Effect of Pupal Cold Storage on Reproductive Performance of Microplitis manilae (Hymenoptera: Braconidae), a Larval Parasitoid of Spodoptera frugiperda (Lepidoptera: Noctuidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Investigation of Field Parasitism Rate
2.3. Experimental Set-Up of M. manilae Pupal Cold Storage
2.4. Determinations of Parasitoid Emergence Rate and Female Proportion
2.5. Determination of Parasitism Rate
2.6. Determination of Parasitoid Longevity
2.7. Statistical Analysis
3. Results
3.1. Field Parasitism Rate and Biological Parameters of M. manilae
3.1.1. Field Parasitism Rate Investigation of M. manilae
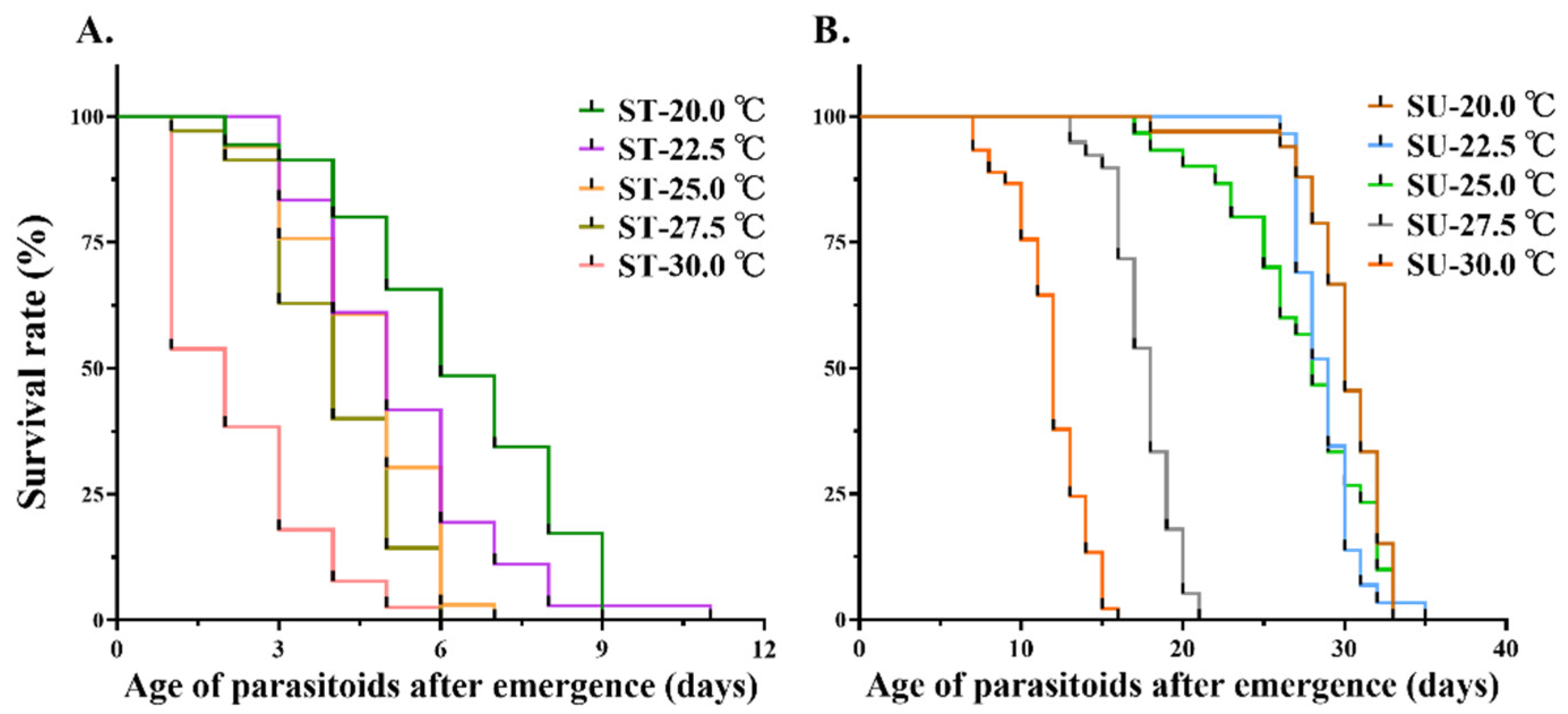
3.1.2. Effects of Food and Temperature on Adult Longevity of M. manilae
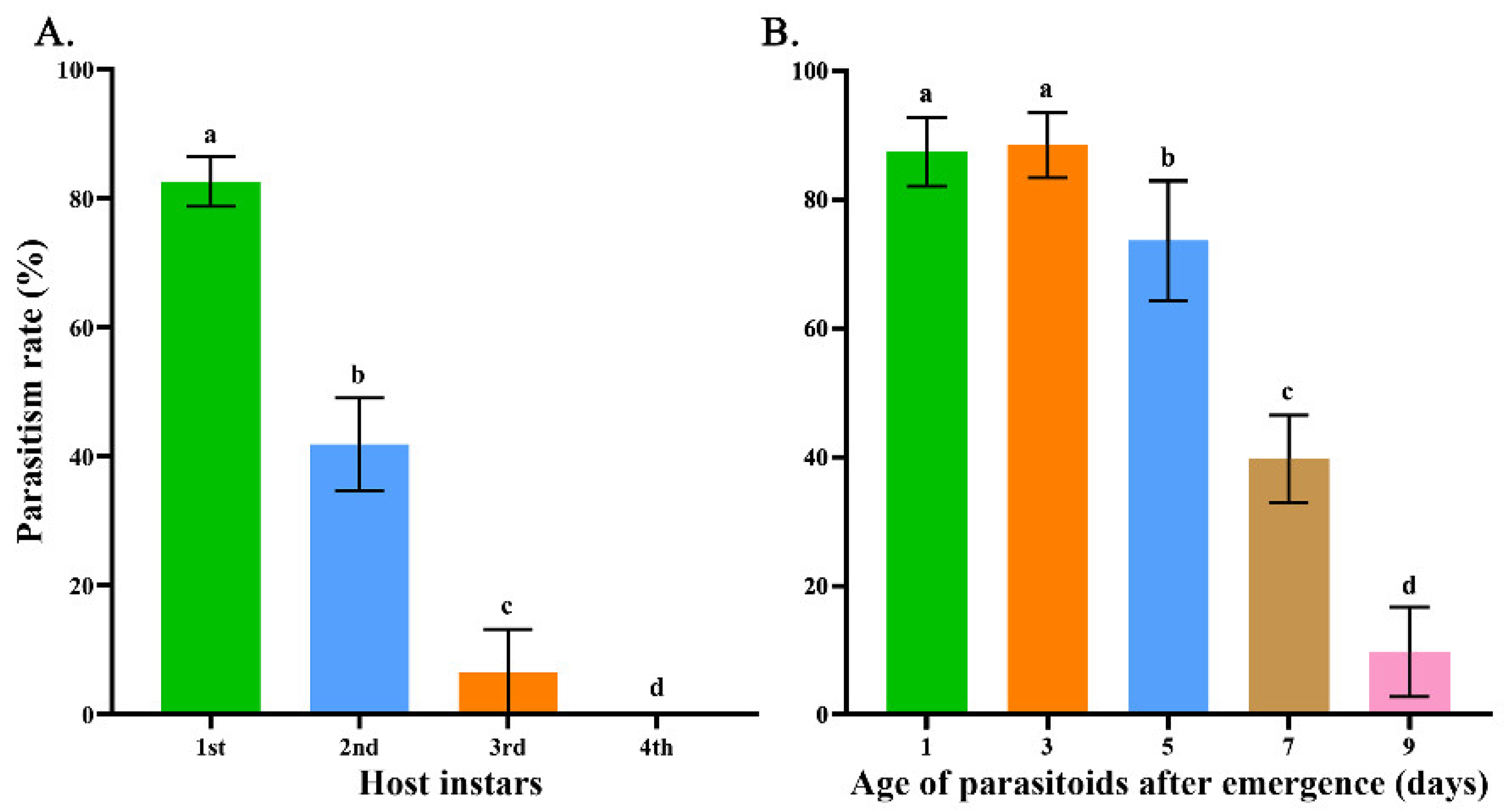
3.1.3. Determination of Parasitism Rate of M. manilae
3.2. Effects of Pupal Cold Storage on the Biological Parameters of M. manilae
3.2.1. Determination of M. manilae Emergence Rate
3.2.2. Determination of M. manilae Parasitism Rate
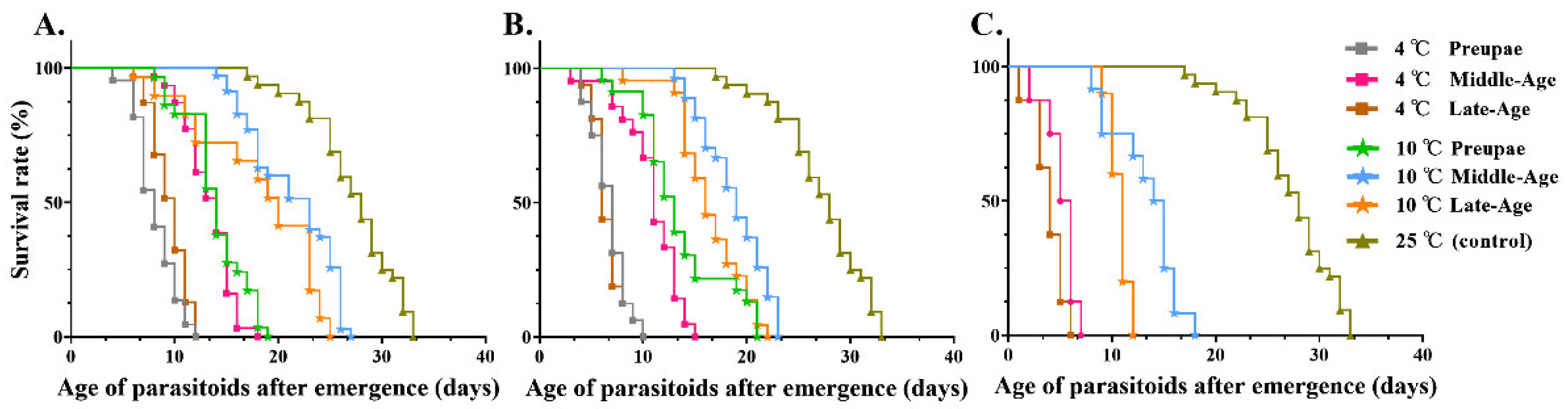
3.2.3. Determination of M. manilae Adult Longevity
3.2.4. Determination of Biological Parameters in F2 M. manilae
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, W.; Hang, C.; Li, C.Y.; Zhou, H.X.; Ren, Y.L.; Li, Z.Y.; XIing, L.S.; Zhang, B.; Xi, Q.; Bo, L. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.A.; Wilson, B.H.; Graves, J.B. Influence of host plant on the susceptibility of the fall armyworm to insecticides. J. Econ. Entomol. 1981, 74, 96–98. [Google Scholar] [CrossRef]
- Yu, S.J.; McCord, E., Jr. Lack of cross-resistance to indoxacarb in insecticide-resistant Spodoptera frugiperda (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Yponomeutidae). Pest Manag. Sci. 2007, 63, 63–67. [Google Scholar] [CrossRef]
- Yang, L.; Xing, B.L.; Li, F.; Wang, L.K.; Yuan, L.L.; Mbuji, A.L.; Peng, Z.Q.; Malhat, F.; Wu, S.Y. Full-length transcriptome analysis of Spodoptera frugiperda larval brain reveals detoxification genes. PeerJ 2021, 9, e12069. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Liu, Y.Q.; Min, S.; Huang, J.H.; Chen, X.X. Parasitoid wasps as effective biological control agents. J. Integr. Agr. 2019, 18, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Ando, K.; Inoue, R.; Maeto, K.; Tojo, S. Effects of Temperature on the Life History Traits of Endoparasitoid, Microplitis manilae Ashmead (Hymenoptera: Braconidae), Parasitizing the Larvae of the Common Cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Jpn. J. Appl. Entomol. Zool. 2006, 50, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Azidah, A.A. Population study of Spodoptera exigua (Lepidoptera: Noctuidae) larva and its affecting factors in Sekinchan, Selangor. Pak. J. Biol. Sci. 2007, 10, 2152–2158. [Google Scholar] [CrossRef]
- Nakai, M.; Cuc, N.T.T. Field application of an insect virus in the Mekong Delta: Effects of a Vietnamese nucleopolyhedrovirus on Spodoptera litura (Lepidoptera: Noctuidae) and its parasitic natural enemies. Biocontrol Sci. Technol. 2005, 15, 443–453. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, Z.S.; Luo, S.P.; Xu, Z.F. Effect of temperature on development, survival, and fecundity of Microplitis manilae (Hymenoptera: Braconidae). Environ. Entomol. 2012, 41, 657–664. [Google Scholar] [CrossRef]
- Rajapakse, R.H.S.; Ashley, T.R.; Waddill, V.H. Biology and host acceptance of Microplitis manilae (Hymenoptera: Braconidae) raised on fall armyworm larvae Spodoptera frugiperda (Lepidoptera: Noctuidae). Fla. Entomol. 1985, 68, 653–657. [Google Scholar] [CrossRef]
- Ashmead, W.H. A list of the Hymenoptera of the Philippine Islands, with descriptions of new species. J. N. Y. Entomol. Soc. 1904, 12, 1–22. [Google Scholar]
- Sun, J.S.; Huang, S.S. Evaluation of potential control ability of Snellenius manilae (Ashmead) against Spodoptera exigua (Hübner). Acta Ecol. Sin. 2010, 30, 1494–1499. [Google Scholar]
- Rajapakse, R.H.S.; Ashley, T.R.; Waddill, V.H. Interspecific competition between parasitoids of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Int. J. Trop. Insect Sci. 1991, 12, 473–480. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Matsumoto, H.; Ochiai, M.; Tsuzuki, S.; Hayakawa, Y. Enhanced expression of stress-responsive cytokine-like gene retards insect larval growth. Insect Biochem. Mol. Biol. 2012, 42, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, D.Q.; Hao, X.X.; Cai, P.M.; Guo, Y.Q.; Shi, S.; Liu, C.M.; Ji, Q. Effect of cold storage on the quality of Psyttalia incisi (Hymenoptera: Braconidae), a larval parasitoid of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2021, 12, 558. [Google Scholar] [CrossRef]
- Colinet, H.; Boivin, G. Insect parasitoids cold storage: A comprehensive review of factors of variability and consequences. Biol. Control 2011, 58, 83–95. [Google Scholar] [CrossRef]
- Venkatesan, T.; Singh, S.P.; Jalali, S.K. Effect of cold storage on cocoons of Goniozus nephantidis Muesebeck (Hymenoptera: Bethylidae) stored for varying periods at different temperature regimes. J. Entomol. Res. 2000, 24, 43–47. [Google Scholar]
- Hance, T.; Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef]
- Rathee, M.; Ram, P. Impact of cold storage on the performance of entomophagous insects: An overview. Phytoparasitica 2018, 46, 421–429. [Google Scholar] [CrossRef]
- Ghazy, N.; Suzuki, T.; Amano, H.; Ohyama, K. Air temperature optimisation for humidity-controlled cold storage of the predatory mites Neoseiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Pest Manag. Sci. 2014, 70, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Han, S.C.; Li, J.; Liu, J.S.; Li, Z.G. Effects of cold storage on the quality of Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) reared on artificial medium. Pest Manag. Sci. 2019, 75, 1328–1338. [Google Scholar]
- Rezaei, M.; Talebi, A.A.; Fathipour, Y.H.; Karimzadeh, J.; Mehrabadi, M.; Reddy, G.V.P. Effects of cold storage on life-history traits of Aphidius matricariae. Entomol. Exp. Appl. 2020, 168, 800–807. [Google Scholar] [CrossRef]
- Ayvaz, A.; Karasu, E.; Karabörklü, S.; Tunçbilek, A. Effects of cold storage, rearing temperature, parasitoid age and irradiation on the performance of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). J. Stored Prod. Res. 2008, 44, 232–240. [Google Scholar] [CrossRef]
- Nadeem, S.; Ashfaq, M.; Hamed, M.; Ahmed, S. Optimization of short and long term storage duration for Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) at low temperatures. Pak. J. Zool. 2010, 42, 63–67. [Google Scholar]
- Al-Tememi, N.A.M. Effect of low temperature storage on the fecundity and parasitizing efficacy of Bracon hebetor (Say). J. Agric. Res. 2005, 43, 155–160. [Google Scholar]
- Amice, G.; Vernon, P.; Outreman, Y.; Van Alphen, J.; Van Baaren, J. Variability in responses to thermal stress in parasitoids. Ecol. Entomol. 2008, 33, 701–708. [Google Scholar] [CrossRef]
- Chen, W.L.; Leopold, R.A. Progeny quality of Gonatocerus ashmeadi (Hymenoptera: Mymaridae) reared on stored eggs of Homalodisca coagulata (Hemiptera: Cicadellidae). J. Econ. Entomol. 2007, 100, 685–694. [Google Scholar] [CrossRef]
- Chen, W.L.; Leopold, R.A.; Harris, M.O. Cold storage effects on maternal and progeny quality of Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Biol. Control 2008, 46, 122–132. [Google Scholar] [CrossRef]
- Jackson, C.G. Effects of cold storage of adult Anaphes ovijentatus on survival, longevity, and oviposition. Southwest. Entomol. 1986, 11, 149–153. [Google Scholar]
- Krishnamoorthy, A. Effect of cold-storage on the emergence and survival of the adult exotic parasitoid, Leptomastix dactylopii Howard (Hymenoptera: Encyrtidae). Entomon 1989, 14, 313–318. [Google Scholar]
- Colinet, H.; Hance, T. Interspecific variation in the response to low temperature storage in different aphid parasitoids. Ann. Appl. Biol. 2010, 156, 147–156. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Lee, R.E. Temperature sensitivity in insects and application in integrated pest management. In Physiology of Cold Sensitivity, 1st ed.; CRC Press: New York, NY, USA, 1998; pp. 55–95. [Google Scholar]
- Van Baaren, J.; Outreman, Y.; Boivin, G. Effect of low temperature exposure on oviposition behaviour and patch exploitation strategy in parasitic wasps. Anim. Behav. 2005, 70, 153–163. [Google Scholar] [CrossRef]
- Marwan, I.A.; Tawfiq, M.M. Response of Aphidius matricariae Haliday (Hymenoptera: Aphidiidae) from mummified Myzus persicae Sulzer (Homoptera: Aphididae) to short term cold storage. Int. Pest Control 2006, 48, 262–265. [Google Scholar]
- Tang, Y.Q.; Yokomi, R.K. Temperature–Dependent Development of Three Hymenopterous Parasitoids of Aphids (Homoptera: Aphididae) Attacking Citrus. Environ. Entomol. 1995, 24, 1736–1740. [Google Scholar] [CrossRef]
- López, S.N.; Botto, E. Effect of cold storage on some biological parameters of Eretmocerus corni and Encarsia formosa (Hymenoptera: Aphelinidae). Biol. Control 2005, 33, 123–130. [Google Scholar] [CrossRef]
- Lysyk, T.J. Effects of Cold Storage on Development and Survival of Three Species of Parasitoids (Hymenoptera: Pteromalidae) of House Fly, Musca domestica L. Environ. Entomol. 2004, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xing, B.L.; Wang, L.K.; Yuan, L.L.; Manzoor, M.; Li, F.; Wu, S.Y. Identification of serine protease, serine protease homolog and prophenoloxidase genes in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2021, 24, 1144–1152. [Google Scholar] [CrossRef]
- Vieira, N.F.; Pomari-Fernandes, A.; Lemes, A.A.F.; Vacari, A.M.; De Bortoli, S.A.; De Freitas Bueno, A. Cost of production of Telenomus remus (Hymenoptera: Platygastridae) grown in natural and alternative hosts. J. Econ. Entomol. 2017, 110, 2724–2726. [Google Scholar] [CrossRef]
- Yang, L.; Qiu, L.M.; Fang, Q.; Stanley, D.W.; Ye, G.Y. Cellular and humoral immune interactions between Drosophila and its parasitoids. Insect Sci. 2020, 28, 1208–1227. [Google Scholar] [CrossRef]
- Su, H.; Lv, B.Q.; Zhang, B.Q.; Wu, Q.Q.; Lu, H.; Tang, J.H.; Wu, X.S. Biological Characteristics of Tetrastichus howardi, a Parasitoid of Spodoptera frugiperda. Chin. J. Biol. Control 2021, 37, 406–411. [Google Scholar]
- Lu, Z.Y.; Zhong, Y.; Qu, G.Z.; Liu, W.X.; Li, J.C. Influence of Temperature and Nutrition on Adult Longevity of Microplitis tuberculifer Wesmael. J. Hebei Agric. Sci. 2010, 14, 75–76. [Google Scholar]
- Zhong, Y.; Lu, Z.Y.; Qu, Z.G.; Liu, W.X.; Liu, X.X.; Li, J.C. Effects of Female Adult Age and Parasitism Experience on the Parasitic Rate and Offspring of Microplitis tuberculifer Wesmael. J. Hebei Agric. Sci. 2010. [Google Scholar] [CrossRef]
- Mackauer, M. Host selection and host suitability in Aphidius smithi (Hy menoptera: Aphididae). Bull. Entomol. Soc. Nz. 2013, 1973, 20–29. [Google Scholar]
- Salt, G. The effects of hosts upon their insect parasites. Biol. Rev. 1941, 16, 239–264. [Google Scholar] [CrossRef]
- Sequeira, R.; Mackauer, M. Nutritional ecology of an insect host-parasitoid association: The pea aphid-Aphidius ervi system. Ecology 1992, 73, 183–189. [Google Scholar] [CrossRef]
- Vinson, S.B.; Iwantsch, G.F. Host suitability for insect parasitoids. Annu. Rev. Entomol. 1980, 25, 397–419. [Google Scholar] [CrossRef]
- King, B.H.; Lee, H.E. Test of the adaptiveness of sex ratio manipulation in a parasitoid wasp. Behav. Ecol. Sociobiol. 1994, 35, 437–443. [Google Scholar] [CrossRef]
- Wang, J.Y.; Qu, Z.G. Selection on host size and reproduction ability of Microplitis tuberculifer. Chin. J. Biol. Control 2005, 21, 85–87. [Google Scholar]
- Wang, K.W.; Jiang, J.X.; You, L.S. Preference of Microplitis sp. to host age. J. Hunan Agric. Univ. 2001, 27, 367–369. [Google Scholar]
- Li, J.C.; Zhang, Q.W.; Liu, X.X.; Pan, W.L. Effects of parasitoid age and host age on the parasitism of Microplitis mediator (Hymenoptera: Braconidae), an endoparasitoid of Leucania separata. Chin. J. Biol. Control 2005, 21, 14–17. [Google Scholar]
- Zhao, J.; Wang, S.; Guo, X.J.; Zhang, F.S.A.S. Progress in research of cold storage of insect parasitoids. Sci. Agric. Sin. 2014, 47, 482–494. [Google Scholar]
- Neven, L.G.; Hansen, L.D. Effects of temperature and controlled atmospheres on codling moth metabolism. Ann. Entomo. Soc. Am. 2010, 103, 418–423. [Google Scholar] [CrossRef]
- Long, X.Z.; Chen, K.W.; Xian, J.D.; Lu, Y.Y.; Zeng, L. Cold storage technique of Diachasmimorpha longicaudata (Ashmead). J. Econ. Entomol. 2014, 36, 115–121. [Google Scholar]
- Luczynski, A.; Nyrop, J.P.; Shi, A. Influence of cold storage on pupal development and mortality during storage and on post-storage performance of Encarsia formosa and Eretmocerus eremicus (Hymenoptera: Aphelinidae). Biol. Control 2007, 40, 107–117. [Google Scholar] [CrossRef]
- Yocum, G.D.; Žďárek, J.; Joplin, K.H.; Lee Jr, R.E.; Smith, D.C.; Manter, K.D.; Denlinger, D.L. Alteration of the eclosion rhythm and eclosion behavior in the flesh fly, Sarcophaga crassipalpis, by low and high temperature stress. J. Insect Physiol. 1994, 40, 13–21. [Google Scholar] [CrossRef]
- Kelty, J.D.; Killian, K.A.; LEE, R.E. Cold shock and rapid cold-hardening of pharate adult flesh flies (Sarcophaga crassipalpis): Effects on behaviour and neuromuscular function following eclosion. Physiol. Entomol. 1996, 21, 283–288. [Google Scholar] [CrossRef]
- Alam, M.S.; Alam, M.Z.; Alam, S.N.; Miah, M.R.U.; Mian, M.I.H. Effect of storage duration on the stored pupae of parasitoid Bracon hebetor (Say) and its impact on parasitoid quality. Bangladesh J. Agric. Res. 2016, 41, 297–310. [Google Scholar] [CrossRef]
- Yan, Z.; Yue, J.J.; Bai, C.; Peng, Z.Q.; Zhang, C.H. Effects of cold storage on the biological characteristics of Microplitis prodeniae (Hymenoptera: Braconidae). Bull. Entomol. Res. 2017, 107, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Foerster, L.A.; Doetzer, A.K.; Castro, L.C.F. Emergence, longevity and fecundity of Trissolcus basalis and Telenomus podisi after cold storage in the pupal stage. Pesqui. Agropecu. Bras. 2004, 39, 841–845. [Google Scholar] [CrossRef] [Green Version]
| Collection Time | Location | Coordinate | Number of Collected S. frugiperda | Number of Parasitized Larvae | Parasitism Rate (%) |
|---|---|---|---|---|---|
| 2020.11.10 | Dongfang | 108°64′ E, 18°84′ N | 199 | 38 | 19.10 |
| 2020.11.03 | Ledong1 | 108°91′ E, N18°47′ N | 131 | 18 | 13.74 |
| 2020.10.23 | Qiongzhong | 109°90′ E, N19°13′ N | 131 | 16 | 12.21 |
| 2020.11.01 | Sanya | 109°18′ E, N18°38′ N | 238 | 15 | 6.30 |
| 2020.10.30 | Ledong2 | 108°83′ E, N18°51′ N | 53 | 3 | 5.66 |
| 2021.05.13 | Danzhou | 109°48′ E, N19°51′ N | 235 | 19 | 8.09 |
| 2021.05.10 | Sanya | 109°18′ E, N18°38′ N | 81 | 0 | 0.00 |
| 2020.10–2021.05 | In total | 1068 | 109 | 10.21 | |
| Food | Survival Time (d) | F | p | ||||
|---|---|---|---|---|---|---|---|
| 20 °C | 22.5 °C | 25 °C | 27.5 °C | 30 °C | |||
| 10% sucrose water | 29.66 ± 0.98 a | 28.24 ± 0.81 ab | 26.51 ± 1.18 b | 18.09 ± 0.55 c | 12.26 ± 1.04 d | 206.05 | <0.001 |
| Sterile water | 6.31 ± 0.68 e | 5.22 ± 0.42 ef | 4.75 ± 0.37 ef | 4.06 ± 0.12 fg | 2.39 ± 0.44 g | ||
| Storage Time (d) | Storage Temperature (°C) | Emergence Rate (%) | F | p | ||
|---|---|---|---|---|---|---|
| Prepupa | Middle-Aged Pupa | Late-Aged Pupa | ||||
| 5 | 4 | 65.66 ± 1.01 e | 82.32 ± 0.50 c | 74.13 ± 1.40 d | 108.95 | <0.001 |
| 10 | 76.55 ± 2.11 d | 91.88 ± 0.21 b | 83.33 ± 0.00 c | |||
| 25 (control) | 98.68 ± 0.94 a | |||||
| 10 | 4 | 26.26 ± 4.40 f | 63.64 ± 0.00 cd | 47.22 ± 2.78 e | 138.02 | <0.001 |
| 10 | 58.33 ± 0.00 d | 74.88 ± 1.21 b | 65.66 ± 1.01 c | |||
| 25 (control) | 98.68 ± 0.94 a | |||||
| 20 | 4 | 0 f | 27.27 ± 0.00 c | 19.39 ± 0.61 e | 1027.39 | <0.001 |
| 10 | 0 f | 36.56 ± 1.93 b | 29.29 ± 2.02 c | |||
| 25 (control) | 98.68 ± 0.94 a | |||||
| Storage Time (d) | Storage Temperature (°C) | Parasitism Rate (%) | H | p | ||
|---|---|---|---|---|---|---|
| Prepupa | Middle-Aged Pupa | Late-Aged Pupa | ||||
| 5 | 4 | 23.08(23.08–26.92) f | 42.31(38.46–42.31) d | 26.92(26.92–26.92) e | 83.12 | <0.001 |
| 10 | 50.00(46.15–53.85) c | 69.23(65.38–73.08) b | 53.85(50.00–57.69) c | |||
| 25 (control) | 88.46(84.62–92.31) a | |||||
| 10 | 4 | 7.69(7.69–11.54) d | 30.77(26.92–34.62) c | 11.54(11.54–11.54) d | 69.64 | <0.001 |
| 10 | 34.62(34.62–38.46) c | 61.54(57.69–65.38) b | 38.46(34.62–42.31) c | |||
| 25 (control) | 88.46(84.62–92.31) a | |||||
| Storage Time (d) | Storage Temperature (°C) | Survival Time of Adults (d) | F | p | ||
|---|---|---|---|---|---|---|
| Prepupa | Middle-Aged Pupa | Late-Aged Pupa | ||||
| 5 | 4 | 8.22 ± 1.05 e | 13.58 ± 0.11 d | 10.54 ± 0.47 de | 34.21 | <0.001 |
| 10 | 14.00 ± 0.08 cd | 21.32 ± 0.03 b | 17.87 ± 1.80 c | |||
| 25 (control) | 26.51 ± 1.18 a | |||||
| 10 | 4 | 6.63 ± 0.63 e | 10.88 ± 0.43 d | 6.39 ± 0.72 e | 51.62 | <0.001 |
| 10 | 12.87 ± 0.14 cd | 18.78 ± 0.86 b | 16.35 ± 0.65 bc | |||
| 25 (control) | 26.51 ± 1.18 a | |||||
| 20 | 4 | - | 5.30 ± 0.70 c | 3.88 ± 0.63 c | 76.51 | <0.001 |
| 10 | - | 13.32 ± 0.12 b | 10.63 ± 0.38 b | |||
| 25 (control) | 26.51 ± 1.18 a | |||||
| Storage Time (d) | Storage Temperature (°C) | Emergence Rate (%) | F | p | ||
|---|---|---|---|---|---|---|
| Prepupa | Middle-Aged Pupa | Late-Aged Pupa | ||||
| 5 | 4 | 86.62 ± 1.41 b | 87.84 ± 1.70 b | 85.47 ± 1.17 b | 2.96 | 0.011 |
| 10 | 85.69 ± 1.42 b | 85.51 ± 1.84 b | 86.37 ± 1.89 b | |||
| 25 (control) | 98.68 ± 0.94 a | |||||
| 10 | 4 | 86.17 ± 1.51 b | 87.74 ± 1.57 b | 86.55 ± 1.64 b | 2.88 | 0.013 |
| 10 | 86.76 ± 1.53 b | 84.77 ± 1.81 b | 86.63 ± 1.54 b | |||
| 25 (control) | 98.68 ± 0.94 a | |||||
| Storage Time (d) | Storage Temperature (°C) | Female Proportion (%) | F | p | ||
|---|---|---|---|---|---|---|
| Prepupa | Middle-Aged Pupa | Late-Aged Pupa | ||||
| 5 | 4 | 68.12 ± 3.29 a | 67.21 ± 2.87 a | 67.67 ± 3.26 a | 0.06 | 0.999 |
| 10 | 67.01 ± 2.97 a | 68.42 ± 4.38 a | 66.62 ± 3.64 a | |||
| 25 (control) | 68.82 ± 1.46 a | |||||
| 10 | 4 | 67.33 ± 4.96 a | 68.06 ± 2.51 a | 68.93 ± 4.38 a | 0.07 | 0.999 |
| 10 | 68.59 ± 4.42 a | 67.73 ± 3.88 a | 66.12 ± 3.75 a | |||
| 25 (control) | 68.82 ± 1.46 a | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, B.; Yang, L.; Gulinuer, A.; Li, F.; Wu, S. Effect of Pupal Cold Storage on Reproductive Performance of Microplitis manilae (Hymenoptera: Braconidae), a Larval Parasitoid of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 449. https://doi.org/10.3390/insects13050449
Xing B, Yang L, Gulinuer A, Li F, Wu S. Effect of Pupal Cold Storage on Reproductive Performance of Microplitis manilae (Hymenoptera: Braconidae), a Larval Parasitoid of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects. 2022; 13(5):449. https://doi.org/10.3390/insects13050449
Chicago/Turabian StyleXing, Binglin, Lei Yang, Ahamaijiang Gulinuer, Fen Li, and Shaoying Wu. 2022. "Effect of Pupal Cold Storage on Reproductive Performance of Microplitis manilae (Hymenoptera: Braconidae), a Larval Parasitoid of Spodoptera frugiperda (Lepidoptera: Noctuidae)" Insects 13, no. 5: 449. https://doi.org/10.3390/insects13050449
APA StyleXing, B., Yang, L., Gulinuer, A., Li, F., & Wu, S. (2022). Effect of Pupal Cold Storage on Reproductive Performance of Microplitis manilae (Hymenoptera: Braconidae), a Larval Parasitoid of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects, 13(5), 449. https://doi.org/10.3390/insects13050449






