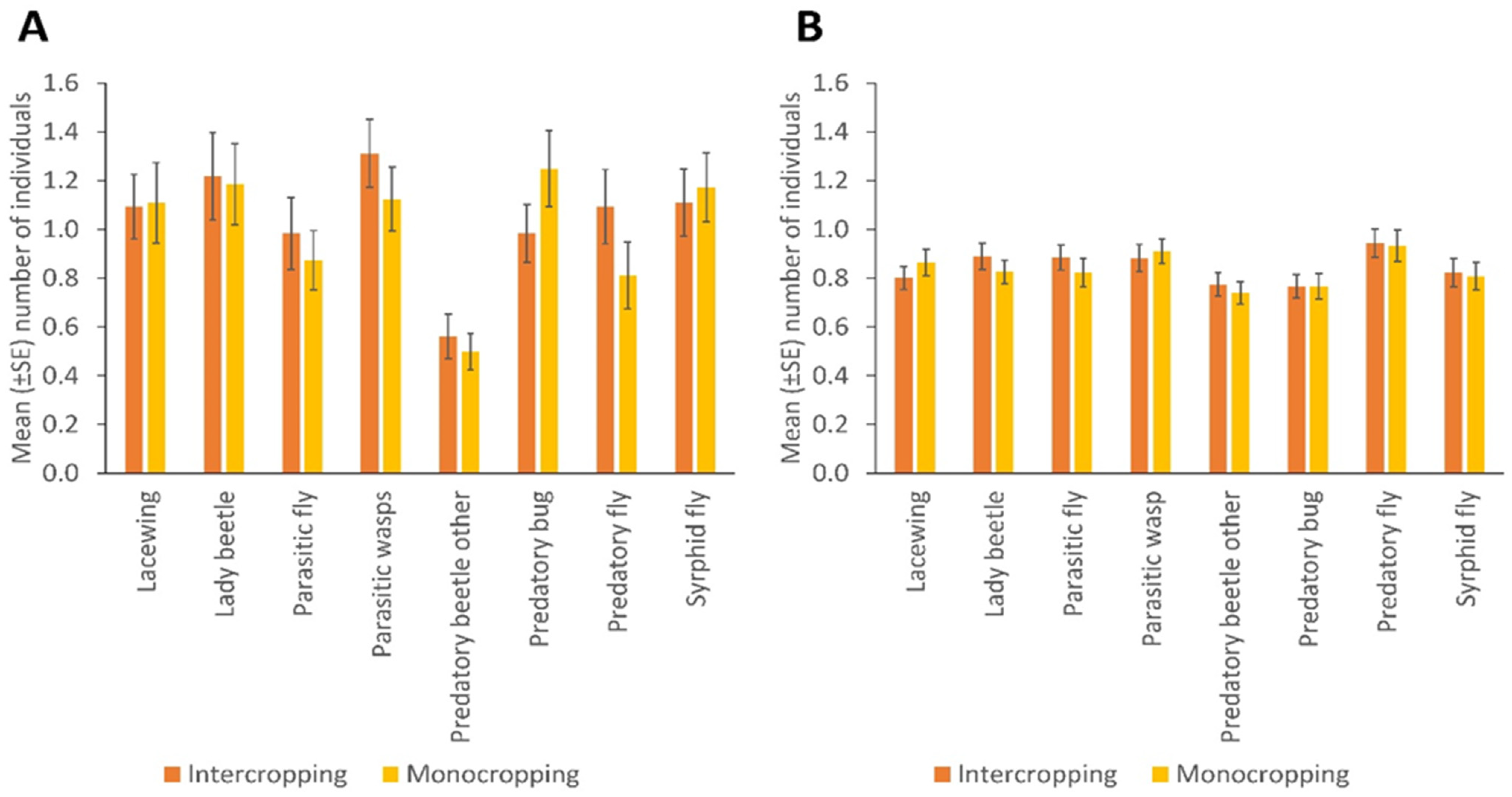
NE populations differed over the duration of the experiment in terms of catches of parasitic wasps (p = 0.004) and syrphid flies (p = 0.031) by the sticky trapping. There were no differences in the number of lady beetles, predatory flies, parasitic flies and other predatory beetles in different months. Parasitic wasps and syrphid flies were significantly more numerous in the flowering stage of the crop (p = 0.001; p = 0.032); fruiting stage and early maturity stages of the crop (p = 0.005; 0.008); and late maturity stage of the crop (p = 0.009; 0.018) than in the late seedling and vegetative stages of the crop, respectively. Predatory bugs were significantly more frequent in the fruiting stage and early maturity stages of the crop (p = 0.032) than in the flowering stage of the crop (Figure 2; Supplementary Table S3). More parasitic wasps (p = 0.001), lacewings (p = 0.006), syrphid flies (p = 0.009), parasitic flies (p = 0.001) and predatory flies (p = 0.001) were caught via sweep netting from high plant diversity fields compared to fields with low plant diversity in margins, but other insect taxa did not differ in abundance according to margin type (Figure 1B; Supplementary Table S2). No significant differences were observed between cropping systems (mono-cropping versus intercropping) for both sticky trap and sweep netting collections (Figure 3A,B).
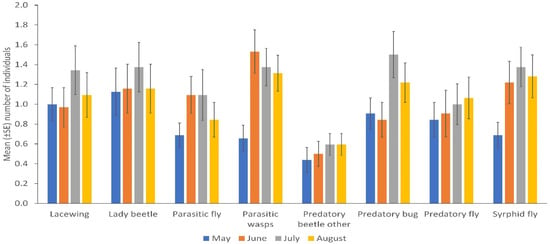
Figure 2.
Natural enemies’ distribution in different months collected by sticky trapping. May corresponds to the late seedling and vegetative stage; June corresponds to the flowering stage; July corresponds to the fruiting stage and early maturity stages and August corresponds to the late maturity stage of the crop near to harvest (Error bars = s.e.m.).
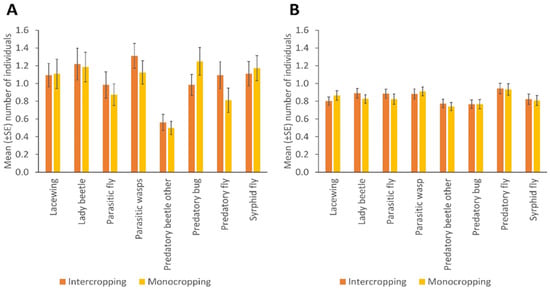
Figure 3.
The number of natural enemies in bean monocropped and intercropped fields, collected by (A) sticky trapping and (B) sweep netting (Error bars = s.e.m.).
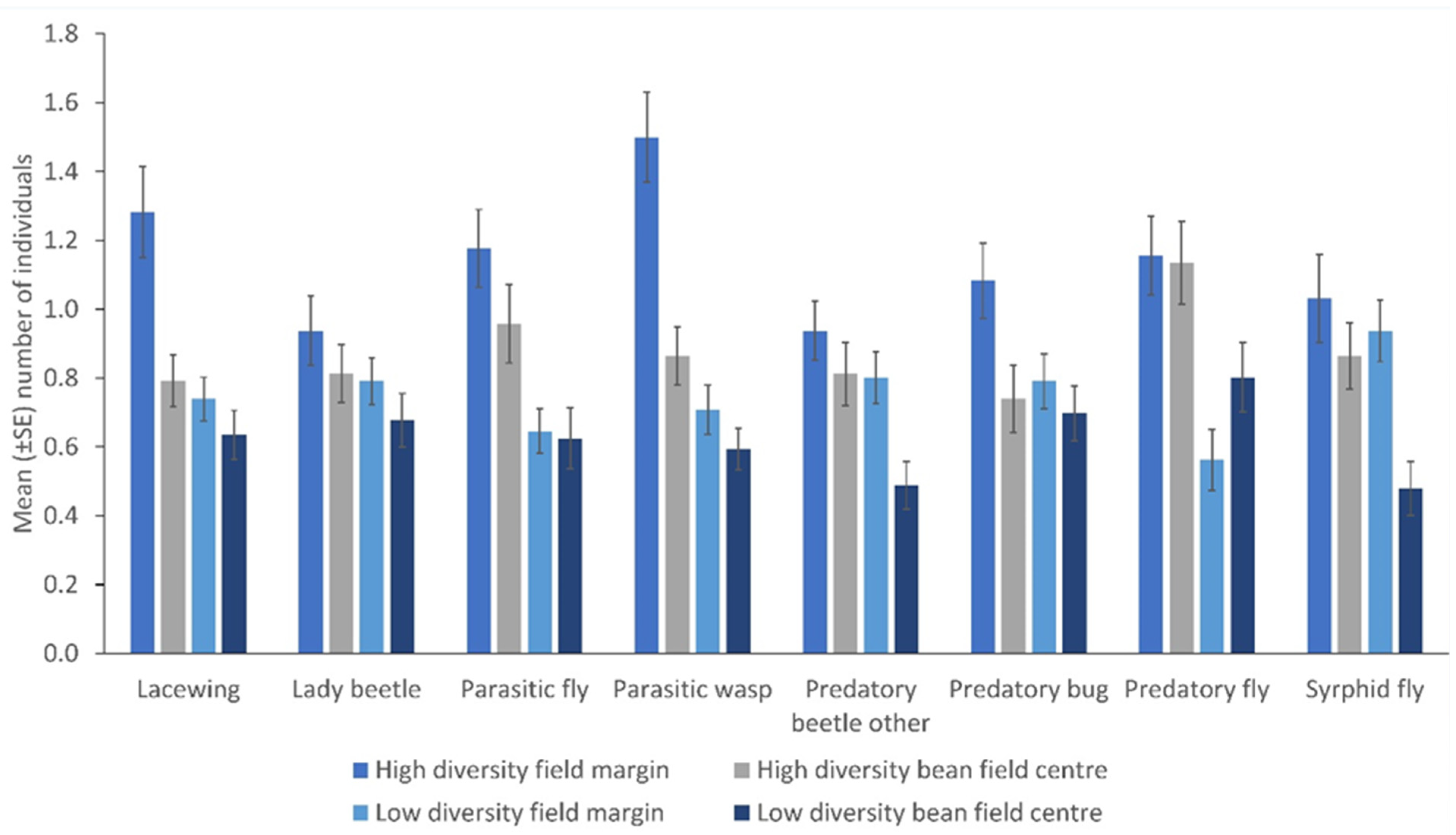
There were more NEs in the margin relative to the crop edge (lacewings, p = 0.046; parasitic wasps, p = 0.041; predatory bugs, p = 0.004), except for syrphid flies, other predatory beetles, parasitic flies and predatory flies. Moreover, there were more insects in the margin relative to the centre of the field (parasitic wasps, p = 0.001; syrphid flies, p = 0.002; lacewings, p = 0.005; other predatory beetles, p = 0.043) except for parasitic flies, predatory bugs, and predatory flies (Table 1). There were few consistent differences in the number of NEs within fields but consistently higher counts from high plant diversity fields were observed (other predatory beetles, p = 0.008; parasitic wasps, p = 0.001; predatory fly, p = 0.046; syrphid fly, p = 0.001) (Figure 4).

Table 1.
Mean ± (SEM) numbers of natural enemies in different field locations collected by sweep netting.
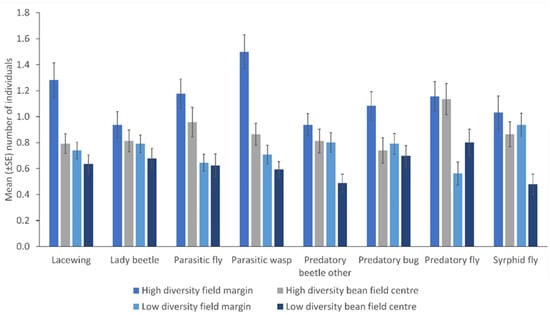
Figure 4.
The number of natural enemies in the field margin and the centre of the fields from low and high plant diversity fields as sampled by sweep nets (Error bars = s.e.m.).
Plant diversity significantly influenced the abundance of all NE groups caught by sticky trapping (lady beetles, predatory flies, hoverflies, predatory bugs, parasitic flies, other predatory beetles, p = 0.001; lacewings, p = 0.005), except for parasitic wasps; while, with sweep netting, the plant diversity had significant effects on the abundance of parasitic wasps, parasitic flies, predatory flies (p = 0.001), lacewings (p = 0.006), and syrphid flies (p = 0.009), except lady beetles, predatory bugs, other predatory beetles. Cropping systems had no significant influence on any of the NE groups. Parasitic wasps’ and syrphids’ abundance were influenced significantly by the time of sampling (p = 0.004; p = 0.031 respectively), while lady beetles, predatory flies, predatory bugs, parasitic flies and other predatory beetles’ abundances were not affected. Syrphids, lacewings and predatory bugs were influenced significantly by field location (p = 0.001; p = 0.010; p = 0.027 respectively) except for other predatory beetles, lady beetles, predatory flies, parasitic flies and parasitic wasps.
Significant effects of the crop development stage on the distribution of NEs for sweep netting collections were observed for lady beetles (p = 0.039); other predatory beetles (p = 0.020); syrphid flies (p = 0.001) and predatory flies (p = 0.001) (Figure 5A). There were no significant differences in the mean number of aphids (A. fabae) observed among different crop development stages and between high and low plant diversity fields. The A. fabae population was high in the flowering stage compared to other crop developmental stages and in low fields compared to the high plant diversity fields (Figure 5B).
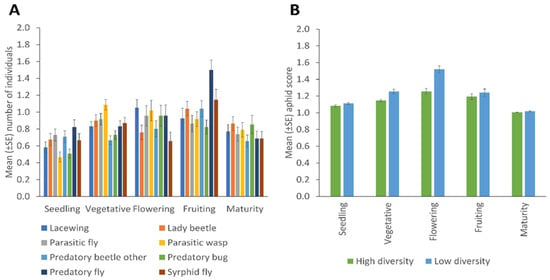
Figure 5.
Changes across the crop’s development stages in (A) the number of natural enemies (collected by sweep netting) of the various taxa, and (B) aphid infestation score, according to margin plant diversity (Error bars = s.e.m.).
4. Discussion
The classic assumptions of conservation biological control are that flowering plants and an abundance of non-crop habitat near a crop will enhance populations of NEs, that the NEs will move into the crop from this habitat, and that those NEs will eat or parasitise pests of the crop, resulting in better pest management and ultimately reduced yield losses. We set out to test these assumptions by comparing smallholder farms with different levels of plant diversity in field margins in terms of the populations of NEs they support and then evaluating whether those predatory NEs controlled key pests within the cropping area.
NEs that were collected in bean fields included predatory bugs, lacewings, predatory flies, parasitic flies, parasitic wasps, lady beetles and diverse other predatory beetles. In accordance with the expectations of conservation biocontrol theories, plant diversity in field margins had a positive impact on the number of NEs but notably, this showed up in the sticky trap data more than in the sweep netting data. This suggests that either method alone may not give an accurate indication of NEs; sticky traps may oversample volant insects relative to non-volant insects (including larvae), indicating that whether or not populations were higher in the rich margins, flight activity (implying perhaps movement within the crop) may have benefitted from richer margins. In no cases did the rich margins reduce populations of any NE taxa. Sweep netting and sticky traps have been used and are common for the collection of NEs. Sticky traps and sweep nets, for instance, have been used to collect different taxa of NEs (parasitoids, lady beetles, hoverflies, true bugs and lacewings) [35,52,53,59,60,61].
Our findings that field margins promote NE activity and/or populations concur with other studies on smallholder farms and studies such as Arnold et al. [19] and Mkenda et al. [22], which found a strong association between flower strips and plant-rich patches with NE communities. As reported by Rebek et al. [52], we found that parasitic wasps were the most abundant of the groups studied and that highly mobile individuals, such as parasitic flies, syrphid flies, and lady beetles, were caught in large numbers by sticky cards [52]. (Figure 1A; Supplementary Table S1). With the yellow sticky traps, different insect behaviours might have affected the number of NEs caught [53]. For example, yellow traps were more likely to trap Hymenoptera and Diptera, whereas blue is favoured by Thysanoptera [62,63]. There was no significant difference in the number of parasitic wasps collected through sticky trapping, and no significant differences observed in the number of lady beetles, predatory bugs, and other predatory beetles collected through sweep netting, between high and low plant diversity fields. These NEs might have been influenced by other factors like the presence of host (aphids) in the field crop [64]. Other between-site differences in NE populations, even where the plant abundance was similar, may be explained by wider differences in field management [65]. Disturbances, such as pesticide applications and cutting, have impacts on the activities of NEs and could affect populations of prey for NEs [66].
Higher numbers of parasitic wasps (from both sweep netting and sticky trapping), syrphid flies (sweep netting), syrphid flies (sticky trapping), and predatory bugs in July mostly corresponded to the bean flowering stage and changes in the frequency of catching of lacewings (sweep netting) might be due to the biotic and abiotic factors contributing to seasonal dynamics in arthropod abundance [67,68]. NE communities respond to environmental factors differently [69]. The variations may also be explained by changing prevailing environmental conditions, for example, an increase in floral resources towards the flowering stage of the bean crop. Our work adds to existing research findings showing a high abundance of NEs is associated with the provision of floral resources from plants [52,67,70,71,72,73,74]. Including a mixture of plants in agricultural systems can provide varied and complementary resources that play specific roles to NEs [75]. NEs depend on other local and landscape characteristics such as fertilizer and pesticide application, crop rotation, tillage practices, and the composition of the field surroundings [38,60,76,77]. Some studies have shown the negative effects of chemical pesticide application on the NEs of pests. Thus field margins can be used to mitigate the negative effects of insecticides on populations of NEs [78,79,80,81,82,83,84,85,86,87]. Lethal and non-lethal effects such as mortality and feeding deterrents on NEs have been associated with the application of chemical pesticides [81,82,83,84,85,86]. Generally, there was an increase in mean aphid populations around the flowering stage of the bean crop and this might have corresponded to the availability of quality host plants, although no significant differences were observed among different crop stages. High populations of aphids have been observed in the flowering stage by Azimi and Amini [88]. The study by Birch [89] found the lowest A. fabae populations in the crop maturity stage, probably due to older plants that are lower quality hosts and also due to increased predation/parasitism by NEs. The survival and reproduction of aphids depend on high-quality hosts for food sources [90].
For most insect taxa, we found consistently higher numbers in the margin relative to the crop. This agreed with most other studies, showing limited movement into the crop of insects with margin-based communities [22,91,92,93,94,95]. However, a few taxa also occurred in high numbers in the centre of the field including other predatory beetles, parasitic wasps, predatory flies and syrphid flies; as these readily enter the crop, they could be an ideal focus for future biocontrol research.
We saw a subtle effect of intercropping versus monocropping on the natural enemy numbers: while overall populations were higher in intercropped systems, no individual taxon was more abundant in intercropped fields. Parasitic wasps, for instance, come out higher in intercrops on sticky traps but their numbers are high in monocrops with sweep nets. Thus, with no consistent patterns, the effects of mono v intercropping were not significant. A few studies have found populations of NEs enhanced through intercropping [88,96]. However, based on our evidence intercropping alone as a method to support NE populations may not yield improved pest management benefits and needs to be combined with other agroecological interventions. NEs have been associated with the field margins and non-crop habitats for resources such as pollen and nectar. In addition, these habitats may offer alternative prey, corridors for their dispersal and places for overwintering and reproduction [19,22,52,92,97,98,99,100,101]. Thus, with habitat disturbances and loss due to agricultural intensification, field margins could play a key role in conserving NE communities and consequently, enhancing biological control of pests in bean fields, especially for resource-constrained smallholder farmers [18].
5. Conclusions
Field margins are valuable in minimizing the negative impacts of agricultural intensification on NE populations; therefore, bringing resilience at local and landscape scales. The abundance of plants within field margins can provide a wide range of seasonal resources to NEs. These resources may enhance NEs’ survival, longevity, and fecundity, and in turn, facilitate them in providing pest suppression. We found evidence that lacewing and lady beetle larvae and adult assassin bugs were consuming the major crop pest in this case; these should be a primary focus of future biological conservation efforts in these agricultural systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13070569/s1, Table S1. Mean ± (SEM) numbers of natural enemies in fields collected by sticky traps. Table S2. Mean ± (SEM) numbers of natural enemies in fields collected by sweep nets. Table S3. Mean ± (SEM) numbers of natural enemies in fields collected by sticky traps.
Author Contributions
Conceptualization, B.J.N., E.R.M., P.A.N., S.R.B., S.E.J.A., V.C.W. and P.C.S.; methodology, B.J.N., E.R.M., P.A.N., S.R.B., S.E.J.A., V.C.W. and P.C.S.; formal analysis, B.J.N., S.R.B., V.C.W.; investigation, B.J.N.; resources, B.J.N., E.R.M., P.A.N., S.R.B., S.E.J.A., V.C.W. and P.C.S.; data curation, B.J.N., S.R.B., V.C.W.; writing—original draft preparation, B.J.N.; writing—review and editing, B.J.N., E.R.M., P.A.N., S.R.B., S.E.J.A., V.C.W. and P.C.S.; visualization, B.J.N., E.R.M., P.A.N., S.R.B., S.E.J.A., V.C.W. and P.C.S.; supervision, E.R.M., P.A.N., S.R.B., S.E.J.A., V.C.W. and P.C.S.; project administration, E.R.M., P.A.N., S.R.B., S.E.J.A. and P.C.S.; funding acquisition, S.R.B., S.E.J.A. and P.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the UKRI GCRF project: Natural Pest Regulation in Orphan Crop Legumes in Africa (NaPROCLA) to PCS, grant number BB/R020361/1, the McKnight Foundation to SRB, grant number 20-034 and the World Bank through The Africa Centre for Research, Agricultural Advancement, Teaching Excellence and Sustainability (CREATES) at the Nelson Mandela African Institution of Science and Technology (NM-AIST).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained in supplementary material.
Acknowledgments
The authors wish to thank the smallholder farmers of Kwa Sadala village in Kilimanjaro where we conducted this study for their cooperation in each stage of the field activities, allowing the use of their farms for data collection. Also, the authors are grateful to field assistants, agricultural officers in the study area, village and ward leaders, field assistants, field labourers and NM-AIST and TPRI technicians for their utmost contribution to various aspects of the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Broughton, W.J.; Hernandez, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)–Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Nedumaran, S.; Abinaya, P.; Jyosthnaa, P.; Shraavya, B.; Rao, P.P.; Bantilan, M.C.S. Grain Legumes Production, Consumption and Trade Trends in Developing Countries; Working Paper Series No. 60; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 2015; p. 64. [Google Scholar]
- Hillocks, R.J.; Madata, C.S.; Chirwa, R.; Minja, E.M.; Msolla, S. Phaseolus bean improvement in Tanzania, 1959–2005. Euphytica 2006, 150, 215–231. [Google Scholar] [CrossRef]
- Abate, T.; Ampofo, J.K. Insect pests of beans in Africa: Their ecology and management. Annu. Rev. Entomol. 1996, 41, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Karel, A.K.; Autrique, A. Insects and other pests in Africa. In Bean Production Problems in the Tropics; Schwartz, H.F., Pastor-Corrales, M.A., Eds.; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 1989; pp. 455–504. [Google Scholar]
- Nyiira, Z.M. Pests of grain legumes and their control in Uganda. In Pests of Grain Legumes: Ecology and Control; Taylor, T.A., Singh, S.R., van Emden, H.F., Eds.; Academic Press: London, UK, 1978; pp. 118–121. [Google Scholar]
- Swaine, G. Studies on the biology and control of pests of seed beans (Phaseolus vulgaris) in northern Tanzania. Bull. Entomol. Res. 1969, 59, 323–338. [Google Scholar] [CrossRef]
- Wamonje, F.O.; Donnelly, R.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.; Mutuku, J.M.; Bruce, T.J.; Gilligan, C.A.; et al. Different plant viruses induce changes in feeding behavior of specialist and generalist aphids on common bean that are likely to enhance virus transmission. Front. Plant. Sci. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Belmain, S.R.; Haggar, J.; Holt, J.; Stevenson, P.C. Managing Legume Pests in Sub-Saharan Africa: Challenges and Prospects for Improving Food Security and Nutrition through Agro-Ecological Intensification; Natural Resources Institute: London, UK, 2013; pp. 1–34. [Google Scholar]
- Otieno, M.; Steffan-Dewenter, I.; Potts, S.G.; Kinuthia, W.; Kasina, M.J.; Garratt, M.P. Enhancing legume crop pollination and natural pest regulation for improved food security in changing African landscapes. Glob. Food Secur. 2020, 26, 100394. [Google Scholar] [CrossRef]
- Mkenda, P.A.; Ndakidemi, P.A.; Stevenson, P.C.; Arnold, S.E.; Darbyshire, I.; Belmain, S.R.; Priebe, J.; Johnson, A.C.; Tumbo, J.; Gurr, G.M. Knowledge gaps among smallholder farmers hinder adoption of conservation biological control. Biocontrol. Sci. Technol. 2020, 30, 256–277. [Google Scholar] [CrossRef]
- El-Heneidy, A.H.; Khidr, A.A.; Taman, A.A. Side-effects of insecticides on non-target organisms: 1-In Egyptian cotton fields. Egypt. J. Biol. Pest Control 2015, 25, 685–690. [Google Scholar]
- Gill, H.K.; Garg, H. Pesticide: Environmental impacts and management strategies. Pestic.-Toxic Asp. 2014, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Rice, P.J.; Arthur, E.L.; Barefoot, A.C. Advances on environmental fate and exposures assessments. J. Agric. Food Chem. 2007, 55, 5367–5376. [Google Scholar] [CrossRef]
- Tamò, M.; Srinivasan, R.; Dannon, E.; Agboton, C.; Datinon, B.; Dabiré, C.; Baoua, I.; Ba, M.; Haruna, B.; Pittendrigh, B. Biological control: A major component for the long-term cowpea pest management strategy. In Improving Livelihoods in the Cowpea Value Chain through Advancements in Science; Proceedings of the 5th World Cowpea Research Conference, Dakar, Senegal, 27 September–1 October 2010; Boukar, O., Coulibaly, C., Fatokun, C., Lopez, K., Tamò, M., Eds.; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2012; pp. 249–259. [Google Scholar]
- Ballal, C.R.; Verghese, A. Role of parasitoids and predators in the management of insect pests. In New Horizons in Insect Science: Towards Sustainable Pest Management; Springer: New Delhi, India, 2015; pp. 307–326. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Altieri, M.A. Ecological engineering: A new direction for agricultural pest management. AFBM J. 2004, 1, 28–35. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Stevenson, P.C. Cost: Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Prot. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Arnold, S.E.; Elisante, F.; Mkenda, P.A.; Tembo, Y.L.; Ndakidemi, P.A.; Gurr, G.M.; Darbyshire, I.A.; Belmain, S.R.; Stevenson, P.C. Beneficial insects are associated with botanically rich margins with trees on small farms. Sci. Rep. 2021, 11, 15190. [Google Scholar] [CrossRef] [PubMed]
- Balzan, M.V.; Bocci, G.; Moonen, A.C. Landscape complexity and field margin vegetation diversity enhance natural enemies and reduce herbivory by Lepidoptera pests on tomato crop. BioControl 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Dyer, L.E.; Landis, D.A. Influence of noncrop habitats on the distribution of Eriborus terebrans (Hymenoptera: Ichneumonidae) in cornfields. Environ. Entomol. 1997, 26, 924–932. [Google Scholar] [CrossRef]
- Mkenda, P.A.; Ndakidemi, P.A.; Stevenson, P.C.; Arnold, S.E.; Belmain, S.R.; Chidege, M.; Gurr, G.M. Field margin vegetation in tropical African bean systems harbours diverse natural enemies for biological pest control in adjacent crops. Sustainability 2019, 11, 6399. [Google Scholar] [CrossRef] [Green Version]
- Tschumi, M.; Albrecht, M.; Entling, M.H.; Jacot, K. High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc. R. Soc. B Biol. Sci. 2015, 282, 1814. [Google Scholar] [CrossRef] [Green Version]
- Tscharntke, T.; Karp, D.S.; Chaplin-kramer, R.; Batáry, P.; Declerck, F.; Gratton, C.; Hunt, L.; Ives, A.R.; Jonsson, M.; Larsen, A.E.; et al. When natural habitat fails to enhance biological pest control–Five hypotheses. Biol. Conserv. 2002, 204, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef]
- González, E.; Štrobl, M.; Janšta, P.; Hovorka, T.; Kadlec, T.; Knapp, M. Artificial temporary non-crop habitats support parasitoids on arable land. Biol. Conserv. 2022, 265, 109409. [Google Scholar] [CrossRef]
- Baggen, L.R.; Gurr, G.M. The influence of food on Copidosoma koehleri (hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculella (lepidoptera: Gelechiidae). Biol. Control 1998, 11, 9–17. [Google Scholar] [CrossRef]
- Long, R.; Corbett, A.; Lamb, C.; Reberg-Horton, C.; Chandler, J.; Stimmann, M. Beneficial insects move from flowering plants to nearby crops. Calif. Agric. 1998, 52, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Thies, C.; Tscharntke, T. Landscape structure and biological control in agroecosystems. Science 1999, 285, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, C.I.; Parrella, M.; Altieri, M.A. The effects of a vegetational corridor on the abundance and dispersal of insect biodiversity within a northern California organic vineyard. Landsc. Ecol. 2001, 16, 133–146. [Google Scholar] [CrossRef]
- Tylianakis, J.; Veddeler, D.; Lozada, T.; Lopez, R.M.; Benítez, P.; Klein, A.M.; De Koning, G.H.J.; Olschewski, R.; Veldkamp, E.; Navarrete, H.; et al. Biodiversity of land-use systems in coastal Ecuador and bioindication using trap-nesting bees, wasps, and their natural enemies. Lyonia 2004, 6, 7–15. [Google Scholar]
- Pywell, R.F.; James, K.L.; Herbert, I.; Meek, W.R.; Carvell, C.; Bell, D.; Sparks, T.H. Determinants of overwintering habitat quality for beetles and spiders on arable farmland. Biol. Conserv. 2005, 123, 79–90. [Google Scholar] [CrossRef]
- Bianchi, F.J.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Olson, D.M.; Wäckers, F.L. Management of field margins to maximize multiple ecological services. J. Appl. Ecol. 2007, 44, 13–21. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Vegetation increases the abundance of natural enemies in vineyards. Biol. Control 2009, 49, 259–269. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, B.; Li, Q.; Sun, M.; Li, M.; Wyckhuys, K.A.; Wang, P.; Lu, Y. Perennial flowering plants sustain natural enemy populations in Gobi Desert Oases of Southern Xinjiang, China. Insects 2022, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.K.; Sigsgaard, L.; Johansen, A.B.; Thorup, K.; Per, K. The impact of reduced tillage and distance to field margin on predator functional diversity. J. Insect Conserv. 2022, 26, 491–501. [Google Scholar] [CrossRef]
- Rand, T.A.; Tylianakis, J.M.; Tscharntke, T. Spillover edge effects: The dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 2006, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Wäckers, F.L. Assessing the suitability of flowering herbs as parasitoid food sources: Flower attractiveness and nectar accessibility. Biol. Control 2004, 29, 307–314. [Google Scholar] [CrossRef]
- Knapp, M.; Milan, Ř. Even the smallest non-crop habitat islands could be beneficial: Distribution of carabid beetles and spiders in agricultural landscape. PLoS ONE 2015, 10, e0123052. [Google Scholar] [CrossRef]
- Aguilera, G.; Roslin, T.; Miller, K.; Tamburini, G.; Birkhofer, K.; Sandra, B.C.; Öckinger, E.; Rundlöf, M.; Rusch, A.; Smith, H.G.; et al. Crop diversity benefits carabid and pollinator communities in landscapes with semi-natural habitats. J. Appl. Ecol. 2020, 57, 2170–2179. [Google Scholar] [CrossRef]
- Bartual, A.M.; Sutter, L.; Bocci, G.; Moonen, A.C.; Cresswell, J.; Entling, M.; Giffard, B.; Jacot, K.; Jeanneret, P.; Holland, J.; et al. The potential of different semi-natural habitats to sustain pollinators and natural enemies in European agricultural landscapes. Agric. Ecosyst. Environ. 2019, 279, 43–52. [Google Scholar] [CrossRef]
- Kovács, G.; Kaasik, R.; Lof, M.E.; van der Werf, W.; Kaart, T.; Holland, J.M.; Luik, A.; Veromann, E. Effects of land use on infestation and parasitism rates of cabbage seed weevil in oilseed rape. Pest Manag. Sci. 2019, 75, 658–666. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef] [Green Version]
- Bohinc, T.; Trdan, S. Sowing mixtures of Brassica trap crops is recommended to reduce Phyllotreta beetles injury to cabbage. Acta Agric. Scand.-B Soil Plant Sci. 2013, 63, 297–303. [Google Scholar] [CrossRef]
- Girma, H.; Rao, M.R.; Sithanantham, S. Insect pests and beneficial hedgerow intercropping systems in semiarid Kenya. Agrofor. Syst. 2000, 50, 279–292. [Google Scholar] [CrossRef]
- Tingey, W.M.; Lamont, W.J. Insect abundance in field beans altered by intercropping. Bull. Entomol. Res. 1988, 78, 527–535. [Google Scholar] [CrossRef]
- Munyuli, M.B.T.; Luther, G.C.; Kyamanywa, S. Effects of cowpea cropping systems and insecticides on arthropod predators in Uganda and the Democratic Republic of the Congo. Crop Prot. 2007, 26, 114–126. [Google Scholar] [CrossRef]
- Ndakidemi, B.J.; Mbega, E.R.; Ndakidemi, P.A.; Belmain, S.R.; Arnold, S.E.J.; Woolley, V.C.; Stevenson, P.C. Field margin plants support natural enemies in sub-Saharan Africa smallholder common bean farming systems. Plants 2022, 11, 898. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963; p. 117. [Google Scholar]
- Rebek, E.J.; Sadof, C.S.; Hanks, L.M. Manipulating the abundance of natural enemies in ornamental landscapes with floral resource plants. Biol. Control 2005, 33, 203–216. [Google Scholar] [CrossRef]
- Sutherland, J.P.; Sullivan, M.S.; Poppy, G.M. Distribution and abundance of aphidophagous hoverflies (Diptera: Syrphidae) in wildflower patches and field margin habitats. Agric. For. Entomol. 2001, 3, 57–64. [Google Scholar] [CrossRef]
- Nabirye, J.; Nampala, P.; Ogenga-Latigo, M.W.; Kyamanywa, S.; Wilson, H.; Odeke, V.; Iceduna, C.; Adipala, E. Farmer-participatory evaluation of cowpea integrated pest management (IPM) technologies in Eastern Uganda. Crop Prot. 2003, 22, 31–38. [Google Scholar] [CrossRef]
- Tena, A.; Pekas, A.; Cano, D.; Wäckers, F.L.; Urbaneja, A. Sugar provisioning maximizes the biocontrol service of parasitoids. J. Appl. Ecol. 2015, 52, 795–804. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book; John Wiley: New York, NY, USA, 2007; pp. 627–685. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2018. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 17 June 2022).
- Woolley, V.C.; Tembo, Y.L.B.; Ndakidemi, B.; Obanyi, J.N.; Sarah, E.J.; Belmain, S.R.; Ndakidemi, P.A.; Ogendo, J.O.; Stevenson, P.C. The diversity of aphid parasitoids in East Africa and implications for biological control. Pest Manag. Sci. 2022, 78, 1109–1116. [Google Scholar] [CrossRef]
- Mccravy, K.W. A Review of Sampling and Monitoring Methods for Beneficial Arthropods in Agroecosystems. Insects 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, L.; van der Werf, W.; Bianchi, F.J.J.A. Sweep netting samples, but not sticky trap samples, indicate beneficial arthropod abundance is negatively associated with landscape wide insecticide use. J. Appl. Ecol. 2022, 59, 942–952. [Google Scholar] [CrossRef]
- Bannerman, J.A.; Costamagna, A.C.; Mccornack, B.P.; Ragsdale, D.W. Comparison of Relative Bias, Precision, and Efficiency of Sampling Methods for Natural Enemies of Soybean Aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2015, 108, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.D.; Zhao, H.Y.; Fu, B.L.; Han, Y.; Liu, K.; Wu, J.H. Coloured sticky traps to selectively survey thrips in cowpea ecosystem. Neotrop. Entomol. 2016, 45, 96–101. [Google Scholar] [CrossRef]
- Pobozniak, M.; Tokarz, K.; Musynov, K. Evaluation of sticky trap colour for thrips (Thysanoptera) monitoring in pea crops (Pisum sativum L.). J. Plant Dis. Prot. 2020, 127, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, P.A. Conservation Biological Control; Academic Press: San Diego, CA, USA, 1998; p. 396. [Google Scholar]
- Martin, E.A.; Dainese, M.; Clough, Y.; Báldi, A.; Bommarco, R.; Gagic, V.; Garratt, M.P.; Holzschuh, A.; Kleijn, D.; Kovács-Hostyánszki, A.; et al. The interplay of landscape composition and configuration: New pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 2019, 22, 1083–1094. [Google Scholar] [CrossRef] [Green Version]
- Vandereycken, A.; Durieux, D.; Joie, E.; Francis, F.; Haubruge, E.; Verheggen, F.J. Aphid species and associated natural enemies in field crops: What about the invasive ladybird Harmonia axyridis (Coleoptera: Coccinellidae)? Entomol. Faun. 2015, 68, 3–15. [Google Scholar]
- Khodeir, I.A.; Khattab, M.A.; Rakha, O.M.; Sharabash, A.S.; Ueno, T.; Mousa, K.M. Population densities of pest aphids and their associated natural enemies on faba bean in Kafr el–Sheikh, Egypt. J. Fac. Agric. Kyushu Univ. 2020, 65, 97–102. [Google Scholar] [CrossRef]
- Pinheiro, F.; Diniz, I.R.; Coelho, D.; Bandeira, M.P.S. Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecol. 2002, 27, 132–136. [Google Scholar] [CrossRef]
- Kataria, R.; Kumar, D. Population dynamics of Aphis craccivora (Koch) and its natural enemies on bean crop in relation to weather parameters in Vadodara, Gujarat, India. Legume Res. 2016, 40, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Bommarco, R.; Marini, L.; Vaissière, B.E. Insect pollination enhances seed yield, quality, and market value in oilseed rap. Oecologia 2012, 169, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.G. Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol. Control 2009, 51, 294–305. [Google Scholar] [CrossRef]
- Narbona, E.; Dirzo, R. A reassessment of the function of floral nectar in Croton suberosus (Euphorbiaceae): A reward for plant defenders and pollinators. Am. J. Bot. 2010, 97, 672–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsden, M.W.; Menéndez, R.; Leather, S.R.; Wäckers, F. Optimizing field margins for biocontrol services: The relative role of aphid abundance, annual floral resources, and overwinter habitat in enhancing aphid natural enemies. Agric. Ecosyst. Environ. 2014, 199, 94–104. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Walsh, E.; Peck, D.C. Revisiting paternal care in the assassin bug, Atopozelus pallens (Heteroptera: Reduviidae). J. Insect Behav. 2004, 17, 431–436. [Google Scholar] [CrossRef]
- Parolin, P.; Bresch, C.; Desneux, N.; Brun, R.; Bout, A.; Boll, R.; Poncet, C. Secondary plants used in biological control: A review. Int. J. Pest Manag. 2012, 58, 91–100. [Google Scholar] [CrossRef]
- Redlich, S.; Martin, E.A.; Wende, B.; Steffan-dewenter, I. Landscape heterogeneity rather than crop diversity mediates bird diversity in agricultural landscapes. PLoS ONE 2018, 13, e0200438. [Google Scholar] [CrossRef] [Green Version]
- Muneret, L.; Auriol, A.; Bonnard, O.; Richart-Cervera, S.; Thiéry, D.; Rusch, A. Organic farming expansion drives natural enemy abundance but not diversity in vineyard- dominated landscapes. Ecol. Evol. 2019, 9, 13532–13542. [Google Scholar] [CrossRef]
- Bakker, L.; van der Werf, W.; Bianchi, F.J. No significant effects of insecticide use indicators and landscape variables on biocontrol in field margins. Agric. Ecosyst. Environ. 2021, 308, 107253. [Google Scholar] [CrossRef]
- Mkenda, P.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.; Mtei, K.; Belmain, S.R. Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 2015, 10, e0143530. [Google Scholar] [CrossRef]
- Mkindi, A.; Mpumi, N.; Tembo, Y.; Stevenson, P.C.; Ndakidemi, P.A.; Mtei, K.; Machunda, R.; Belmain, S.R. Invasive weeds with pesticidal properties as potential new crops. Ind. Crop. Prod. 2017, 110, 113–122. [Google Scholar] [CrossRef]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front. Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.E.; Araya, M.; Guerrero, M.A. Effects of some insecticides applied in sublethal concentrations on the survival and longevity of Aphidius ervi (Haliday) (Hymenoptera: Aphidiidae) adults. Chil. J. Agric. Res. 2010, 70, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.X.; Chen, T.-Y. Effects of the insect growth regulator fenoxycarb on immature Chrysoperla rufilabris (Neuroptera: Chrysopidae). Fla. Entomol. 2001, 84, 628–633. [Google Scholar] [CrossRef]
- Schneider, M.; Smagghe, G.; Pineda, S.; Vinuela, E. Action of insect growth regulator insecticides and spinosad on life-history parameters and absorption in third-instar larvae of the endoparasitoid Hyposoter didymator. Biol. Control 2004, 31, 189–198. [Google Scholar] [CrossRef]
- Shoeb, M.A. Effect of some insecticides on the immature stages of the egg parasitoid Trichogramma evanescens. Egypt. Acad. J. Biol. Sci. 2010, 3, 31–38. [Google Scholar] [CrossRef]
- Thomson, L.; Glenn, D.; Hoffmann, A. Effects of Sulfur on Trichogramma egg parasitoids in vineyards: Measuring toxic effects and establishing release windows. Anim. Prod. Sci. 2001, 40, 1165–1171. [Google Scholar] [CrossRef]
- Golmohammadi, G.; Torshizi, H.R.R.; Vafaei-Shooshtari, R.; Faravardeh, L.; Rafei-Karehroudi, Z. Lethal and sublethal effects of three insecticides on green lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae) under laboratory conditions. J. Entomol. Soc. Iran 2021, 41, 105–121. [Google Scholar] [CrossRef]
- Azimi, S.; Amini, R. Population density of Aphis fabae Scopoli (Hemiptera, Aphididae) and its natural enemies in intercropping of faba bean (Vicia faba L.) and dragonhead (Dracocephalum moldavica L.). J. Biodivers. Environ. Sci. 2015, 6, 380–388. [Google Scholar]
- Birch, N. Field evaluation of resistance to black bean aphid, Aphis fabae, in close relatives of the Faba bean, Vicia faba. Ann. Appl. Biol. 1985, 106, 561–569. [Google Scholar] [CrossRef]
- Karley, A.J.; Parker, W.E.; Pitchford, J.W.; Douglas, A.E. The mid-season crash in aphid populations: Why and how does it occur? Ecol. Entomol. 2004, 29, 383–388. [Google Scholar] [CrossRef]
- Caballero-López, B.; Bommarco, R.; Blanco-Moreno, J.M.; Sans, F.X.; Pujade-Villar, J.; Rundlöf, M.; Smith, H.G. Aphids and their natural enemies are differently affected by habitat features at local and landscape scales. Biol. Control 2012, 63, 222–229. [Google Scholar] [CrossRef]
- Clem, C.S.; Harmon-Threatt, A.N. Field borders provide winter refuge for beneficial predators and parasitoids: A case study on organic farms. J. Insect Sci. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Lavandero, B.; Wratten, S.D.; Didham, R.K.; Gurr, G. Increasing floral diversity for selective enhancement of biological control agents: A double-edged sward? Basic Appl. Ecol. 2006, 7, 236–243. [Google Scholar] [CrossRef]
- Nilsson, L.; Klatt, B.K.; Smith, H.G.; Campbell, A.J. Effects of flower-enriched ecological focus areas on functional diversity across scales. Front. Ecol. Evol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Samaranayake, K.G.; Costamagna, A.C. Adjacent habitat type affects the movement of predators suppressing soybean aphids. PLoS ONE 2019, 14, e0218522. [Google Scholar] [CrossRef]
- Tiroesele, B.; Obopile, M.; Karabo, O. Insect diversity and population dynamics of natural enemies under sorghum–legume intercrops. Trans. R. Soc. S. Afr. 2019, 74, 258–267. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Wildflower plantings enhance the abundance of natural enemies and their services in adjacent blueberry fields. Biol. Control 2015, 91, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Dover, J.W. The Ecology of Hedgerows and Field Margins; Routledge: Abingdon, UK, 2019. [Google Scholar] [CrossRef]
- Li, P.; Kleijn, D.; Badenhausser, I.; Zaragoza-Trello, C.; Gross, N.; Raemakers, I.; Scheper, J. The relative importance of green infrastructure as refuge habitat for pollinators increases with local land-use intensity. J. Appl. Ecol. 2020, 57, 1494–1503. [Google Scholar] [CrossRef]
- Marshall, E.J.; Moonen, A.C. Field margins in northern Europe: Their functions and interactions with agriculture. Agric. Ecosyst. Environ. 2002, 89, 5–21. [Google Scholar] [CrossRef]
- Pywell, R.F.; Heard, M.S.; Woodcock, B.A.; Hinsley, S.; Ridding., L.; Nowakowski, M.; Bullock, J.M. Wildlife-friendly farming increases crop yield: Evidence for ecological intensification. Proc. R. Soc. B Biol. Sci. 2015, 282, 1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).