Effects of Acibenzolar-S-methyl on the Probing Behaviour and Mortality of Cacopsylla pyri on Pear Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Insects and Plant Material
2.2. ASM Applications
2.3. Gene Expression Analysis after ASM Treatment
2.4. EPG-DC Recording
2.5. Nymphs Mortality Bioassay
3. Results
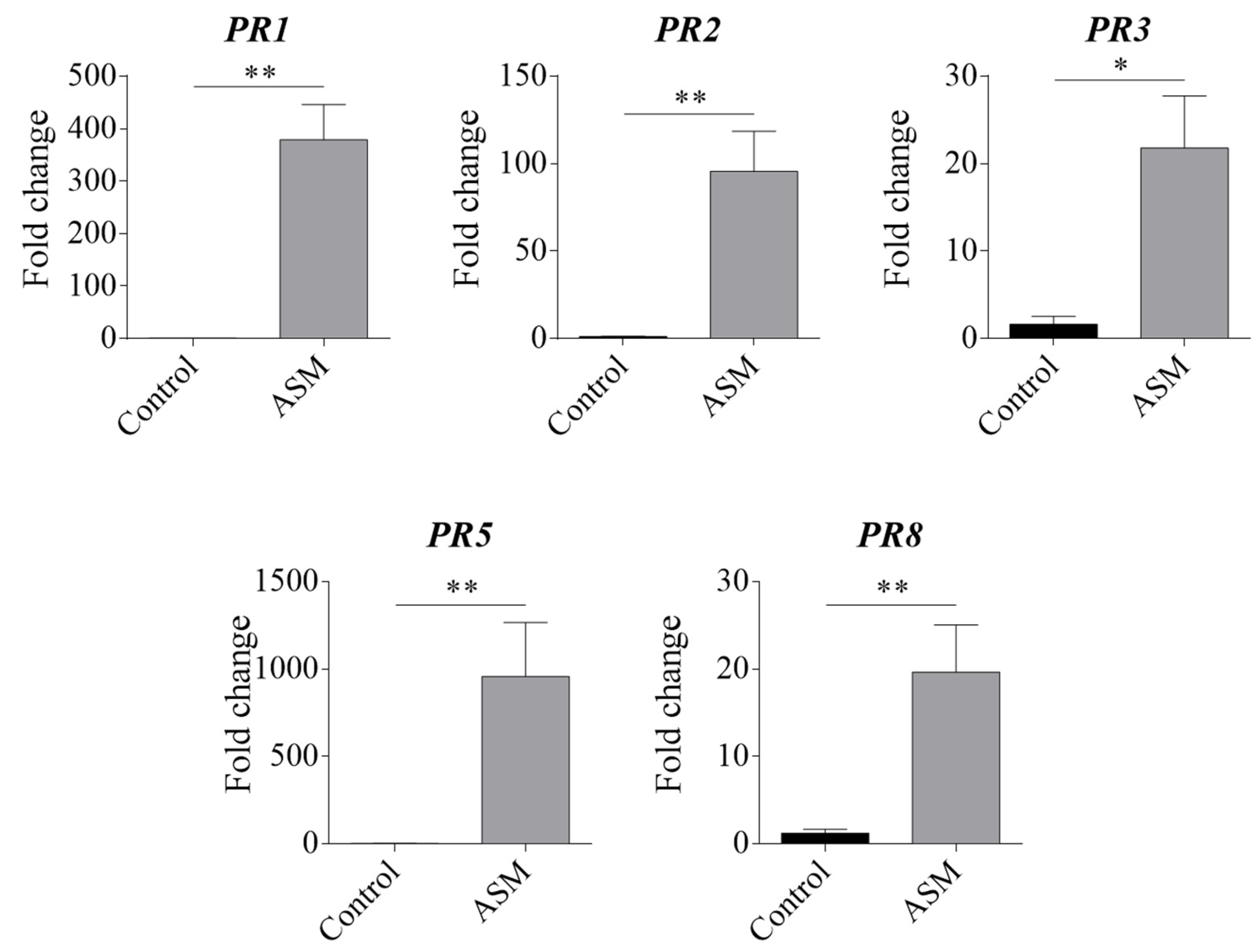
3.1. Effect of ASM Treatment on Pear Pathogenesis-Related (PR) Proteins
3.2. EPG-DC Recording
3.3. Nymph Mortality Bioassay
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Civolani, S. The past and present of pear protection against the pear psylla, Cacopsylla pyri L. In Insecticides—Pest Engineering; Perveen, F., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.A.; Ortin-Angulo, M.C. Abundance and population dynamics of Cacopsylla pyri (Hemiptera: Psyllidae) and its potential natural enemies in pear orchards in Southern Spain. Crop Prot. 2012, 32, 24–29. [Google Scholar] [CrossRef]
- Seemüller, E.; Schneider, B. ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. Int. J. Syst. Evol. Microbiol. 2004, 54, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Civolani, S.; Peretto, R.; Caroli, L.; Pasqualini, E.; Chicca, M.; Leis, M. Preliminary resistance screening on abamectin in pear psylla (Hemiptera: Psyllidae) in Northern Italy. J. Econ. Entomol. 2007, 100, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Civolani, S.; Cassanelli, S.; Rivi, M.; Manicardi, G.C.; Peretto, R.; Chicca, M.; Pasqualini, E.; Leis, M. Survey of susceptibility to abamectin of pear psylla (Hemiptera: Psyllidae) in Northern Italy. J. Econ. Entomol. 2010, 103, 816–822. [Google Scholar] [CrossRef]
- Civolani, S.; Boselli, M.; Butturini, A.; Chicca, M.; Cassanelli, S.; Tommasini, M.G.; Aschonitis, V.; Fano, E.A. Testing spirotetramat as an alternative solution to abamectin for Cacopsylla pyri (Hemiptera: Psyllidae) control: Laboratory and field tests. J. Econ. Entomol. 2015, 108, 2737–2742. [Google Scholar] [CrossRef]
- Daugherty, M.P.; Briggs, C.J.; Welter, S.C. Bottom-up and top-down control of pear psylla (Cacopsylla pyricola): Fertilization, plant quality, and the efficacy of the predator Anthocoris nemoralis. Biol. Control 2007, 43, 257–264. [Google Scholar] [CrossRef]
- Nin, S.; Ferri, A.; Sacchetti, P.; Giordani, E. Pear resistance to psilla (Cacopsylla pyri L.): A review. Adv. Hortic. Sci. 2012, 26, 59–74. [Google Scholar]
- Cooper, R.W.; Horton, D.R. Effects of elicitors of host plant defenses on pear psylla, Cacopsylla pyricola. Entomol. Exp. Appl. 2015, 177, 300–306. [Google Scholar] [CrossRef]
- Cooper, R.W.; Horton, D.R. Elicitors of host plant defenses partially suppress Cacopsylla pyricola (Hemiptera: Psyllidae) populations under field conditions. J. Insect Sci. 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orpet, R.J.; Cooper, W.R.; Beers, E.H.; Nottingham, L.B. Test of plant defense elicitors for arthropod pest suppression and PR-1 gene induction in pear orchards. Entomol. Exp. Appl. 2021, 169, 1137–1146. [Google Scholar] [CrossRef]
- Johnson, K.B.; Temple, T.N. Comparison of Methods of Acibenzolar-S-Methyl application for post-infection fire blight suppression in Pear and Apple. Plant Dis. 2016, 100, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzo, F.; Faoro, F. Systemic acquired resistance (50 years after discovery): Moving from the lab to the field. J. Agric. Food Chem. 2013, 61, 12473–12491. [Google Scholar] [CrossRef]
- Marolleau, B.; Gaucher, M.; Heintz, C.; Degrave, A.; Warneys, R.; Orain, G.; Lemarquand, A.; Brisset, M.N. When a plant resistance inducer leaves the lab for the field: Integrating ASM into routine apple protection practices. Front. Plant Sci. 2017, 8, 1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuzun, S.; Somanchi, A. The possible role of PR proteins in multigenic and induced systemic resistance. In Multigenic and Induced Systemic Resistance in Plants; Tuzun, S., Bent, E., Eds.; Springer: New York, NY, USA, 2006; Chapter 6; pp. 112–142. [Google Scholar]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Ann. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Maxson-Stein, K.; He, S.-Y.; Hammerschmidt, R.; Jones, A.L. Effect of treating apple trees with acibenzolar-S-methyl on fire blight and expression of pathogenesis-related protein genes. Plant Dis. 2002, 86, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norelli, J.L.; Jones, A.L.; Aldwinckle, H.S. Fire blight management in the twenty-first century: Using new technologies that enhance host resistance in apple. Plant Dis. 2003, 87, 756–765. [Google Scholar] [CrossRef] [Green Version]
- Sparla, F.; Rotino, L.; Valgimigli, M.C.; Pupillo, P.; Trost, P. Systemic resistance induced by benzothiadiazole in pear inoculated with the agent of fire blight (Erwinia amylovora). Sci. Hortic. 2004, 101, 269–279. [Google Scholar] [CrossRef]
- Bazzi, C.; Biondi, E.; Berardi, R.; Brunelli, A. Efficacy of bioagents and chemicals against pear shoot blight. Acta Hortic. 2006, 704, 283–288. [Google Scholar] [CrossRef]
- Inbar, M.; Doostdar, H.; Gerling, D.; Mayer, R.T. Induction of systemic acquired resistance in cotton by BTH has a negligible effect on phytophagous insects. Entomol. Exp. Appl. 2001, 99, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.L.; Murphy, J.B.; Felton, G.W. Does salicylic acid act as a signal in cotton for induced resistance to Helicoverpa zea? J. Chem. Ecol. 1997, 23, 1805–1818. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Cooper, W.C.; Jia, L.; Goggin, F.L. Acquired and R-gene-mediated resistance against the potato aphid in tomato. J. Chem. Ecol. 2004, 30, 2527–2542. [Google Scholar] [CrossRef] [PubMed]
- Civolani, S.; Marchetti, E.; Chicca, M.; Castaldelli, G.; Rossi, R.; Pasqualini, E.; Dindo, M.L.; Baronio, P.; Leis, M. Probing behaviour of Myzus persicae on tomato plants containing Mi gene or BTH-treated evaluated by electrical penetration graph. Bull. Insectology 2010, 63, 265–271. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jung, S.; Lee, T.; Cheng, C.H.; Buble, K.; Zheng, P.; Yu, J.; Humann, J.; Ficklin, S.P.; Gasic, K.; Scott, K.; et al. 15 years of GDR: New data and functionality in the Genome Database for Rosaceae. Nucleic Acids Res. 2018, 47, D1137–D1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjallingii, W.F. Electrical nature of recorded signals during stylet penetration by aphids. Entomol. Exp. Appl. 1985, 38, 177–186. [Google Scholar] [CrossRef]
- Civolani, S.; Leis, M.; Grandi, G.; González, E.; Pasqualini, E.; Chicca, M.; Tjallingii, W.F. Plant penetration by Cacopsylla pyri. An electrical penetration graph (EPG) study. J. Insect Physiol. 2011, 57, 1407–1419. [Google Scholar] [CrossRef]
- Civolani, S.; Grandi, G.; Chicca, M.; Pasqualini, E.; Fano, E.A.; Musacchi, S. Probing behaviour of Cacopsylla pyri on a resistant pear selection. J. Appl. Entomol. 2013, 137, 365–375. [Google Scholar] [CrossRef]
- Bonani, J.P.; Fereres, A.; Garzo, E.; Miranda, M.P.; Appezzato-Da-Gloria, B.; Lopes, J.R.S. Characterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol. Exp. Appl. 2010, 134, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Ripamonti, M.; Maron, F.; Cornara, D.; Marzachì, C.; Fereres, A.; Bosco, D. Leafhopper feeding behaviour on three grapevine cultivars with different susceptibilities to Flavescence dorée. J. Insect Physiol. 2022, 137, 104366. [Google Scholar] [CrossRef]
- Backus, E.A.; Cline, A.R.; Ellerseick, M.R.; Serrano, M.A.S. Lygus hesperus (Hemiptera: Miridae) Feeding on Cotton: New Methods and Parameters for Analysis of Nonsequential Electrical Penetration Graph Data. Ann. Entomol. Soc. Am. 2007, 100, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Schneider-Orelli, O. Computation of the level of effectiveness. In Entomologisches Parktikum; 2 Auflage; Sauerländer: Aarau, Switzerland, 1947; p. 237. [Google Scholar]
- Bressan, A.; Purcell, A.H. Effect of benzothiadiazole on transmission of X-disease phytoplasma by the vector Colladonus montanus to Arabidopsis thaliana, a new experimental host plant. Plant Dis. 2005, 89, 1121–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amelio, R.; Marzachi, C.; Bosco, D. Activity of benzothiadiazole on chrysanthemum yellows phytoplasma (‘Candidatus Phytoplasma asteris’) infection in daisy plants. Crop Prot. 2010, 29, 1094–1099. [Google Scholar] [CrossRef]
- Ratchaseema, M.T.N.; Kladsuwan, L.; Soulard, L.; Swangmaneecharern, P.; Punpee, P.; Klomsa-ard, P.; Sriroth, K.; Keawsompong, S. The role of salicylic acid and benzothiadiazole in decreasing phytoplasma titer of sugarcane white leaf disease. Sci. Rep. 2021, 11, 15211. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Murolo, S.; Feliziani, E. Effects of an innovative strategy to contain grapevine bois noir: Field treatment with resistance inducers. Phytopathology 2013, 103, 785–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Accession nr |
|---|---|---|---|
| PR1 | GCACAAAACTACGCCAACCA | CCTTTCCATCAGCACACGAG | JQ965001 |
| PR2 | TCTCCTGCCTGCCATCCAAA | CCCACTGTTGACGAGGAAGT | PCP025709 |
| PR3 | ACCTAGACGGCGCTATCCAA | CCATTGGCTCCAACTGTTCA | JQ965010 |
| PR5 | GCAGTTCCACCAGCAACTTTA | TTAACATTGGCAGGGCACGAT | PCP037574 |
| PR8 | ACCCAGGTGTTCATGGGGTTA | CCGTTGTCATAGAACCTGCTC | PCP012163 |
| EF1α | ACAAGATGGATGCCACCACTC | AGGTTGGTGGACCTCTCAATC | PCP028497 |
| Total Duration in Minutes/Insect | |||||||
|---|---|---|---|---|---|---|---|
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 105.66 ± 15.02 | 161.36 ± 14.89 | 9.08 ± 3.75 | 2.49 ± 2.10 | 3.25 ± 0.57 | 41.62 ± 9.81 | 156.51 ± 27.65 |
| Control | 134.32 ± 21.31 | 164.96 ± 16.60 | 23.83 ± 6.68 | 3.22 ± 2.00 | 1.53 ± 0.31 | 16.20 ± 3.11 | 135.89 ± 25.45 |
| p value | 0.36 | 0.93 | 0.13 | 0.60 | 0.005 * | 0.01 * | 0.73 |
| Number of Events per Insect | |||||||
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 9.41 ± 1.45 | 18.82 ± 2.18 | 0.32 ± 0.12 | 0.41 ± 0.32 | 9.73 ± 1.63 | 10.09 ± 1.66 | 2.14 ± 0.34 |
| Control | 7.82 ± 1.15 | 12.55 ± 1.35 | 0.86 ± 0.22 | 0.23 ± 0.09 | 4.95 ± 0.95 | 4.86 ± 0.95 | 1.77 ± 0.41 |
| p value | 0.47 | 0.04 * | 0.07 | 0.65 | 0.006 * | 0.003 * | 0.26 |
| Duration of Event in Minutes/Insect | |||||||
| Non-probing | PA, PB, C1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (PE2) | |
| ASM | 14.99 ± 3.46 | 10.07 ± 1.17 | 8.05 ± 3.40 | 0.69 ± 0.40 | 0.32 ± 0.03 | 3.45 ± 0.64 | 74.23 ± 23.67 |
| Control | 24.41 ± 5.60 | 15.60 ± 2.16 | 16.12 ± 4.43 | 3.22 ± 2.00 | 0.30 ± 0.02 | 4.20 ± 0.87 | 100.31 ± 22.37 |
| p value | 0.21 | 0.007 * | 0.18 | 0.55 | 1 | 1 | 1 |
| Total Duration in Minutes/Insect | |||||||
|---|---|---|---|---|---|---|---|
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 134.68 ± 28.91 | 173.16 ± 23.38 | 27.17 ± 17.21 | 40.33 ± 29.02 | 3.14 ± 1.35 | 38.29 ± 19.00 | 63.23 ± 33.17 |
| Control | 186.16 ± 23.93 | 127.73 ± 19.23 | 44.23 ± 18.21 | 8.91 ± 4.71 | 1.46 ± 0.35 | 43.13 ± 14.43 | 68.38 ± 28.01 |
| p value | 0.23 | 0.20 | 0.65 | 1 | 0.23 | 0.96 | 0.77 |
| Number of Events per Insect | |||||||
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 13.50 ± 2.25 | 24.83 ± 4.94 | 0.67 ± 0.33 | 3.17 ± 2.01 | 12.17 ± 5.37 | 12.17 ± 5.37 | 2.50 ± 0.67 |
| Control | 15.43 ± 3.44 | 21.79 ± 2.89 | 1.07 ± 0.29 | 1.79 ± 0.59 | 6.21 ± 1.42 | 6.36 ± 1.49 | 1.93 ± 0.56 |
| p value | 0.90 | 0.59 | 0.50 | 1 | 0.40 | 0.43 | 0.30 |
| Duration of Event in Minutes/Insect | |||||||
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 10.70 ± 2.14 | 7.83 ± 1.57 | 24.31 ± 17.21 | 4.61 ± 2.62 | 0.34 ± 0.08 | 3.54 ± 0.91 | 42.66 ± 25.97 |
| Control | 17.43 ± 3.79 | 6.11 ± 0.82 | 22.03 ± 7.74 | 2.48 ± 0.77 | 0.24 ± 0.04 | 4.94 ± 1.02 | 38.49 ± 23.40 |
| p value | 0.34 | 0.34 | 0.83 | 0.86 | 0.10 | 0.43 | 0.96 |
| Total Duration in Minutes/Insect | |||||||
|---|---|---|---|---|---|---|---|
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 172.97 ± 21.13 | 113.40 ± 14.43 | 115.52 ± 13.43 | 62.42 ± 15.95 | 1.815 ± 0.56 | 41.41 ± 3.28 | 68.82 ± 41.92 |
| Control | 146.10 ± 22.20 | 131.13 ± 16.07 | 130.92 ± 20.50 | 81.94 ± 17.56 | 5.02 ± 3.94 | 21.79 ± 8.96 | 23.05 ± 16.77 |
| p value | 0.46 | 0.42 | 0.96 | 0.56 | 0.72 | 0.65 | 0.46 |
| Number of Events per Insect | |||||||
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 10.65 ± 1.79 | 14.65 ± 2.08 | 2.20 ± 0.39 | 2.80 ± 0.83 | 3.00 ± 1.25 | 2.15 ± 0.83 | 0.95 ± 0.45 |
| Control | 11.18 ± 1.72 | 14.63 ± 2.13 | 2.45 ± 0.29 | 4.77 ± 1.29 | 1.50 ± 0.46 | 1.50 ± 0.44 | 0.63 ± 0.25 |
| p value | 0.91 | 1 | 0.44 | 0.15 | 0.94 | 0.87 | 0.84 |
| Duration of Event in Minutes/Insect | |||||||
| Non-probing | PA, PB, PC1 | Xylem ingestion (PG) | PC2 | PD | Phloem salivation (PE1) | Phloem ingestion (E2) | |
| ASM | 35.05 ± 10.24 | 9.42 ± 1.16 | 55.28 ± 10.46 | 17.48 ± 3.74 | 0.22 ± 0.03 | 2.70 ± 0.85 | 12.14 ± 8.40 |
| Control | 19.70 ± 4.60 | 10.56 ± 1.45 | 49.31 ± 4.42 | 14.43 ± 2.34 | 0.95 ± 0.64 | 3.45 ± 0.87 | 20.44 ± 17.15 |
| p value | 0.19 | 0.42 | 0.80 | 0.68 | 0.14 | 0.68 | 0.56 |
| N | Total C. pyri Nymphs per Treatment | Mortality % (±SEM) | % ASM Efficacy (Corrected Mortality) | |
|---|---|---|---|---|
| ASM | 9 | 180 | 27.97 ± 2.44 | 13.94 |
| Control | 9 | 180 | 16.30 ± 2.77 | - |
| p value | 0.009 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civolani, S.; Mirandola, D.; Benetti, L.; Finetti, L.; Pezzi, M.; Bernacchia, G. Effects of Acibenzolar-S-methyl on the Probing Behaviour and Mortality of Cacopsylla pyri on Pear Plants. Insects 2022, 13, 525. https://doi.org/10.3390/insects13060525
Civolani S, Mirandola D, Benetti L, Finetti L, Pezzi M, Bernacchia G. Effects of Acibenzolar-S-methyl on the Probing Behaviour and Mortality of Cacopsylla pyri on Pear Plants. Insects. 2022; 13(6):525. https://doi.org/10.3390/insects13060525
Chicago/Turabian StyleCivolani, Stefano, Daniele Mirandola, Lorenzo Benetti, Luca Finetti, Marco Pezzi, and Giovanni Bernacchia. 2022. "Effects of Acibenzolar-S-methyl on the Probing Behaviour and Mortality of Cacopsylla pyri on Pear Plants" Insects 13, no. 6: 525. https://doi.org/10.3390/insects13060525
APA StyleCivolani, S., Mirandola, D., Benetti, L., Finetti, L., Pezzi, M., & Bernacchia, G. (2022). Effects of Acibenzolar-S-methyl on the Probing Behaviour and Mortality of Cacopsylla pyri on Pear Plants. Insects, 13(6), 525. https://doi.org/10.3390/insects13060525