Characterization of Insecticide Response-Associated Transcripts in the Colorado Potato Beetle: Relevance of Selected Cytochrome P450s and Clothianidin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. RNA Isolation
2.3. Synthesis of cDNA
2.4. qRT-PCR Amplification of Transcripts of Interest
2.5. dsRNA Synthesis
2.6. dsRNA Injection
2.7. Transcript Targets Silencing Assessment
2.8. Clothianidin Treatment in Insects Injected with dsRNA
2.9. Quantification and Statistical Analysis
3. Results
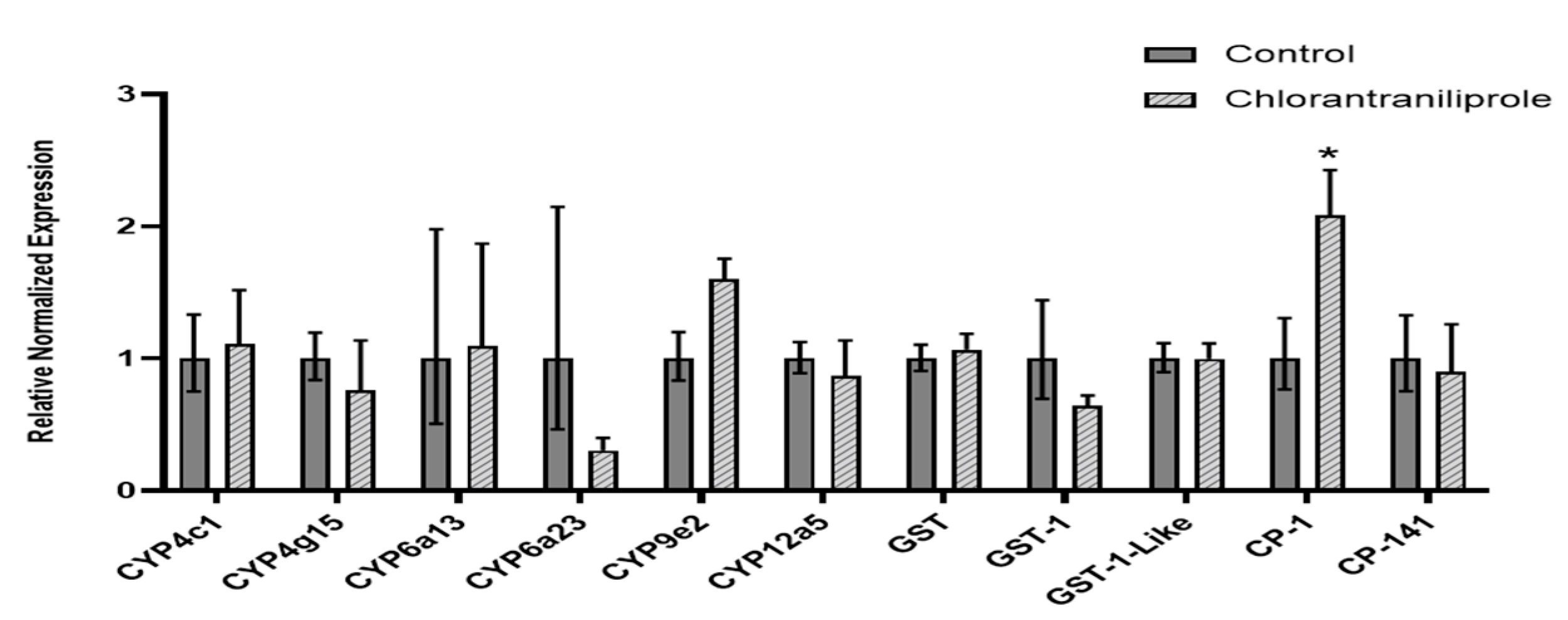
3.1. Transcript Expression in Chlorantraniliprole-Exposed L. decemlineata
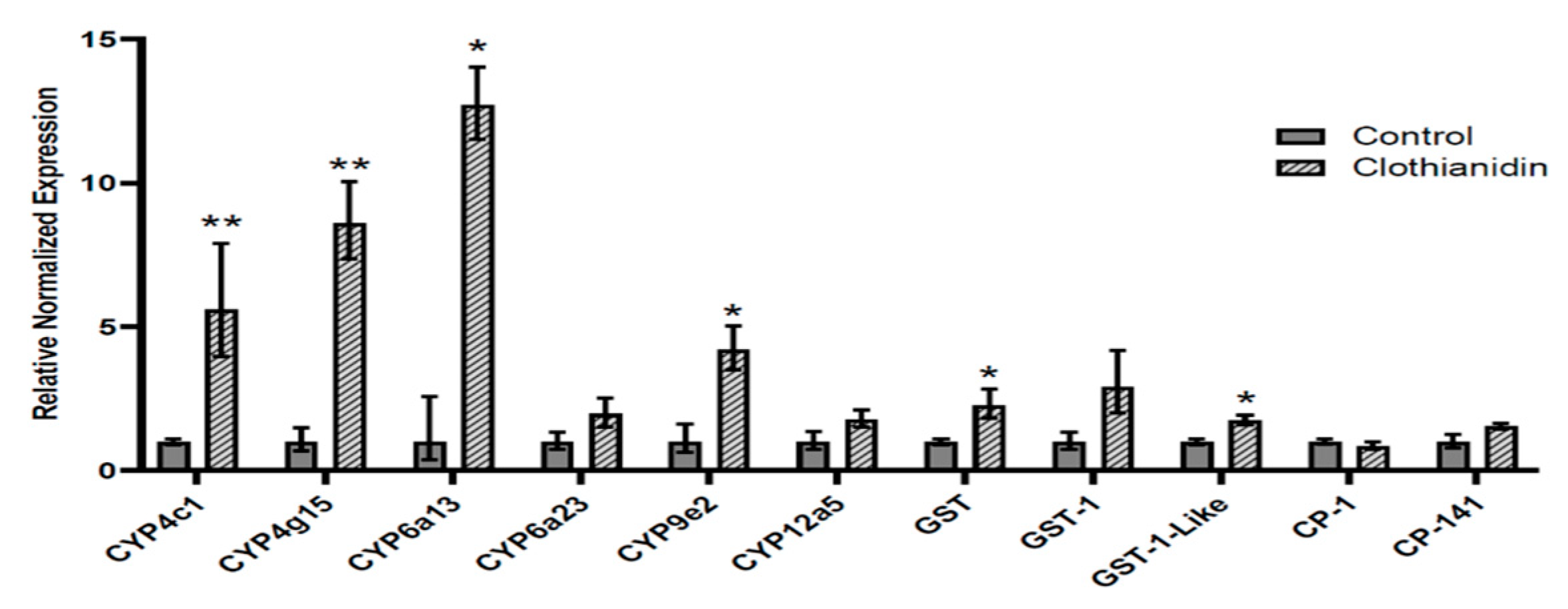
3.2. Transcript Expression in Clothianidin-Exposed L. decemlineata
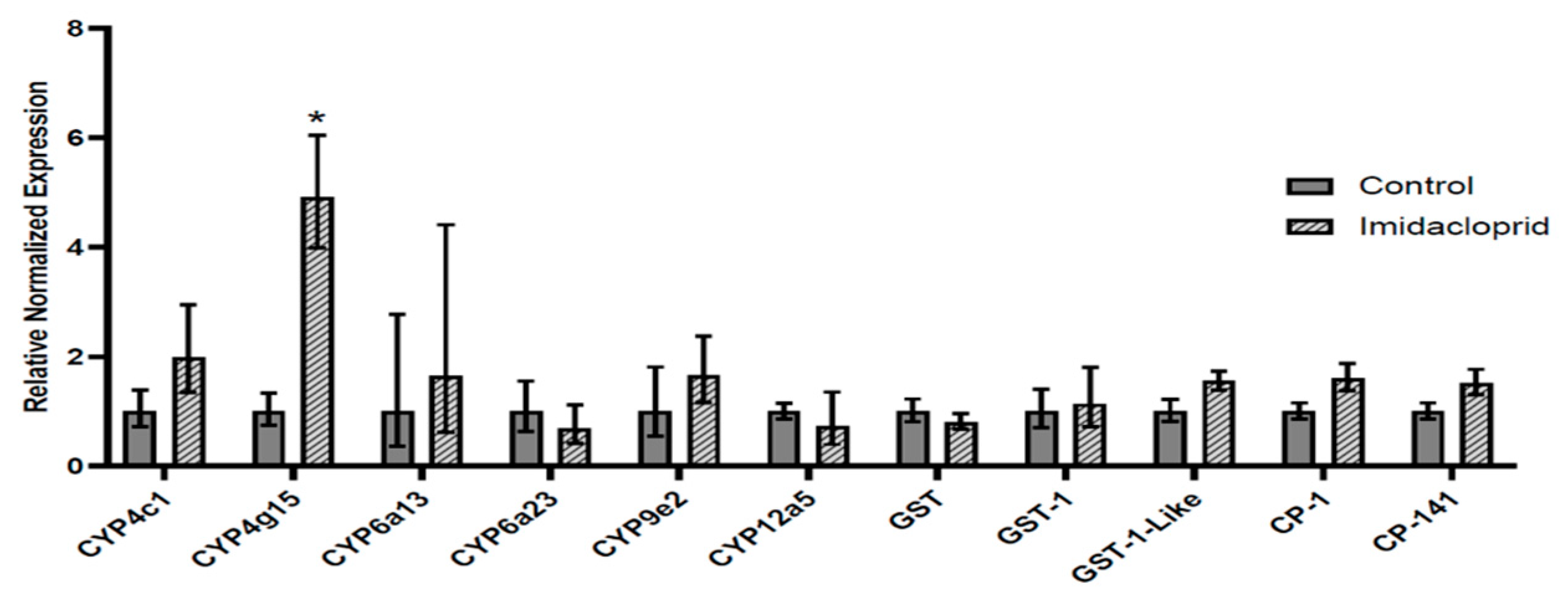
3.3. Transcript Expression in Imidacloprid-Exposed L. decemlineata
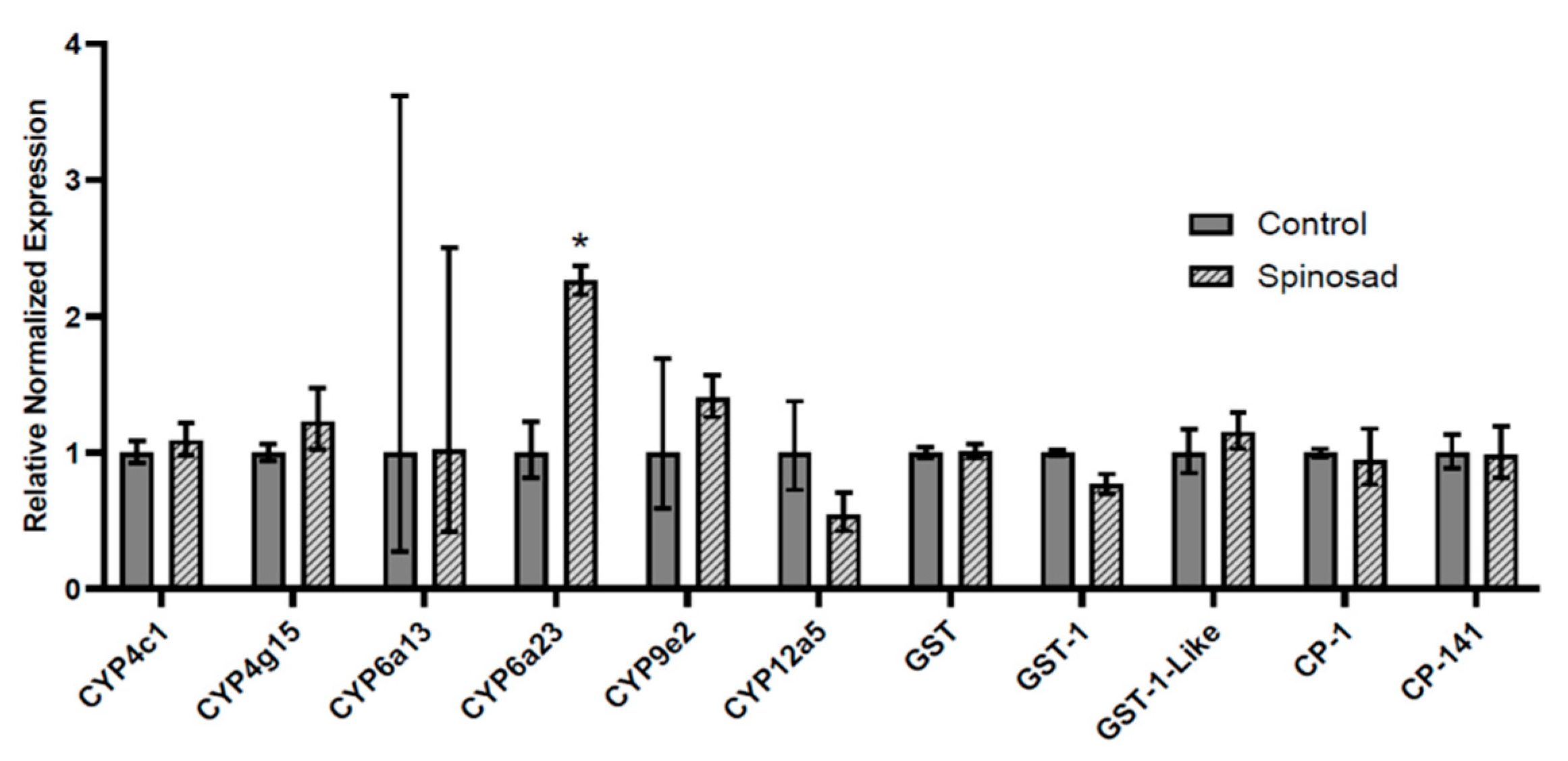
3.4. Transcript Expression in Spinosad-Exposed L. decemlineata
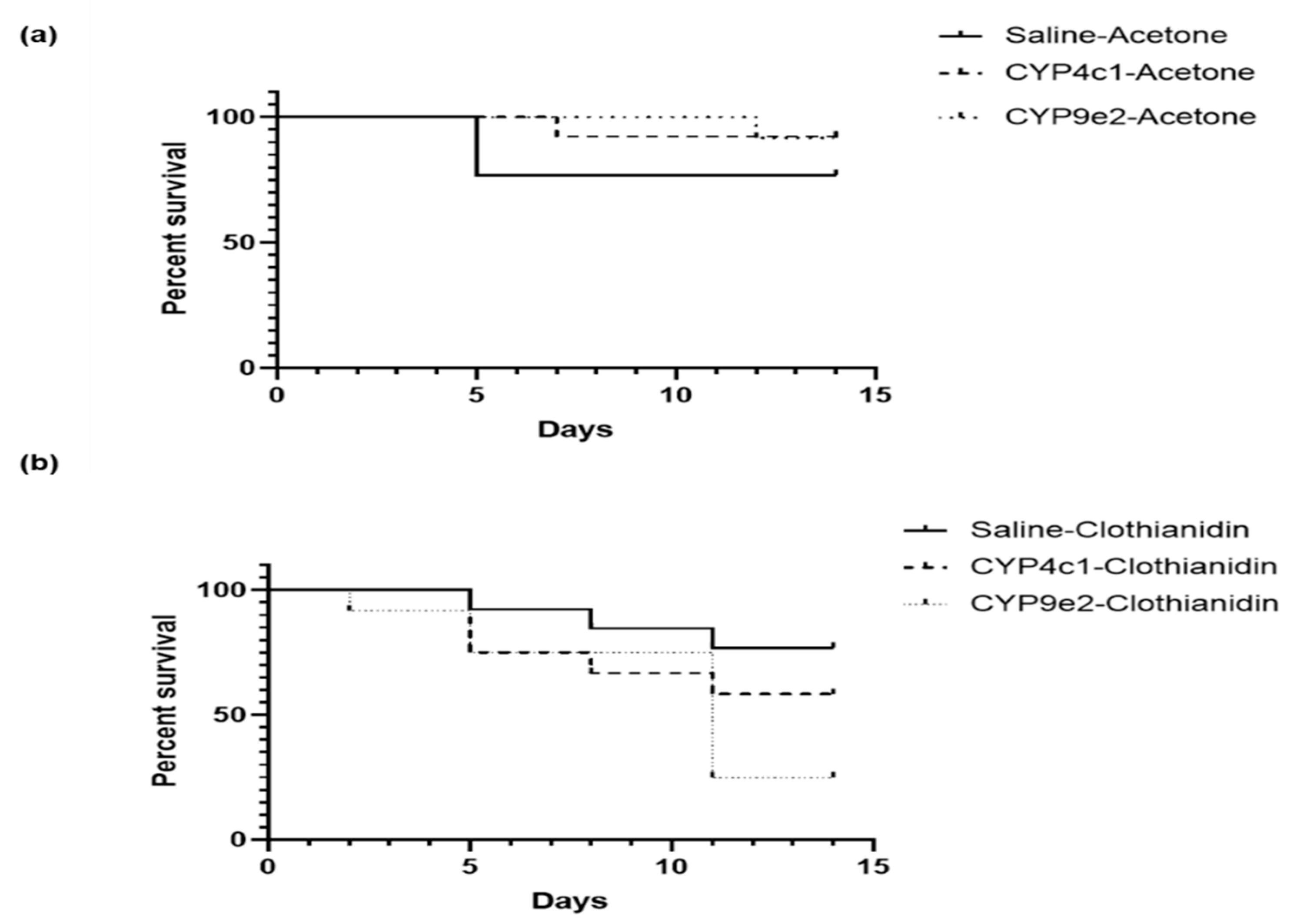
3.5. Mortality of L. decemlineata in dsRNA-Injected Insects Exposed to Clothianidin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, D. Colorado beetle: Pest on the move. Pest. Outlook 2003, 14, 256. [Google Scholar] [CrossRef]
- Ferro, D.N.; Logan, J.A.; Voss, R.H.; Elkinton, J.S. Colorado Potato Beetle (Coleoptera: Chrysomelidae) temperature-dependent growth and feeding rates. Environ. Entomol. 1985, 14, 343–348. [Google Scholar] [CrossRef]
- Alyokhin, A.; Vincent, C.; Giordanengo, P. Insect Pests of Potato: Global Perspectives on Biology and Management; Giordanengo, P., Vincent, C., Alyokhin, A., Eds.; Elsevier: San Diego, CA, USA, 2013; pp. 29–38. [Google Scholar]
- Huseth, A.S.; Frost, K.E.; Knuteson, D.L.; Wyman, J.A.; Groves, R.L. Effects of landscape composition and rotation distance on Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) abundance in cultivated potato. Environ. Entomol. 2012, 41, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Hazzard, R.V.; Ferro, D.N.; Van Driesche, R.G.; Tuttle, A.F. Mortality of eggs of Colorado Potato Beetle (Coleoptera: Chrysomelidae) from predation by Coleomegilla maculate (Coleoptera: Coccinellidae). Environ Entomol. 1991, 20, 841–848. [Google Scholar] [CrossRef]
- Houghgoldstein, J.; Whalen, J. Inundative release of predatory stink bugs for control of Colorado Potato Beetle. Biol. Control 1993, 3, 343–347. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Srinivasan, R.; Cervantes, F.A. Occurrence of the Carabid beetle, Pterostichus melanarius (Illiger), in potato ecosystems of Idaho and its predatory potential on the Colorado potato beetle and aphids. Am. J. Potato Res. 2013, 90, 83–92. [Google Scholar] [CrossRef]
- Alyokhin, A.; Mota-Sanchez, D.; Baker, M.; Snyder, W.E.; Menasha, S.; Whalon, M.; Dively, G.; Moarsi, W.F. The Red Queen in a potato field: Integrated pest management versus chemical dependency in Colorado potato beetle control. Pest Manag. Sci. 2015, 71, 343–356. [Google Scholar] [CrossRef]
- Balaško, M.K.; Mikac, K.M.; Bažok, R.; Lemic, D. Modern techniques in Colorado Potato Beetle (Leptinotarsa decemlineata Say) control and resistance management: History review and future perspectives. Insects 2020, 11, 581. [Google Scholar] [CrossRef]
- Wan, P.-J.; Shi, X.-Q.; Kong, Y.; Zhou, L.-T.; Guo, W.-C.; Ahmat, T.; Li, G.Q. Identification of cytochrome P450 monooxygenase genes and their expression profiles in cyhalothrin-treated Colorado potato beetle, Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2013, 107, 360–368. [Google Scholar] [CrossRef]
- Clements, J.; Schoville, S.; Peterson, N.; Lan, Q.; Groves, R.L. Characterizing molecular mechanisms of imidacloprid resistance in select populations of Leptinotarsa decemlineata in the central sands region of Wisconsin. PLoS ONE 2016, 11, e0147844. [Google Scholar] [CrossRef]
- Clements, J.; Schoville, S.; Peterson, N.; Huseth, A.S.; Lan, Q.; Groves, R.L. RNA interference of three up-regulated transcripts associated with insecticide resistance in an imidacloprid resistant population of Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2017, 135, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, M.N.; Gökçe, A.; Aksoy, E.; Bakhsh, A. Downregulation of imidacloprid resistant genes alters the biological parameters in Colorado potato beetle, Leptinotarsa decemlineata Say (chrysomelidae: Coleoptera). Chemosphere 2020, 240, 124857. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-B.; Li, G.-Q.; Wan, P.-J.; Zhu, T.-T.; Meng, Q.-W. Identification of glutathione S-transferase genes in Leptinotarsa decemlineata and their expression patterns under stress of three insecticides. Pestic. Biochem. Physiol. 2016, 133, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Sanchez-Sedillo, B.; Bradfield, C.A.; Groves, R.L. Transcriptomic analysis reveals similarities in genetic activation of detoxification mechanisms resulting from imidacloprid and chlorothalonil exposure. PLoS ONE 2018, 13, e0205881. [Google Scholar] [CrossRef]
- Bastarache, P.; Wajnberg, G.; Dumas, P.; Chacko, S.; Lacroix, J.; Crapoulet, N.; Moffat, C.E.; Morin, P.J. Transcriptomics-based approach identifies spinosad-associated targets in the Colorado Potato Beetle, Leptinotarsa decemlineata. Insects 2020, 11, 820. [Google Scholar] [CrossRef]
- Dumas, P.; Sambou, M.; Gaudet, J.D.; Morin, M.D.; Moffat, C.E.; Boquel, S.; Morin, P.J. Differential expression of transcripts with potential relevance to chlorantraniliprole response in the Colorado potato beetle, Leptinotarsa decemlineata. Arch. Insect Biochem. Physiol. 2020, 103, e21642. [Google Scholar] [CrossRef]
- Morin, M.D.; Lyons, P.J.; Crapoulet, N.; Boquel, S.; Morin, P.J. Identification of differentially expressed miRNAs in Colorado Potato Beetles (Leptinotarsa decemlineata (Say)) exposed to imidacloprid. Int. J. Mol. Sci. 2017, 18, 2728. [Google Scholar] [CrossRef]
- Jiang, W.-H.; Lu, W.-P.; Guo, W.-C.; Xia, Z.-H.; Fu, W.-J.; Li, G.-Q. Chlorantraniliprole susceptibility in Leptinotarsa decemlineata in the North Xinjiang Uygur autonomous region in China. J. Econ. Entomol. 2012, 105, 549–554. [Google Scholar] [CrossRef][Green Version]
- Kalsi, M.; Palli, S.R. Transcription factor cap « n » collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef]
- Gaddelapati, S.C.; Kalsi, M.; Roy, A.; Palli, S.R. Cap « n » collar C regulates genes responsible for imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Insect Biochem. Mol. Biol. 2018, 99, 54–62. [Google Scholar] [CrossRef]
- Tian, F.; Li, C.; Wang, Z.; Liu, J.; Zeng, X. Identification of detoxification genes in imidacloprid-resistant Asian citrus psyllid (Hemiptera: Lividae) and their expression patterns under stress of eight insecticides. Pest Manag. Sci. 2019, 75, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Lien, N.T.K.; Ngoc, N.T.H.; Lan, N.N.; Hien, N.T.; Tung, N.V.; Ngan, N.T.T.; Hoang, N.H.; Binh, N.T.H. Transcriptome sequencing and analysis of changes associated with insecticide resistance in the dengue mosquito (Aedes aegypti) in Vietnam. Am. J. Trop. Med. Hyg. 2019, 100, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Llorente, L.; Herrero, Ó.; Aquilino, M.; Planelló, R. Prodiamesa olivacea: De novo biomarker genes in a potential sentinel organism for ecotoxicity studies in natural scenarios. Aquat. Toxicol. 2020, 227, 105593. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.M.; Hatten, G.; Tuncer, Y.; Clarke, V.C.; Jurcic, K.; Yeung, K.K.-C. Proteomic analyses detect higher expression of c-type lectins in imidacloprid-resistant Colorado Potato Beetle Leptinotarsa decemlineata (Say). Insects 2021, 12, 3. [Google Scholar] [CrossRef]
- Wan, P.-J.; Guo, W.-Y.; Yang, Y.; Lü, F.-G.; Lu, W.-P.; Li, G.-Q. RNAi suppression of the ryanodine receptor gene results in decreased susceptibility to chlorantraniliprole in Colorado potato beetle Leptinotarsa decemlineata. J. Insect Physiol. 2014, 63, 48–55. [Google Scholar] [CrossRef]
- Gao, Y.; Kim, K.; Kwon, D.H.; Jeong, I.H.; Clark, J.M.; Lee, S.H. Transcriptome-based identification and characterization of genes commonly responding to five different insecticides in the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2018, 144, 1–9. [Google Scholar] [CrossRef]
- Schnaars-Uvino, K.; Baker, M.B. High-level field-evolved resistance to spinosad in Colorado potato beetle, Leptinotarsa decemlineata, in organically managed fields. Pest Manag. Sci. 2021, 77, 4393–4399. [Google Scholar] [CrossRef]
- Petek, M.; Coll, A.; Ferenc, R.; Razinger, J.; Gruden, K. Validating the potential of double-stranded RNA targeting Colorado Potato Beetle Mesh gene in laboratory and field trials. Front. Plant Sci. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Duneau, D.; Sun, H.; Revah, J.; Miguel, K.S.; Kunerth, H.D.; Caldas, I.V.; Messer, P.W.; Scott, J.G.; Buchon, N. Signatures of insecticide selection in the genome of Drosophila melanogaster. G3 Bethesda 2018, 8, 3469–3480. [Google Scholar] [CrossRef]
- Daborn, P.J.; Lumb, C.; Boey, A.; Wong, W.; Ffrench-Constant, R.H.; Batterham, P. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression. Insect Biochem. Mol. Biol. 2007, 37, 512–519. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z.; Wan, H.; Li, J. Characterization of nitenpyram resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhu, Y.C.; Adamczyk, J. Responses of honey bees to lethal and sublethal doses of formulated clothianidin alone and mixtures. J. Econ. Entomol. 2018, 111, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, L.; Ali, F.; Xie, M.; Zhang, G.; Su, W. Using next-generation sequencing to detect differential expression genes in Bradysia odoriphaga after exposure to insecticides. Int. J. Mol. Sci. 2017, 18, 2445. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mei, Y.; Liu, R.; Chen, X.; Li, D.; Wang, C. Transcriptome analysis of Spodoptera litura reveals the molecular mechanism to pyrethroids resistance. Pestic. Biochem. Physiol. 2020, 169, 104649. [Google Scholar] [CrossRef] [PubMed]
- Harrop, T.W.; Denecke, S.; Yang, Y.T.; Chan, J.; Daborn, P.J.; Perry, T.; Batterham, P. Evidence for activation of nitenpyram by a mitochondrial cytochrome P450 in Drosophila melanogaster. Pest Manag. Sci. 2018, 74, 1616–1622. [Google Scholar] [CrossRef]
- Bartling, M.T.; Thümecke, S.; Russert, J.H.; Vilcinskas, A.; Lee, K.Z. Exposure to low doses of pesticides induces an immune response and the production of nitric oxide in honeybees. Sci. Rep. 2021, 11, 6819. [Google Scholar] [CrossRef]
- Dai, W.T.; Li, J.; Ban, L.P. Genome-wide selective signature analysis revealed insecticide resistance mechanisms in Cydia pomonella. Insects 2021, 13, 2. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; He, B.; Zhang, Y.; Cai, T.; Wan, H.; Jin, B.R.; Li, J. Activator protein-1 mediated CYP6ER1 overexpression in the clothianidin resistance of Nilaparvata lugens (Stål). Pest Manag. Sci. 2021, 77, 4476–4482. [Google Scholar] [CrossRef]
- Alyokhin, A.; Divelym, G.; Patterson, M.; Castaldo, C.; Rogers, D.; Mahoney, M.; Wollam, J. Resistance and cross-resistance to imidacloprid and thiamethoxam in the Colorado potato beetle Leptinotarsa decemlineata. Pest Manag. Sci. 2007, 63, 32–41. [Google Scholar] [CrossRef]

| Primer | Sequence | Efficiency | Temperature | |
|---|---|---|---|---|
| CYP4c1 | Fwd Rev | 5′-TGGCTTGGATTGGGATTGCT-3′ 5′-CCATTGCGGCCTCACAAATT-3′ | 102.5% | 60.2 °C |
| CYP4g15 | Fwd Rev | 5′-TGTCGGAGCAAGTGGTGATT-3′ 5′-GGTGACTTTCCGATGCCCAT-3′ | 91.7% | 60.2 °C |
| CYP6a13 | Fwd Rev | 5′-ACCTTAGTGGCCTGCACTTC-3′ 5′-GGATTCCCCTGAGTTTTCCGA-3′ | 103.6% | 60.2 °C |
| CYP6a23 | Fwd Rev | 5′-CCGTTTGCCATTTTGTGTGG-3′ 5′-TGGCTGCAAGTTTCCCCAT-3′ | 96.4% | 60.2 °C |
| CYP9e2 | Fwd Rev | 5′-TCGTTCCCAAGTCCTTGCAA-3′ 5′-CCAACTGTCCCCAAAAAGCC-3′ | 93.3% | 60.2 °C |
| CYP12a5 | Fwd Rev | 5′-GAGTCTAAGCCTCCCGGTTG-3′ 5′-ATACGTAATCTGACCGCCCG-3′ | 86.7% | 57.1 °C |
| GST | Fwd Rev | 5′-TGGCTTGTGTTCCGTAGCAA-3′ 5′-ATCGCCTGGCAAGCAAAATC-3′ | 95.6% | 53.5 °C |
| GST-1 | Fwd Rev | 5′-CTGTCTCACTCGAACGCAGA-3′ 5′-TGGCGGTCCATCTGATACAG-3′ | 97.4% | 62.8 °C |
| GST-1-Like | Fwd Rev | 5′-GCTCAAGGCTTACCAGATGC-3′ 5′-CGTTCAGATGGGCCCTTCTT-3′ | 85.4% | 62.8 °C |
| CP-1 | Fwd Rev | 5′-ATTTTATCGGCGCTCTTGGC-3′ 5′-TGCGTTTCGACCTTCCTTCA-3′ | 109.7% | 60.2 °C |
| CP-141 | Fwd Rev | 5′-CTCCCACCACGTAAGGAAGA-3′ 5′-AGTTGGGTTCCTGGCTCTCT-3′ | 107.7% | 58.3 °C |
| RP-18 | Fwd Rev | 5′-TAGAATCCTCAAAGCAGGTGGCGA-3′ 5′-AGCTGGACCACCGTGTTTCACTGC-3′ | 110.1% | 60.0 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouafoura, R.; Bastarache, P.; Ouédraogo, B.C.; Dumas, P.; Moffat, C.E.; Vickruck, J.L.; Morin, P.J. Characterization of Insecticide Response-Associated Transcripts in the Colorado Potato Beetle: Relevance of Selected Cytochrome P450s and Clothianidin. Insects 2022, 13, 505. https://doi.org/10.3390/insects13060505
Bouafoura R, Bastarache P, Ouédraogo BC, Dumas P, Moffat CE, Vickruck JL, Morin PJ. Characterization of Insecticide Response-Associated Transcripts in the Colorado Potato Beetle: Relevance of Selected Cytochrome P450s and Clothianidin. Insects. 2022; 13(6):505. https://doi.org/10.3390/insects13060505
Chicago/Turabian StyleBouafoura, Raed, Pierre Bastarache, Brigitte Christelle Ouédraogo, Pascal Dumas, Chandra E. Moffat, Jess L. Vickruck, and Pier Jr Morin. 2022. "Characterization of Insecticide Response-Associated Transcripts in the Colorado Potato Beetle: Relevance of Selected Cytochrome P450s and Clothianidin" Insects 13, no. 6: 505. https://doi.org/10.3390/insects13060505
APA StyleBouafoura, R., Bastarache, P., Ouédraogo, B. C., Dumas, P., Moffat, C. E., Vickruck, J. L., & Morin, P. J. (2022). Characterization of Insecticide Response-Associated Transcripts in the Colorado Potato Beetle: Relevance of Selected Cytochrome P450s and Clothianidin. Insects, 13(6), 505. https://doi.org/10.3390/insects13060505






