The Diversity of Aphidlion-like Larvae over the Last 130 Million Years
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Documentation Methods
2.3. Drawings
2.4. Shape Analysis
3. Results
3.1. Short Descriptions of New Fossil Larvae
- (1)
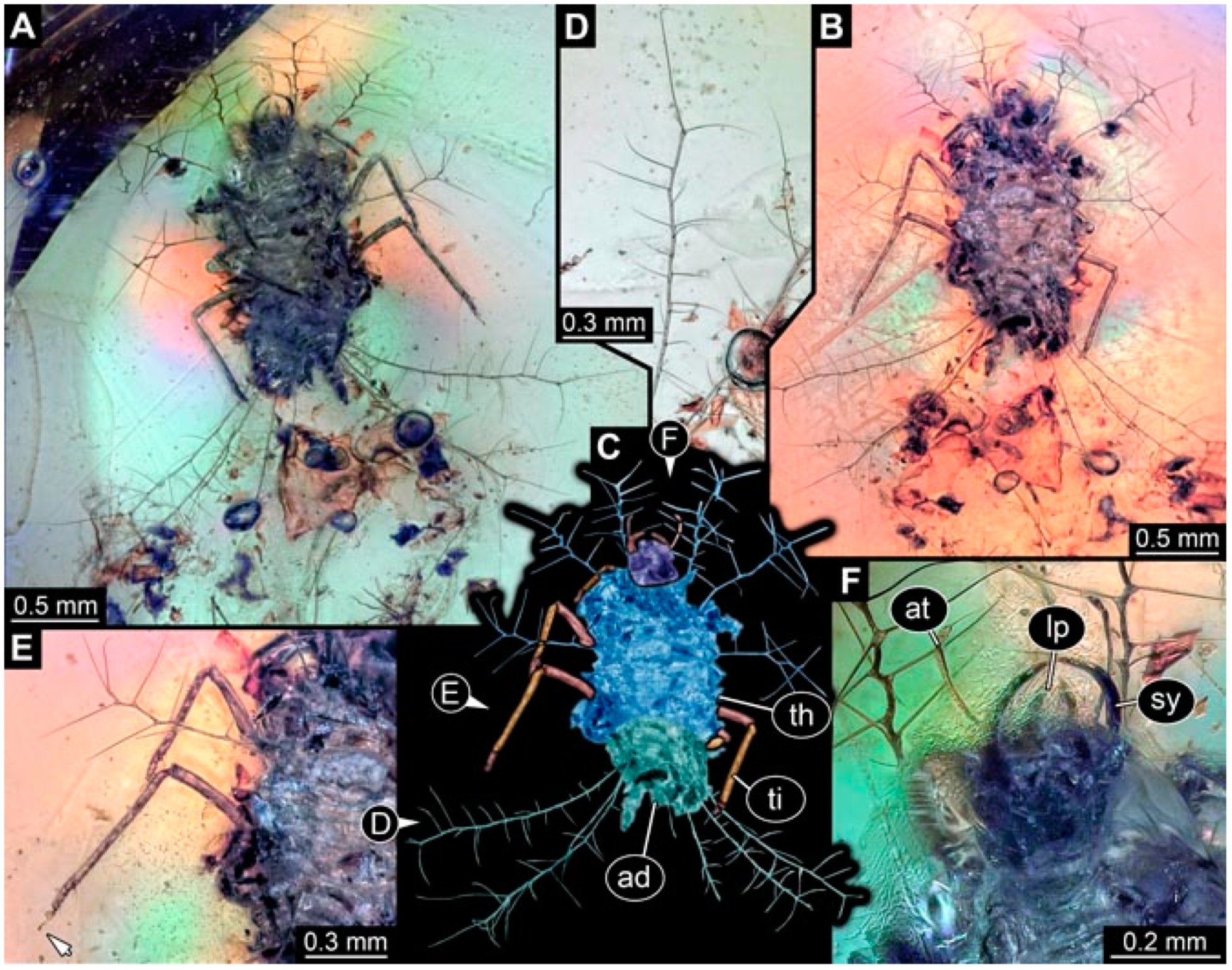
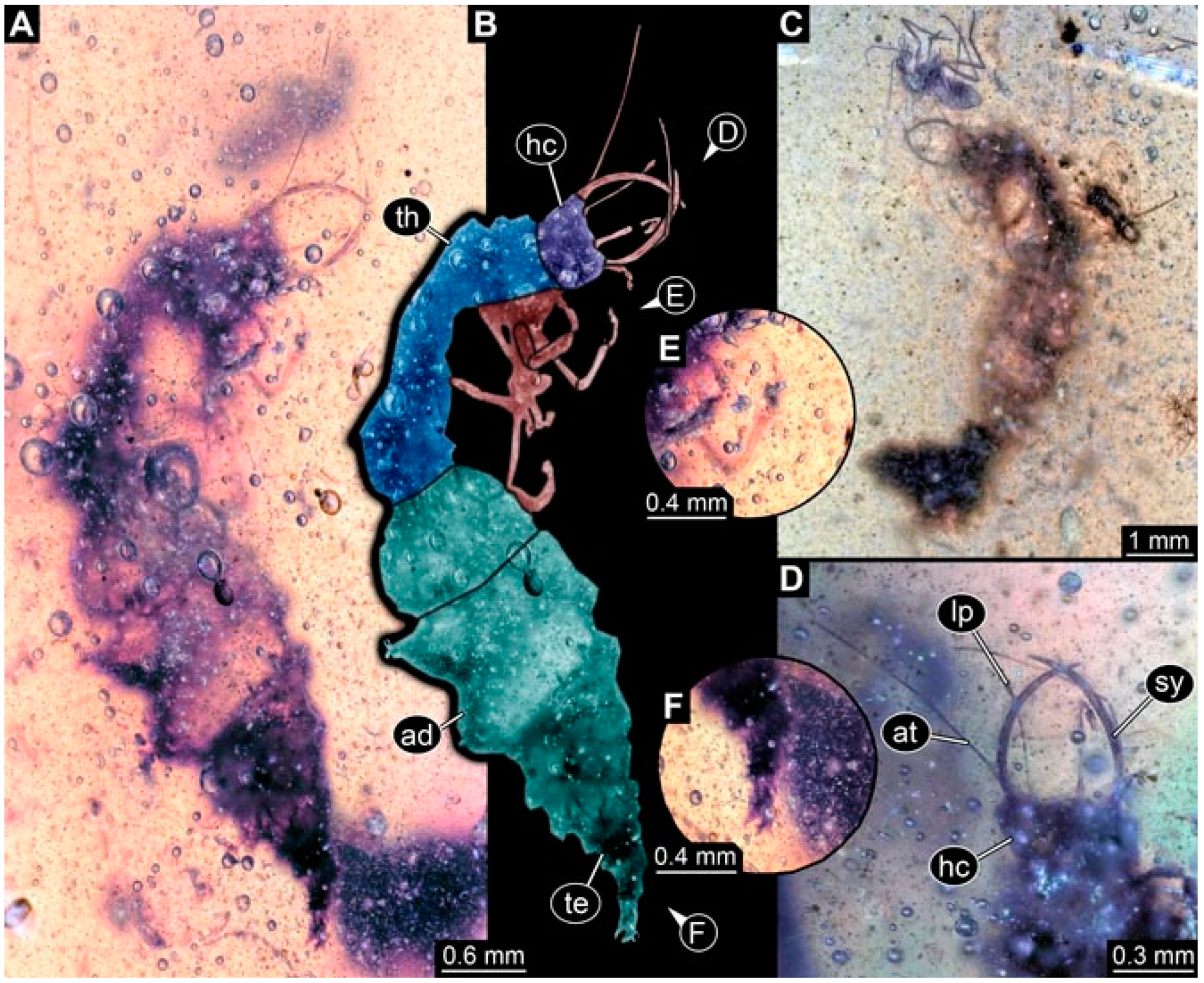
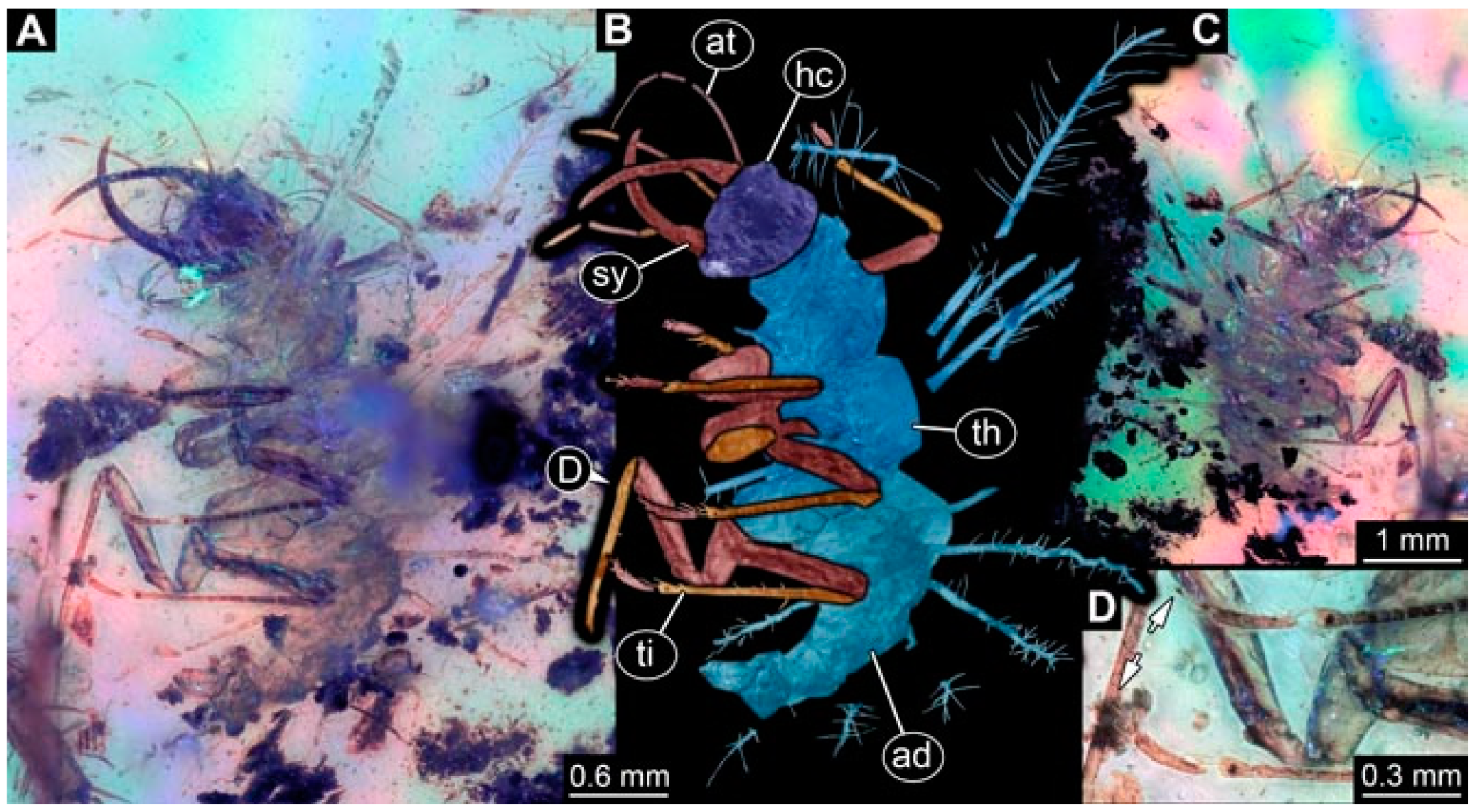
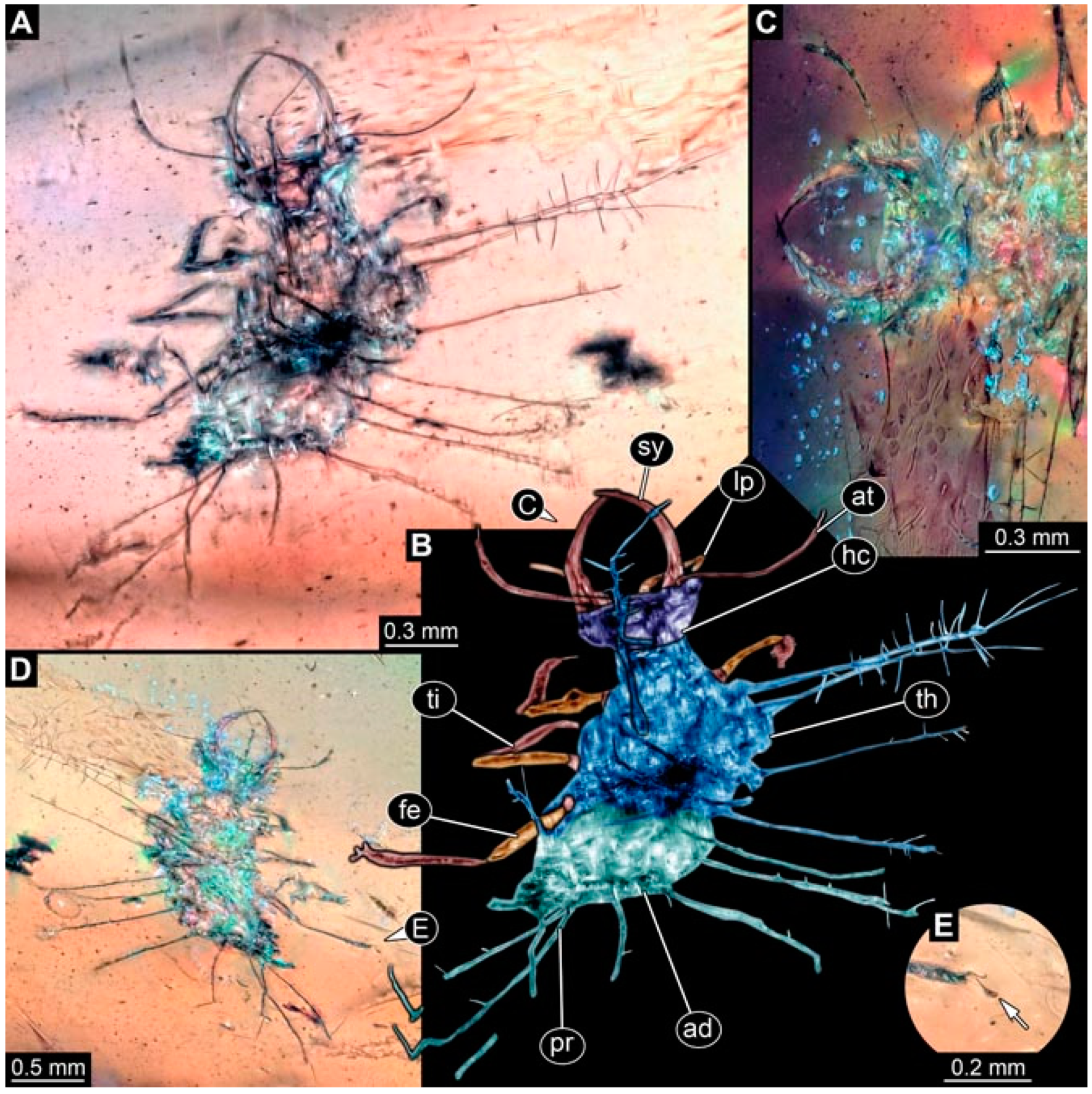
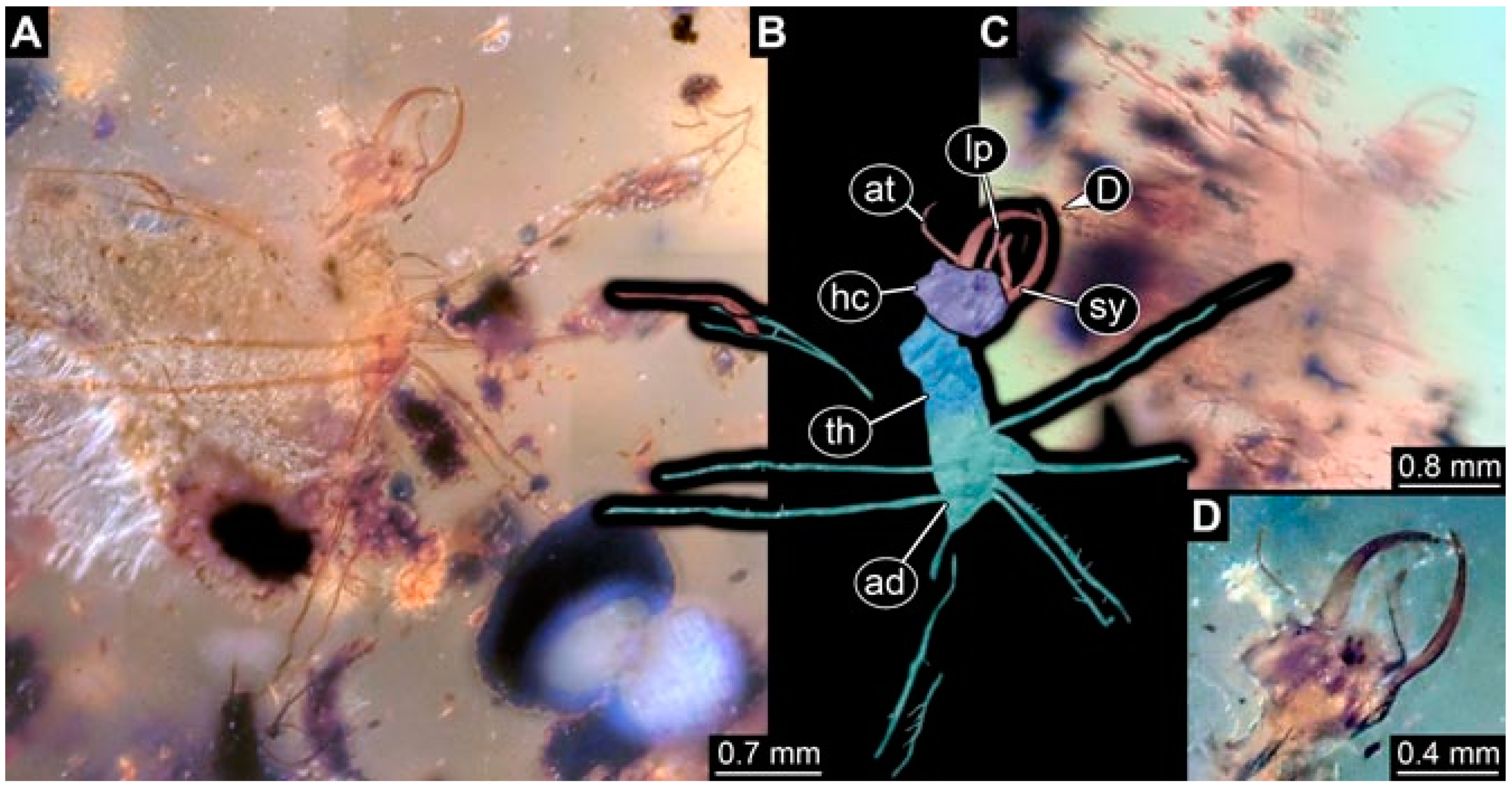
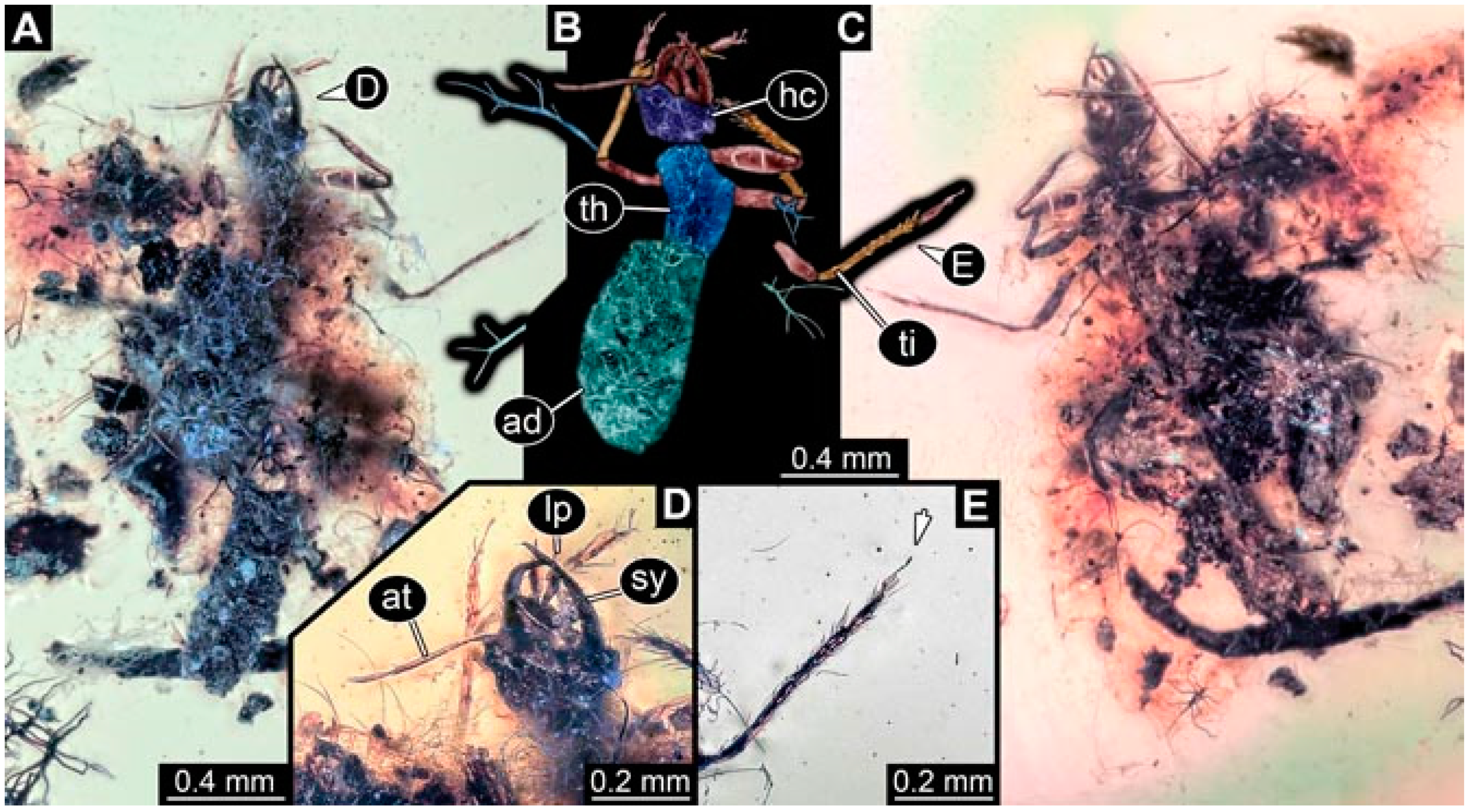
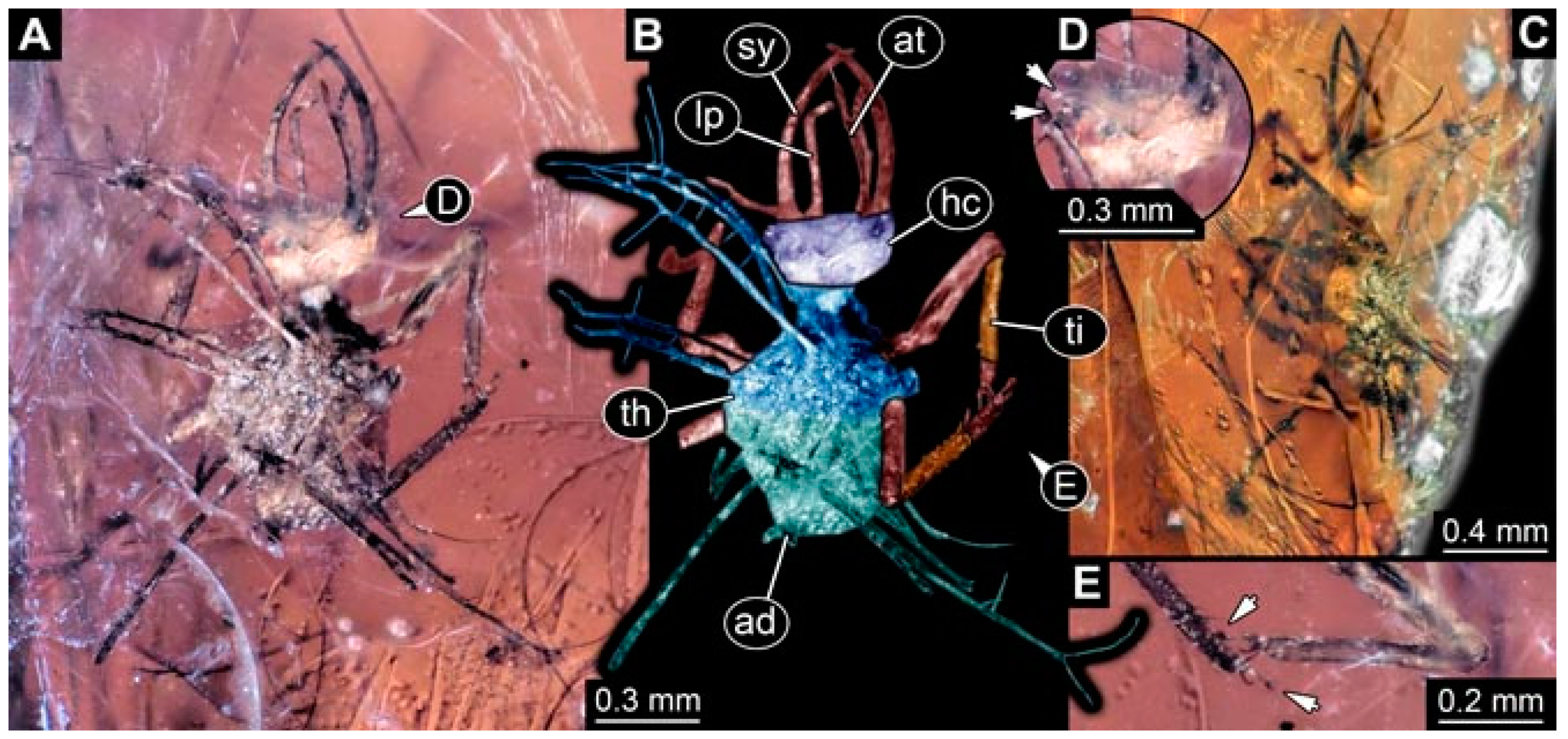
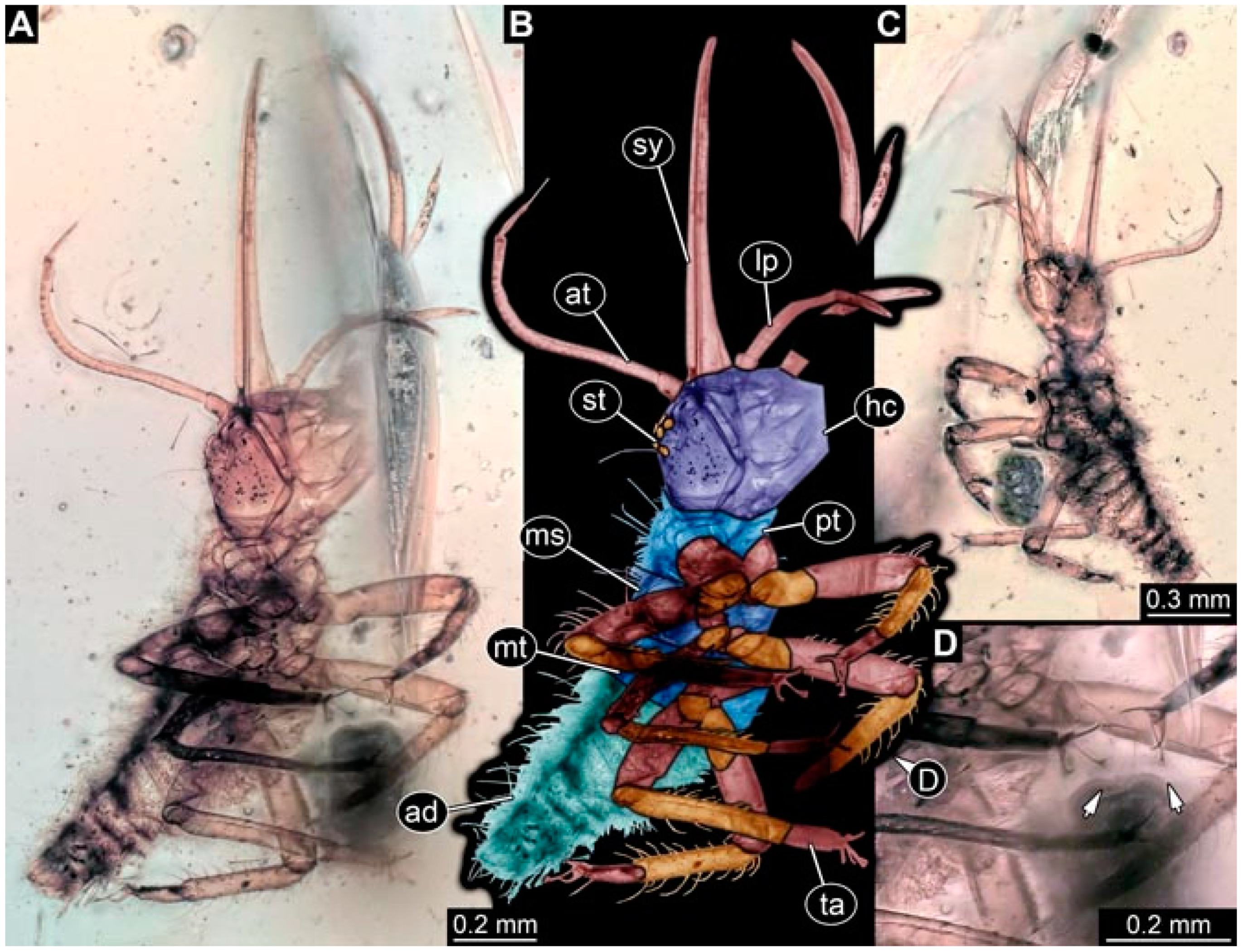
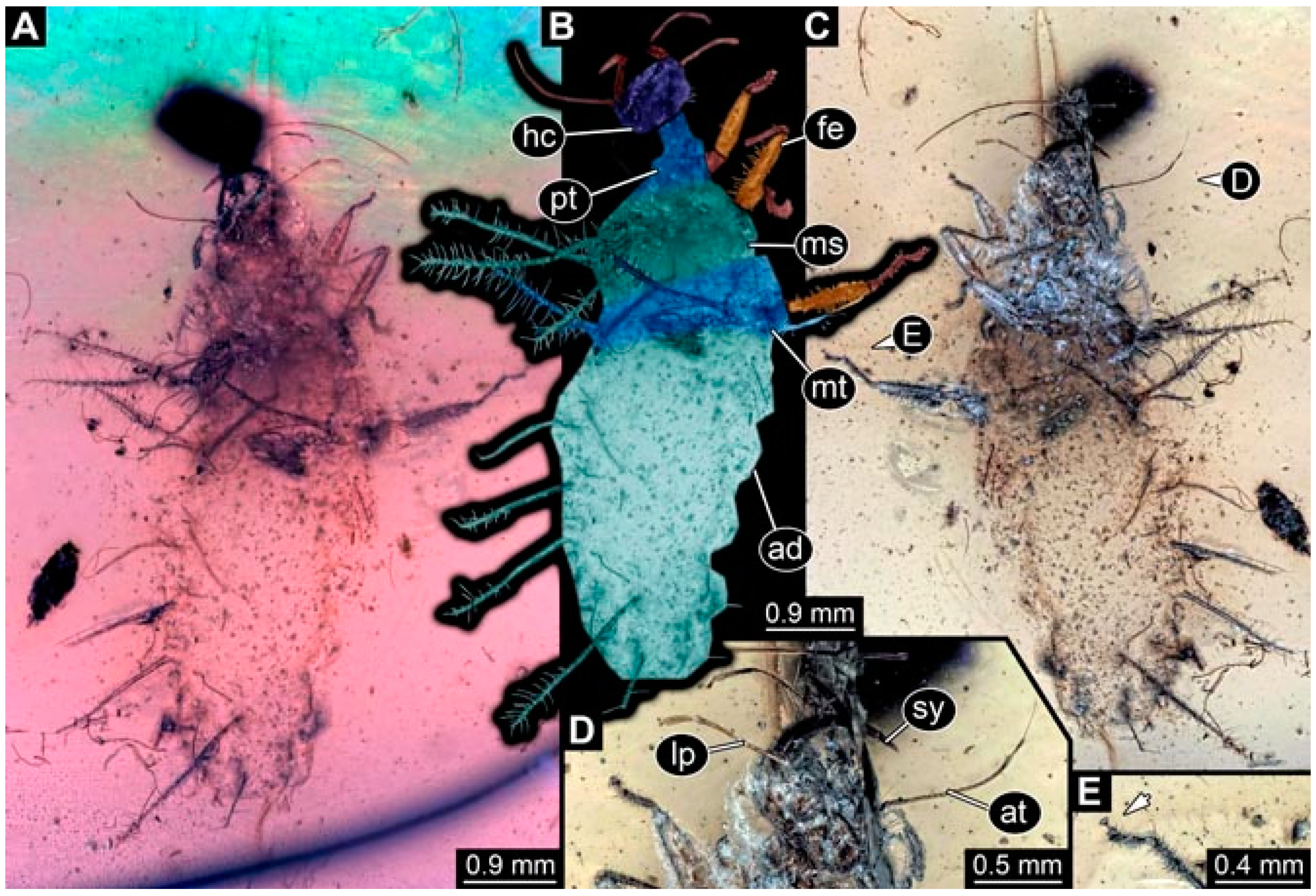
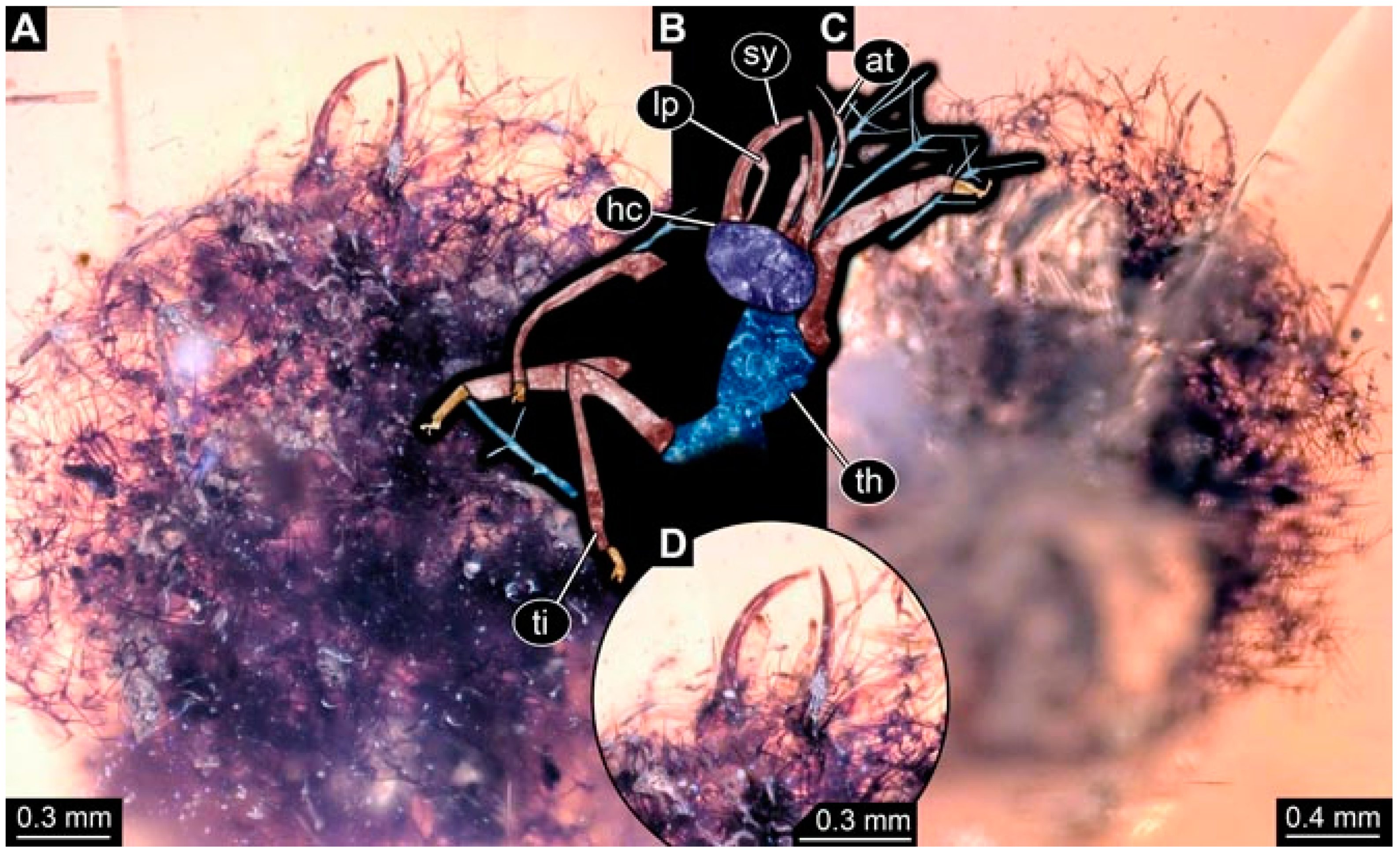
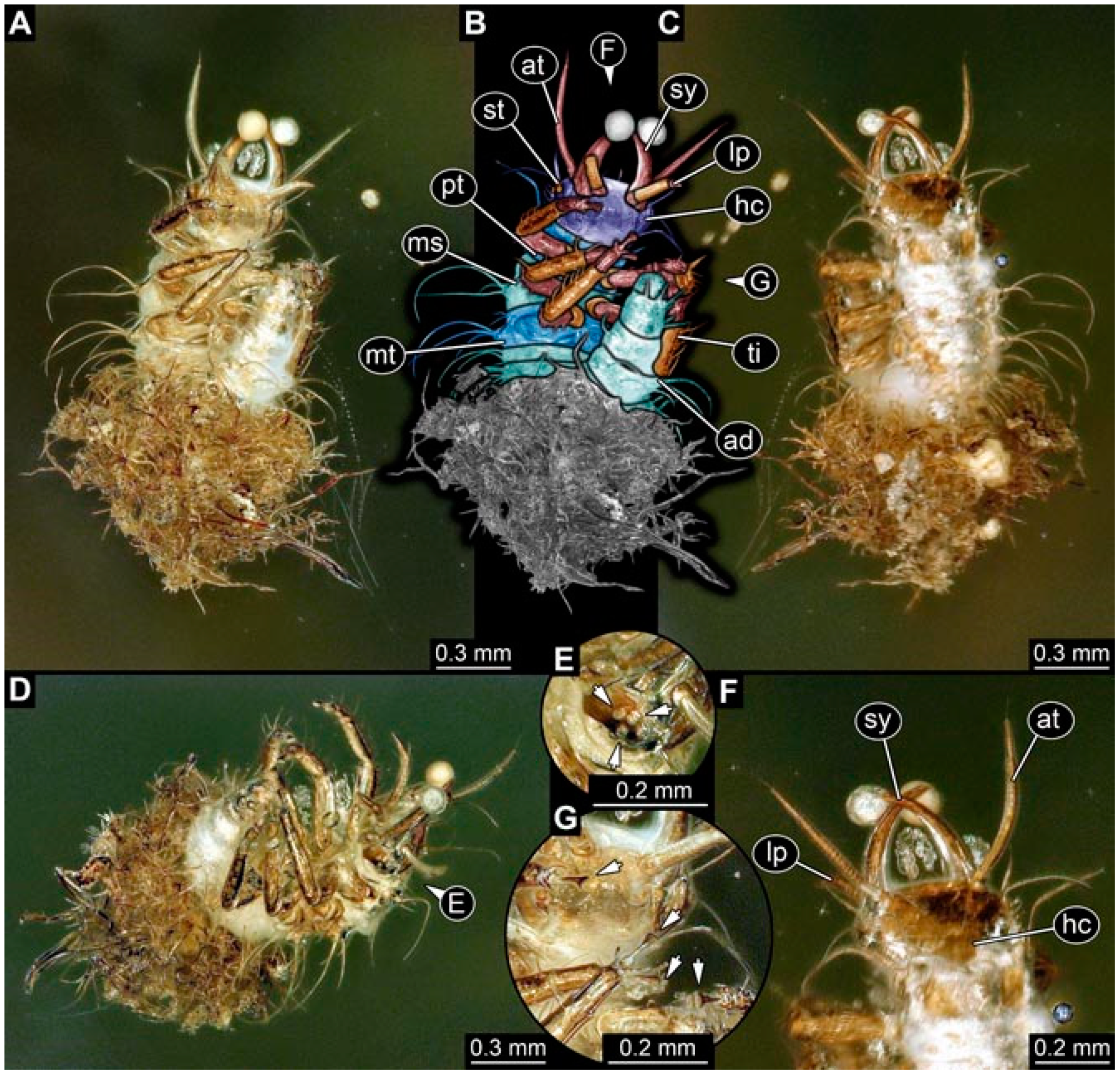
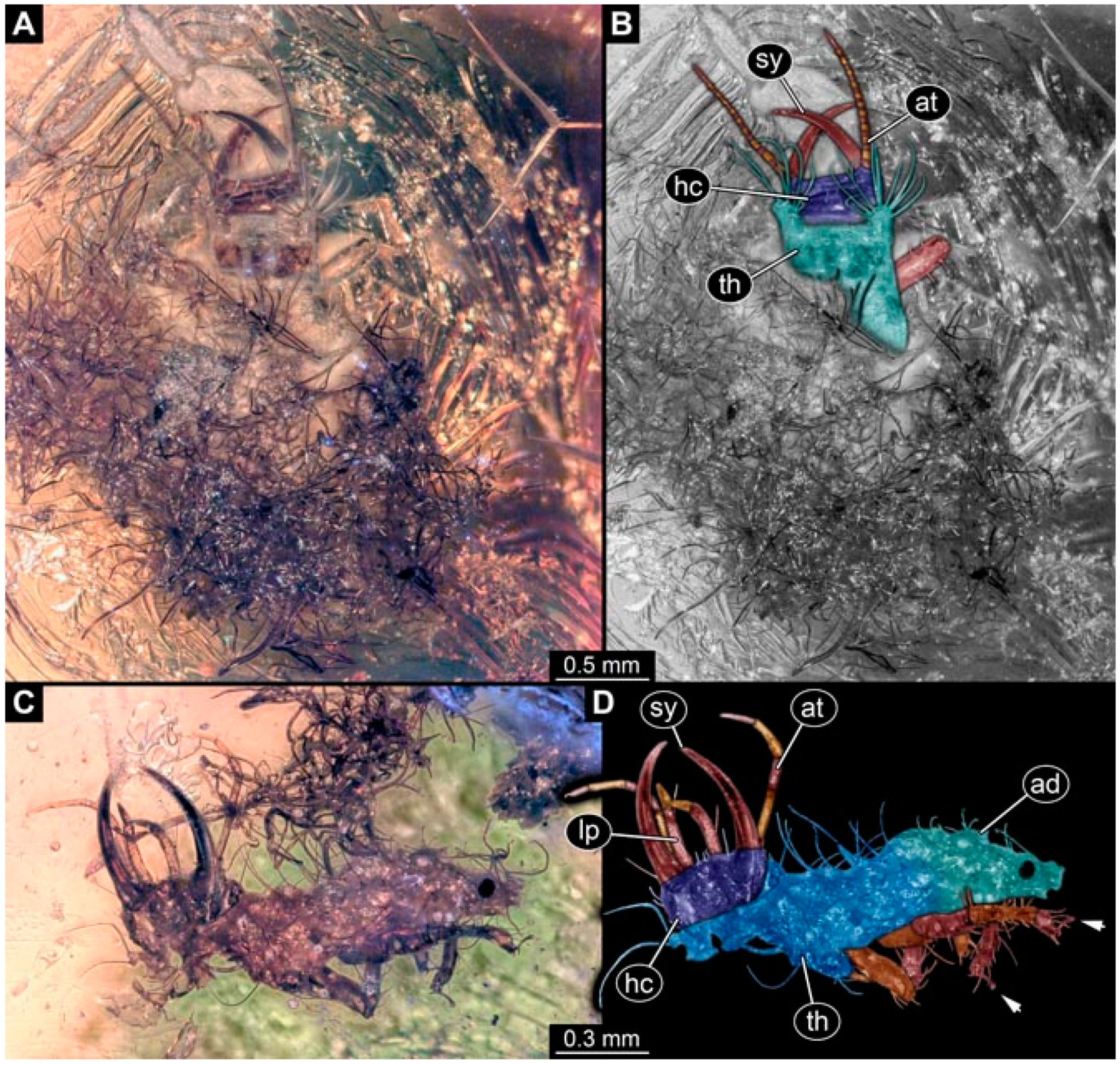
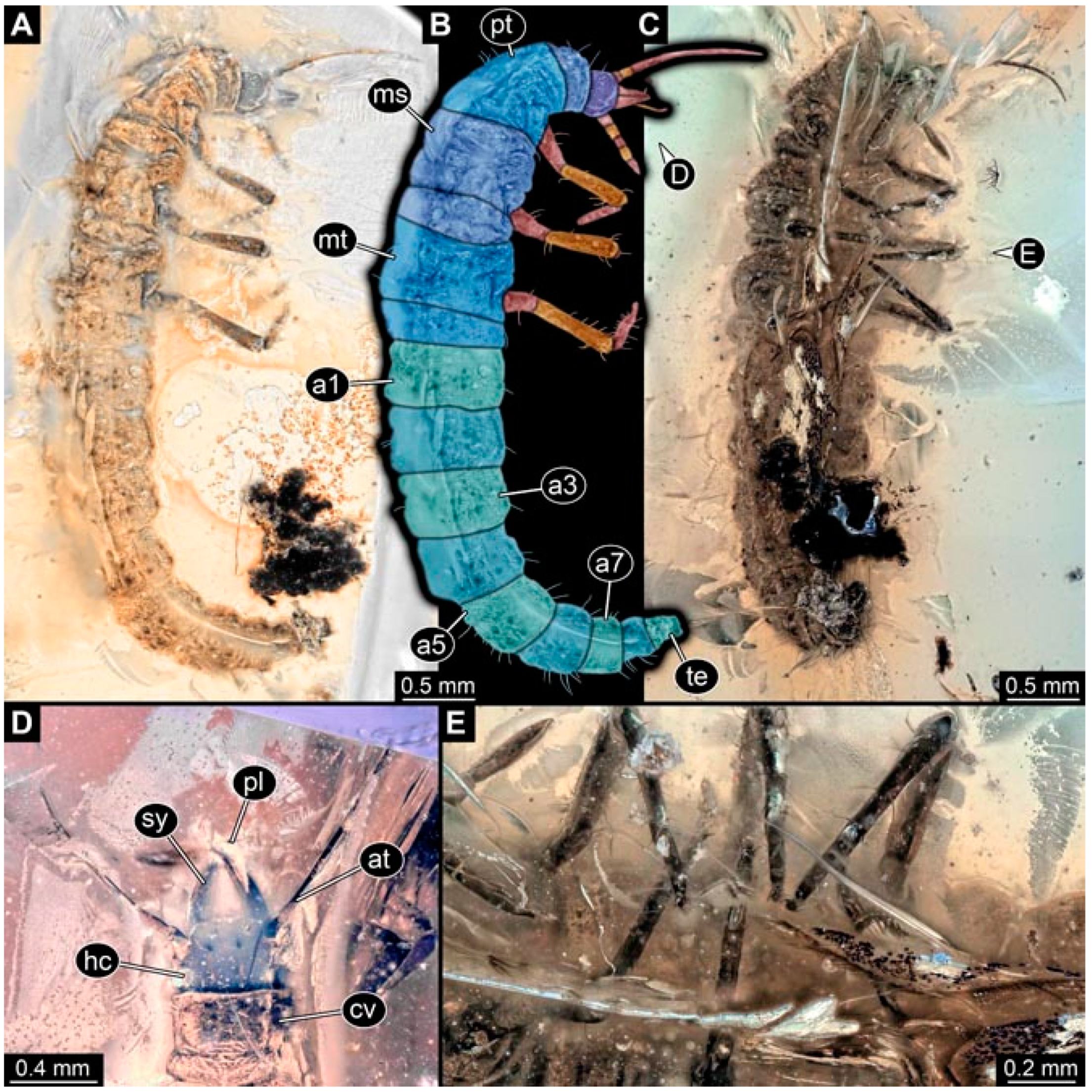
- Specimen 4819 (BUB 3060) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 1B,C) and ventral view (Figure 1A). A prominent camouflaging cloak is present (Figure 1A). In the ventral view, the head capsule is well accessible (Figure 1F). The antenna bears a prominent seta distally (Figure 1F). Each tarsus of the anterior trunk appendages (walking legs) carries a trumpet-shaped empodium (Figure 1E). Prominent protrusions on the back are well apparent (Figure 1D). The overall length of the larva is 1.60 mm.
- (2)
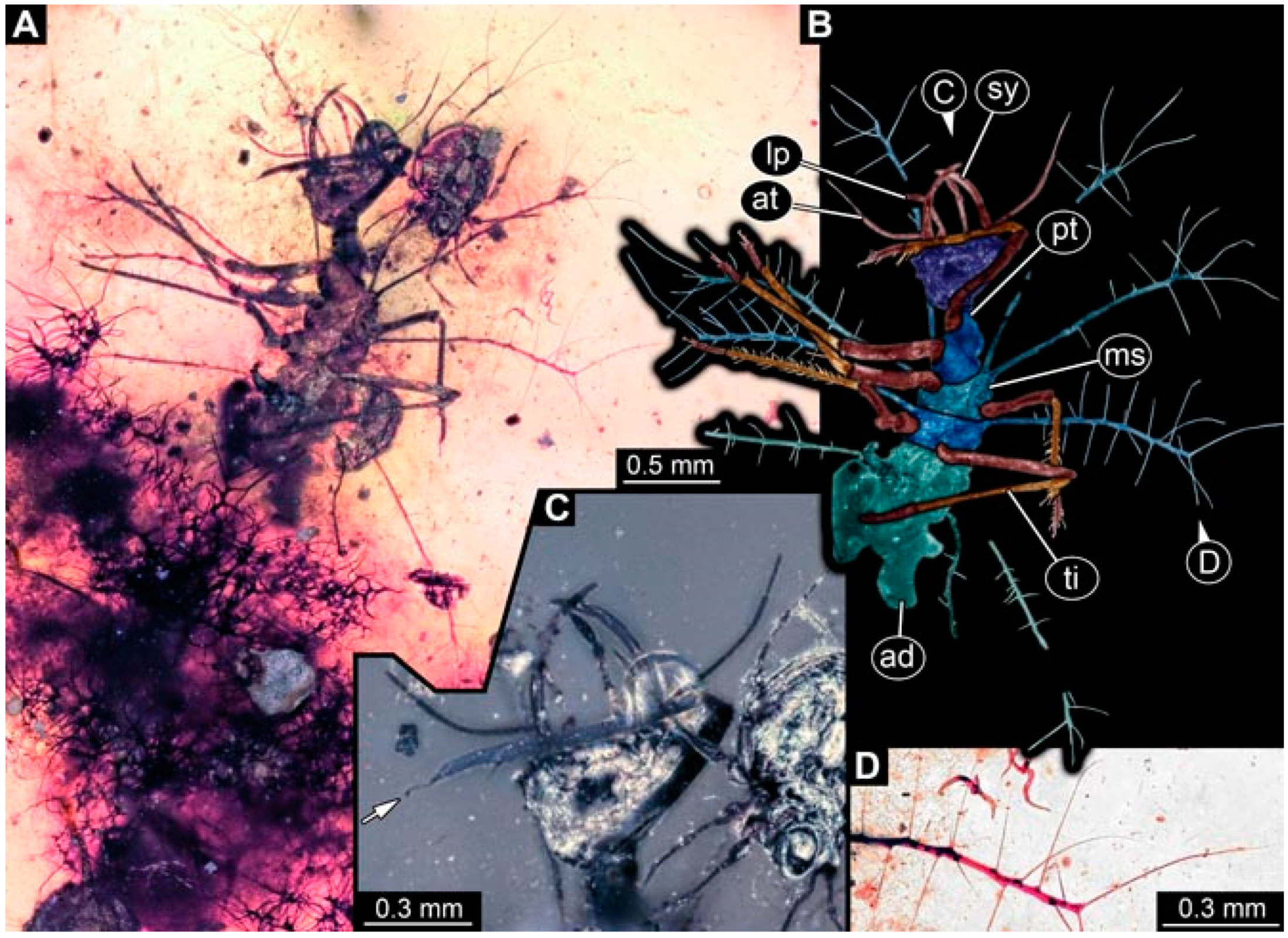
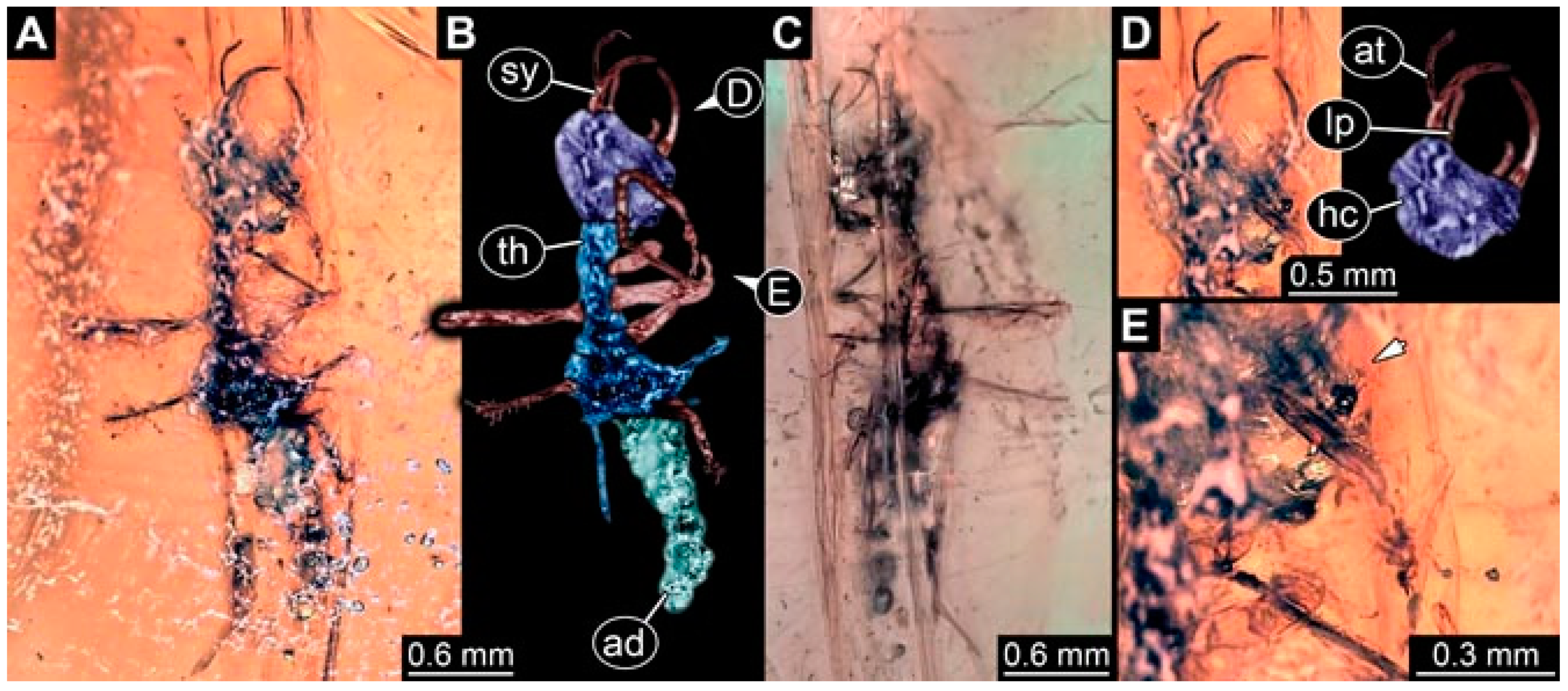
- Specimen 4821 (BUB 3066) is preserved in Myanmar amber. It is accessible in a ventral view (Figure 2A,B). No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 2C). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 2D). Prominent protrusions on the back are well apparent (Figure 2A). The overall length of the larva is 1.69 mm.
- (3)
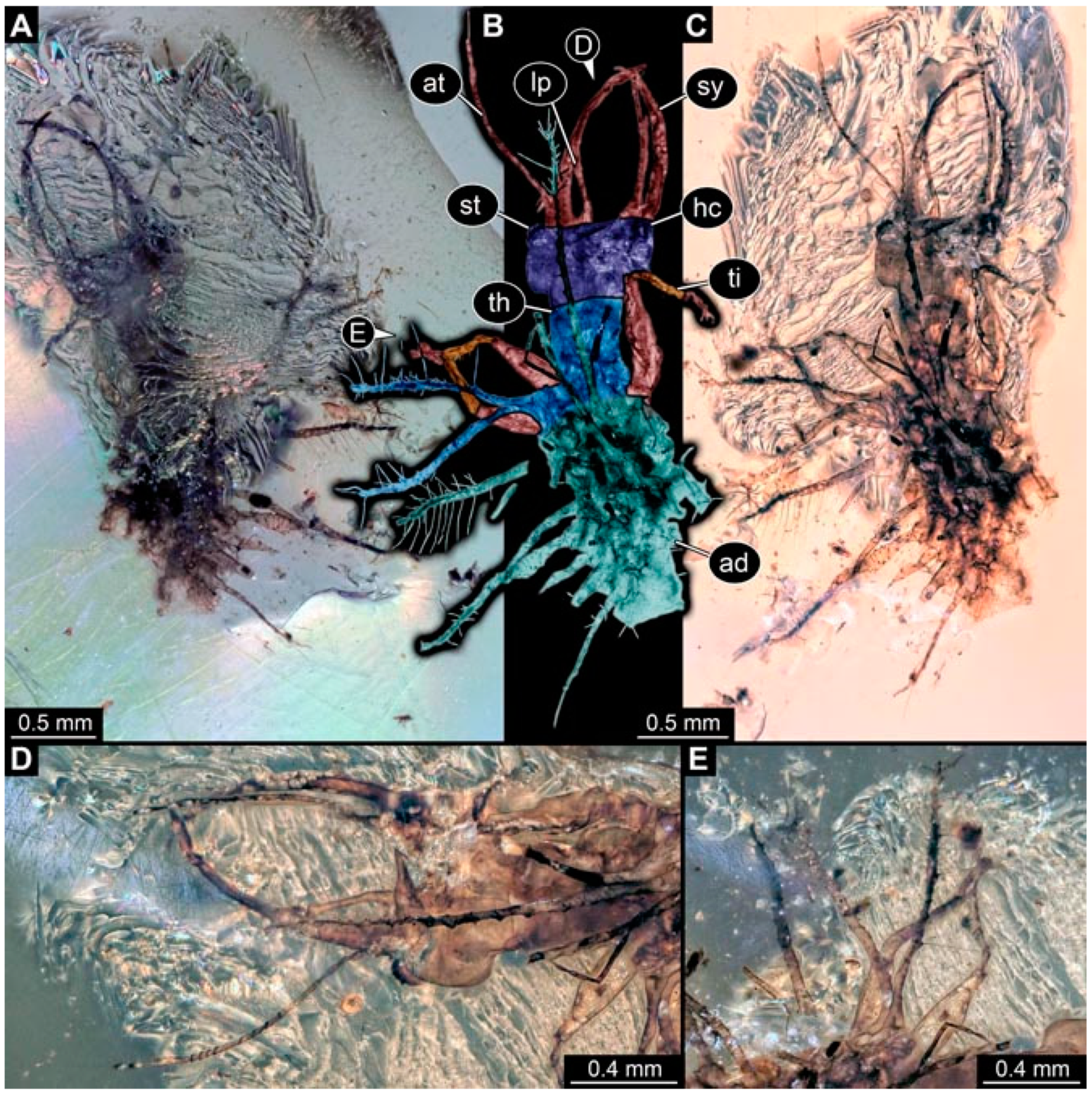
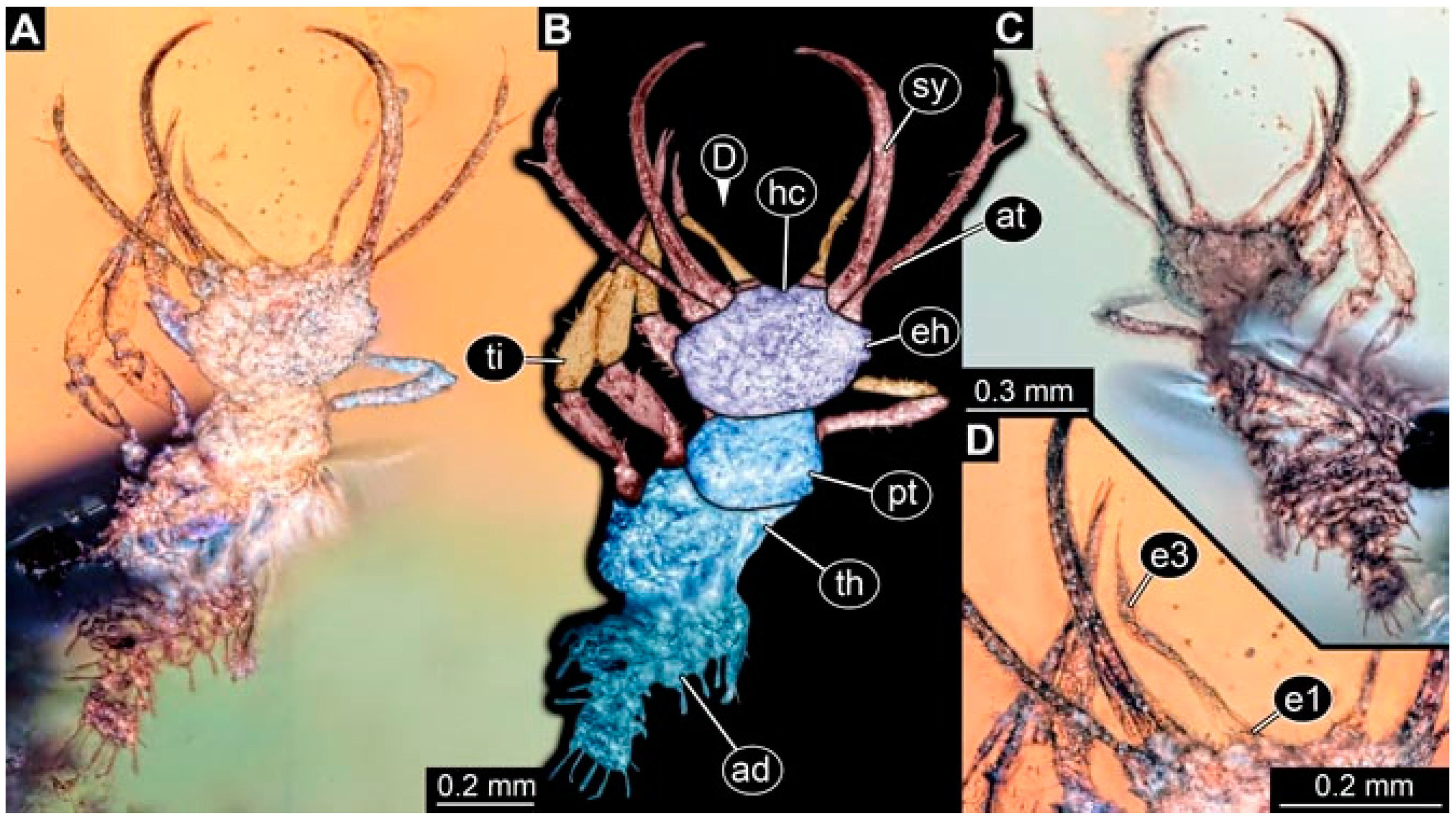
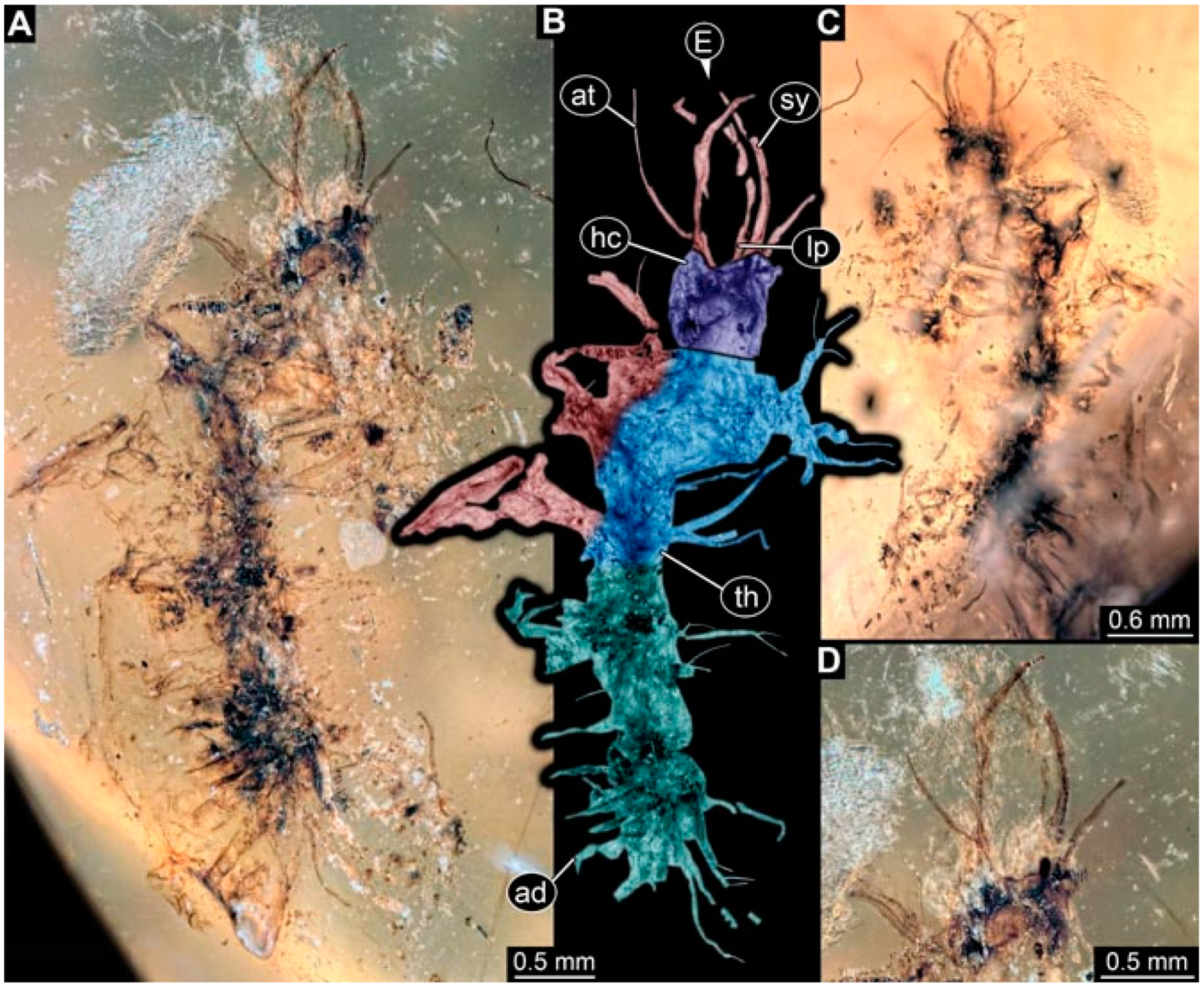
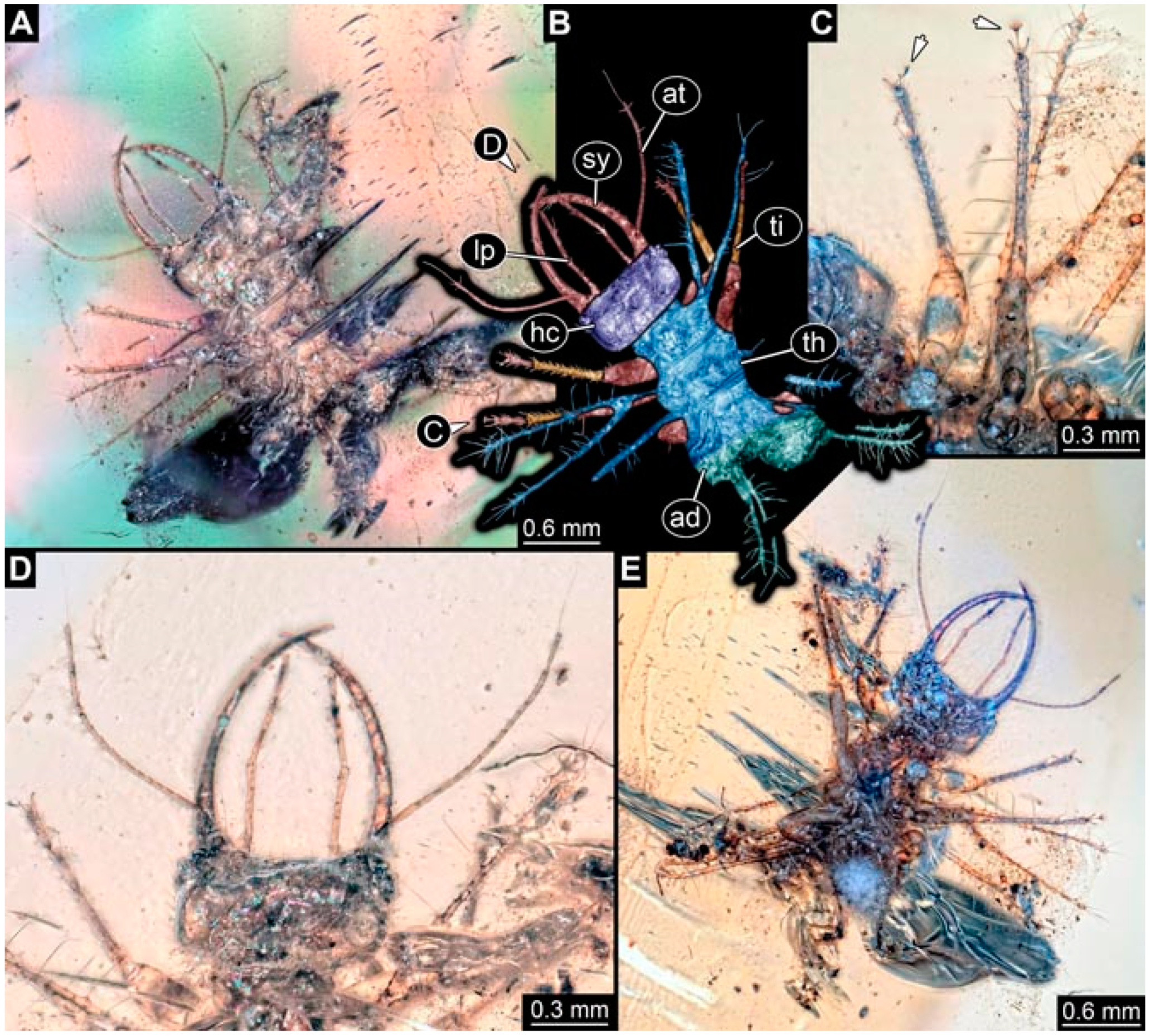
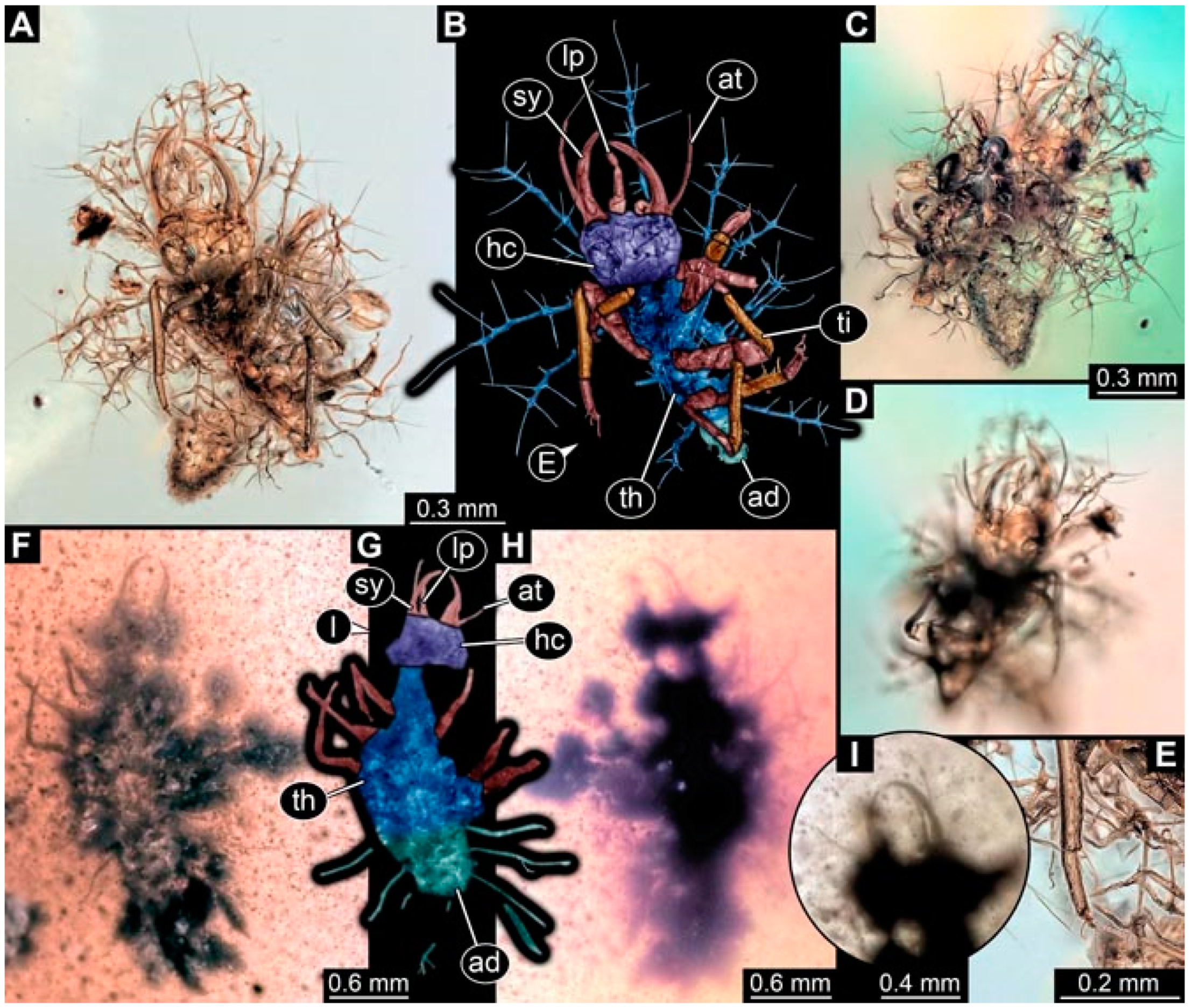
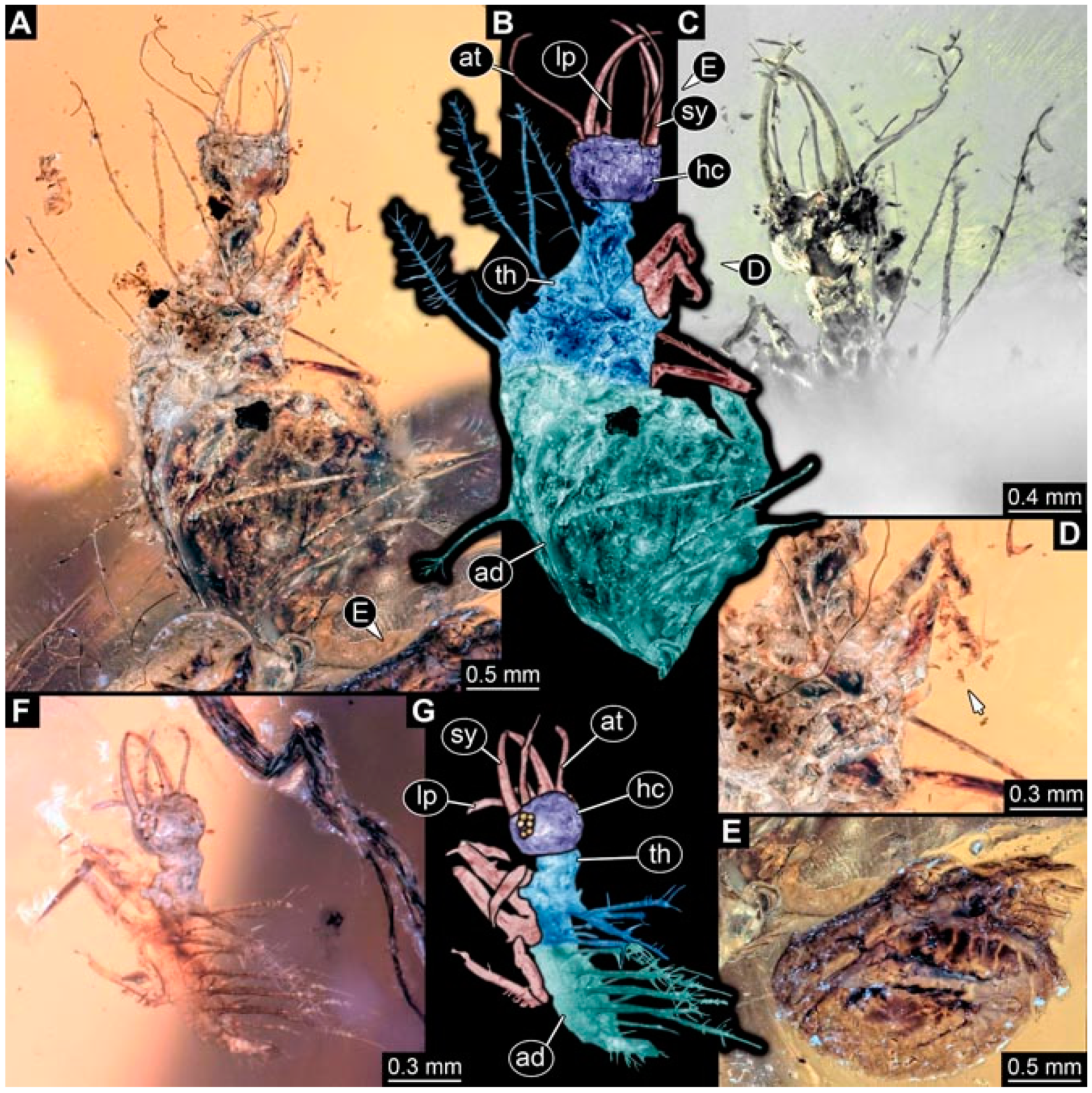
- Specimen 4822 (BUB 3347) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 3B,C) and ventral view (Figure 3A), yet the ventral side is less well accessible due to impurities in the amber. The head capsule is rather rectangular in its outline (Figure 3D). Traces of a camouflaging cloak are present. The antenna bears a prominent seta distally (Figure 3D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 3E). Prominent protrusions on the back are well apparent (Figure 3B,C). The overall length of the larva is 2.12 mm.
- (4)
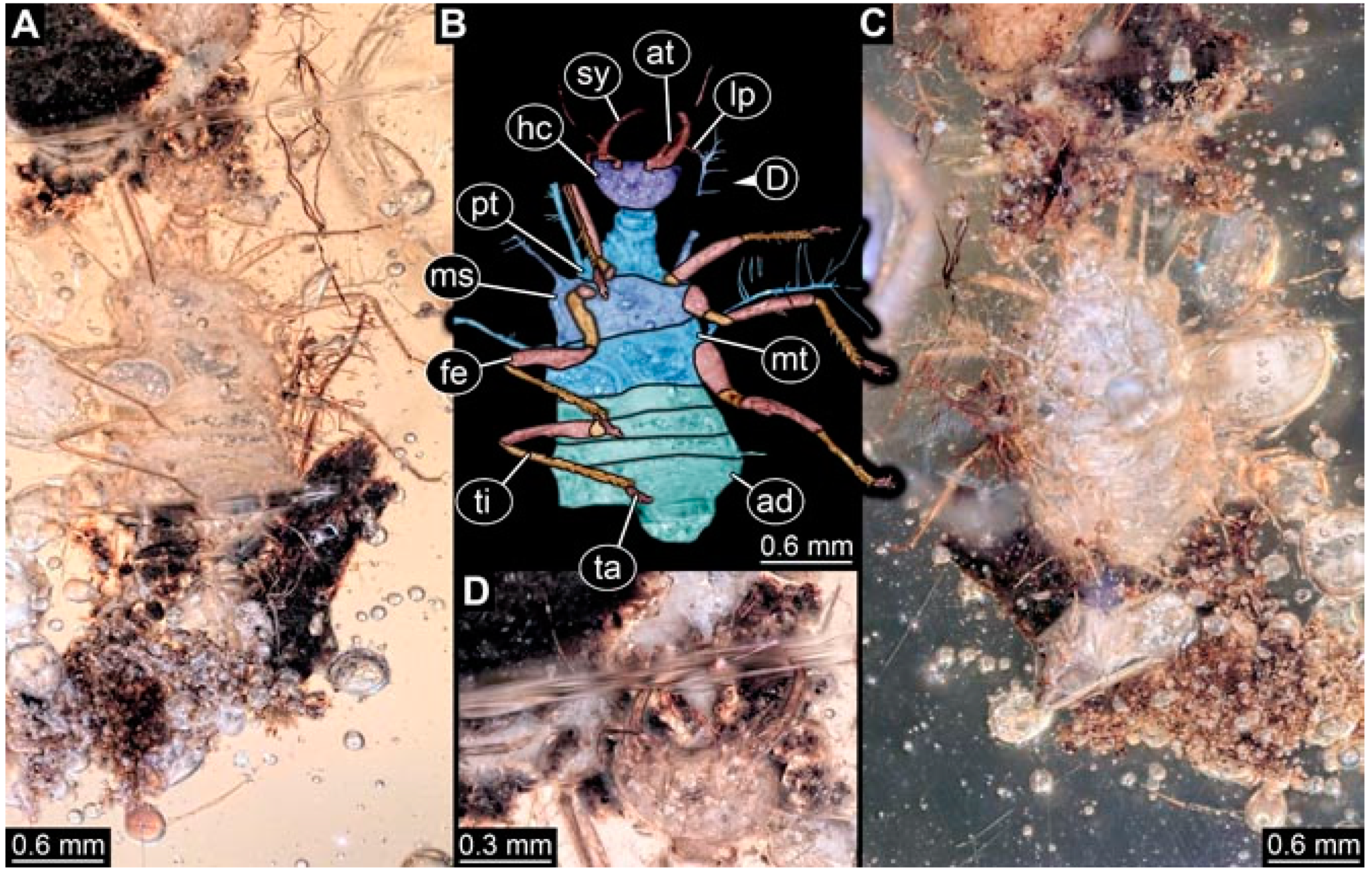
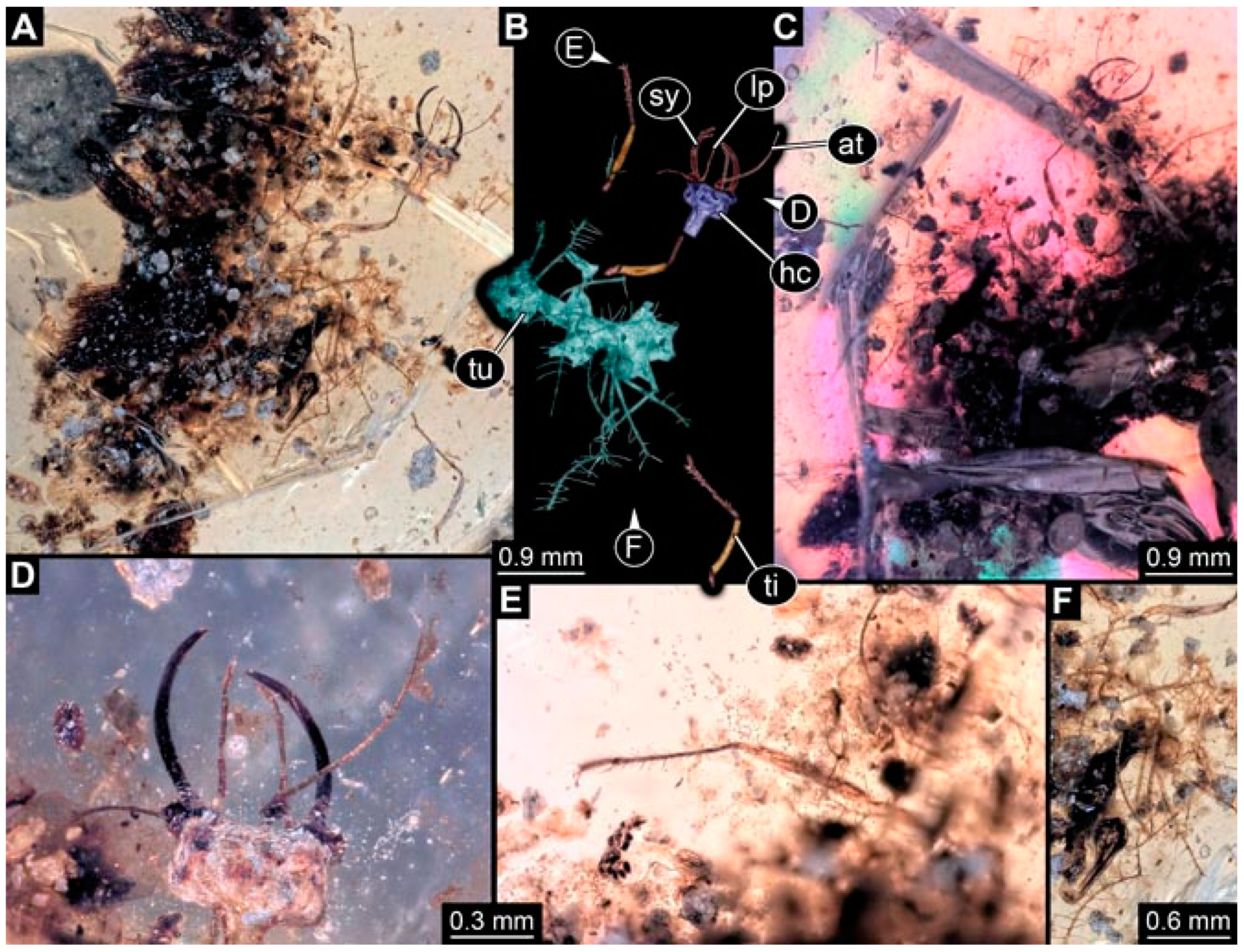
- Specimen 4823 (BUB 3358) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 4A) and ventral view (Figure 4B,C). Parts of the body are partially separated from each other. The head shape is well apparent in dorsal view (Figure 4E). Remains of a camouflaging cloak are apparent, including an appendage of another animal. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 4D). Prominent protrusions on the back are well apparent (Figure 4F). The exact length cannot be measured.
- (5)
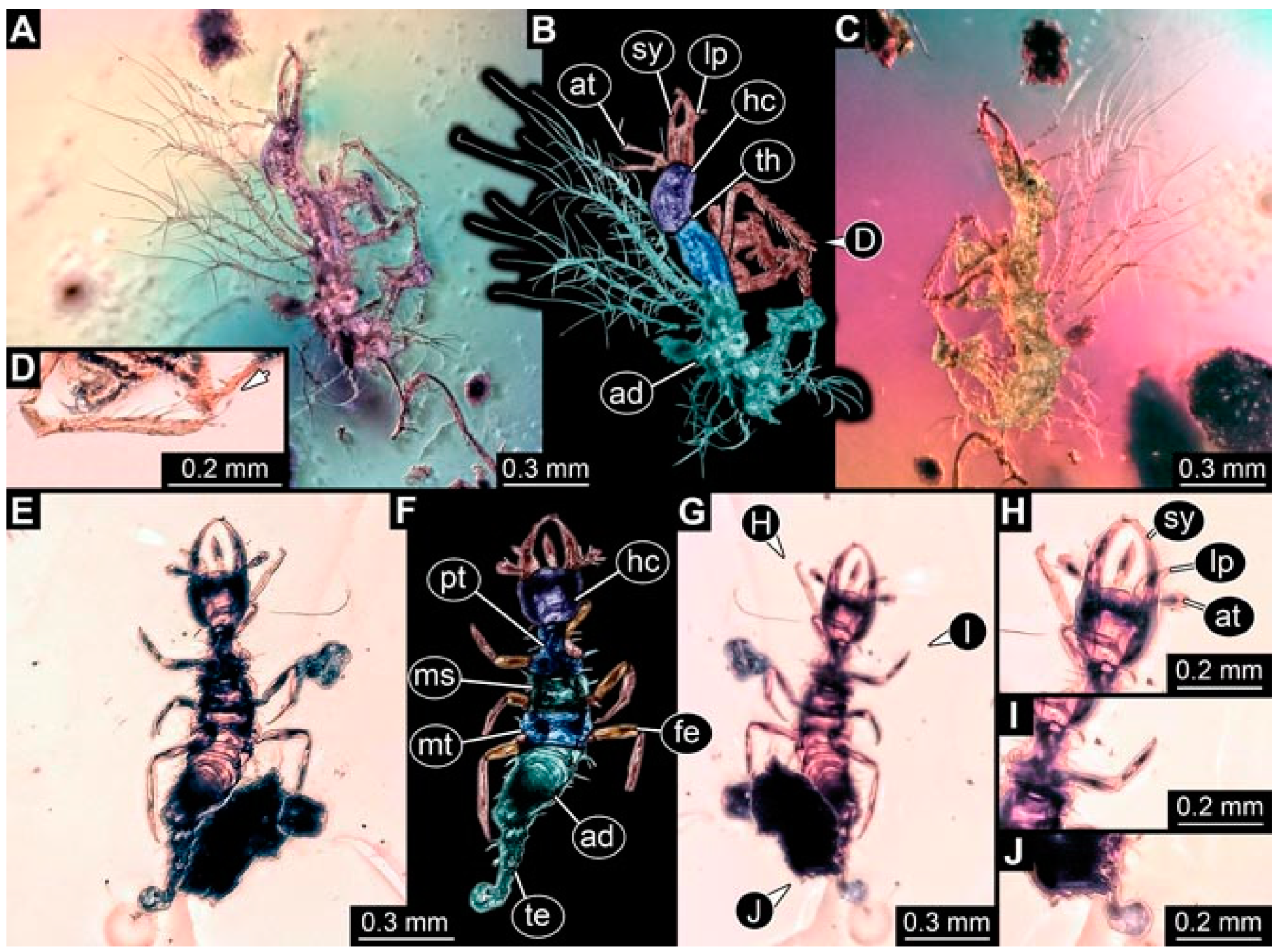
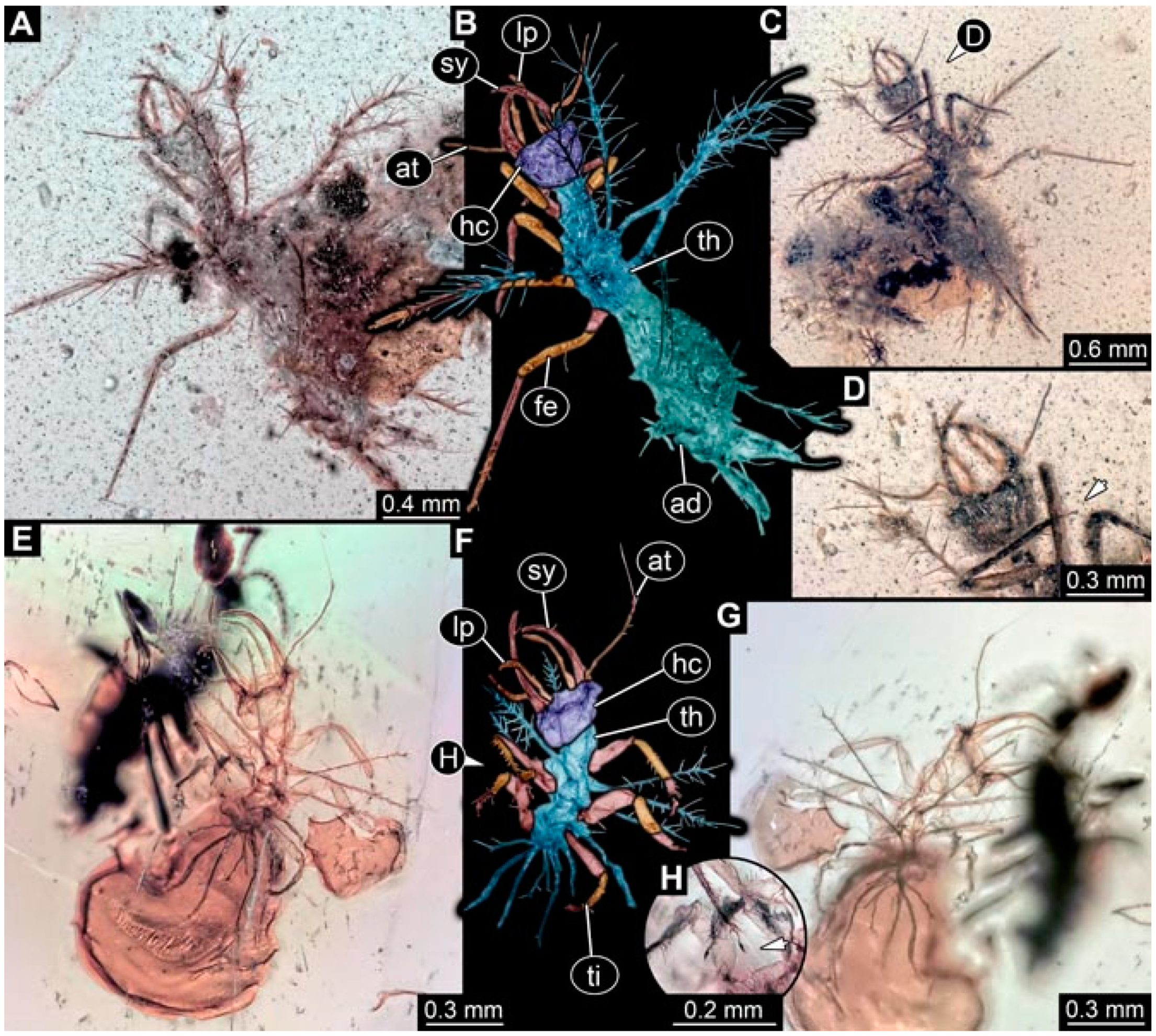
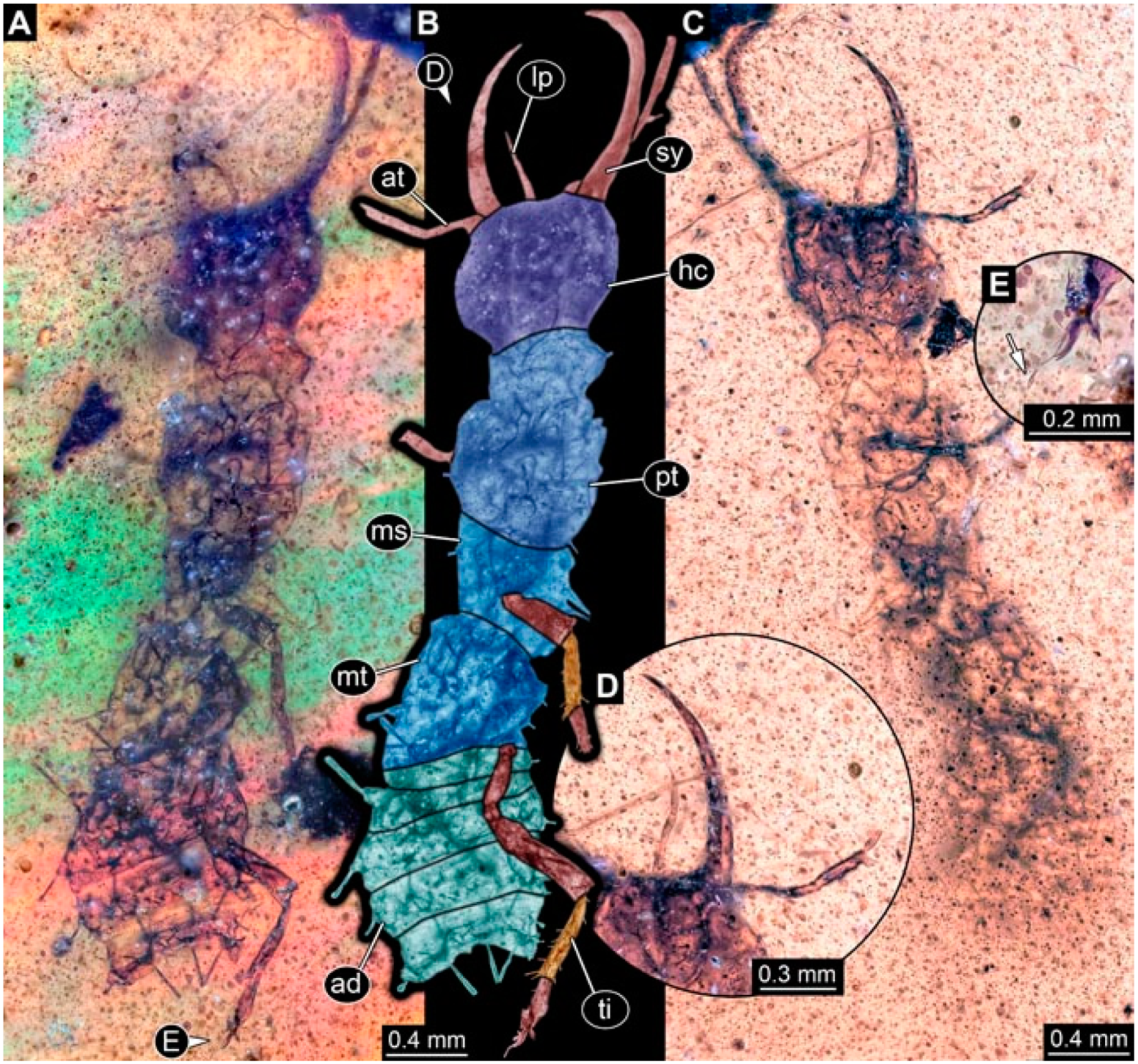
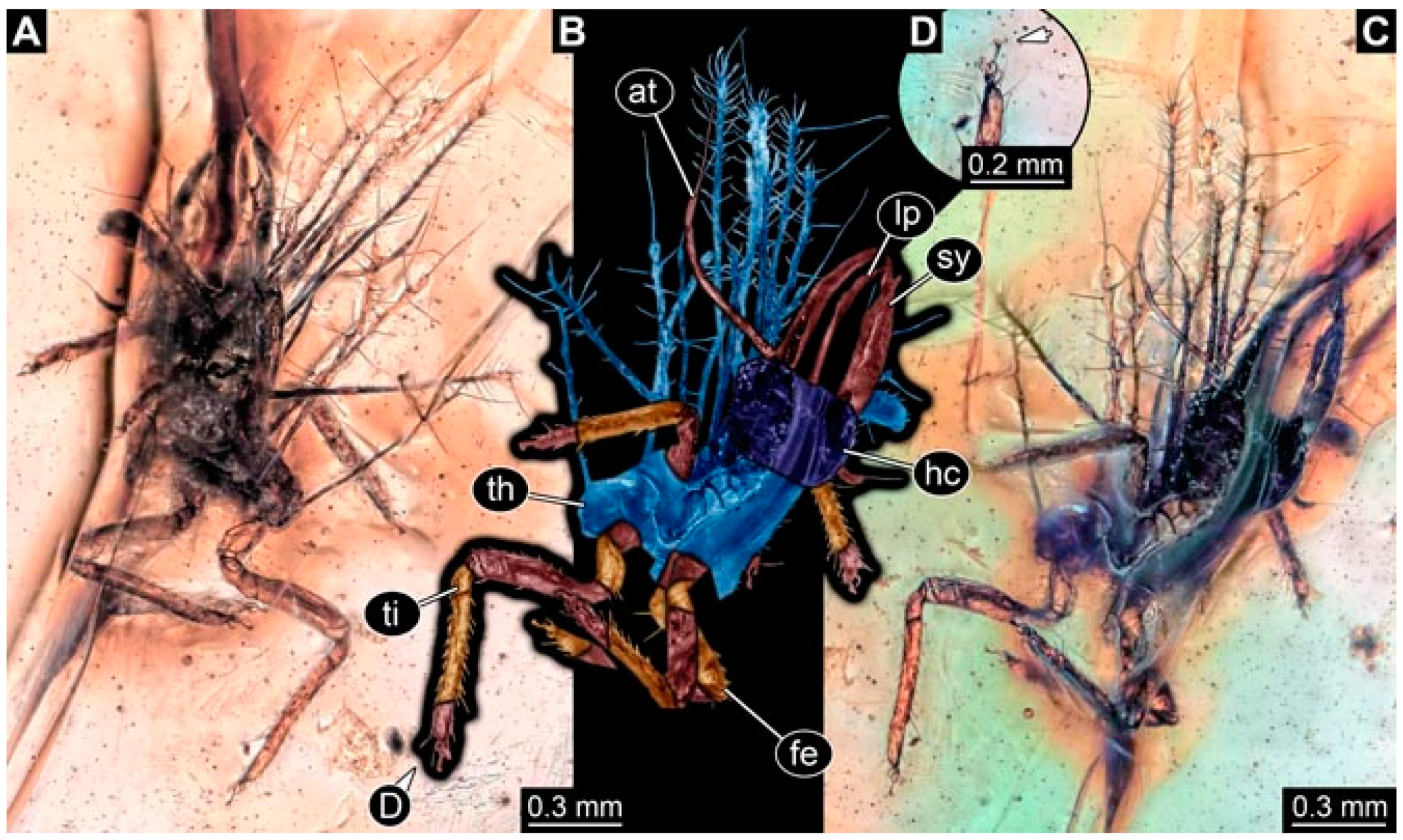
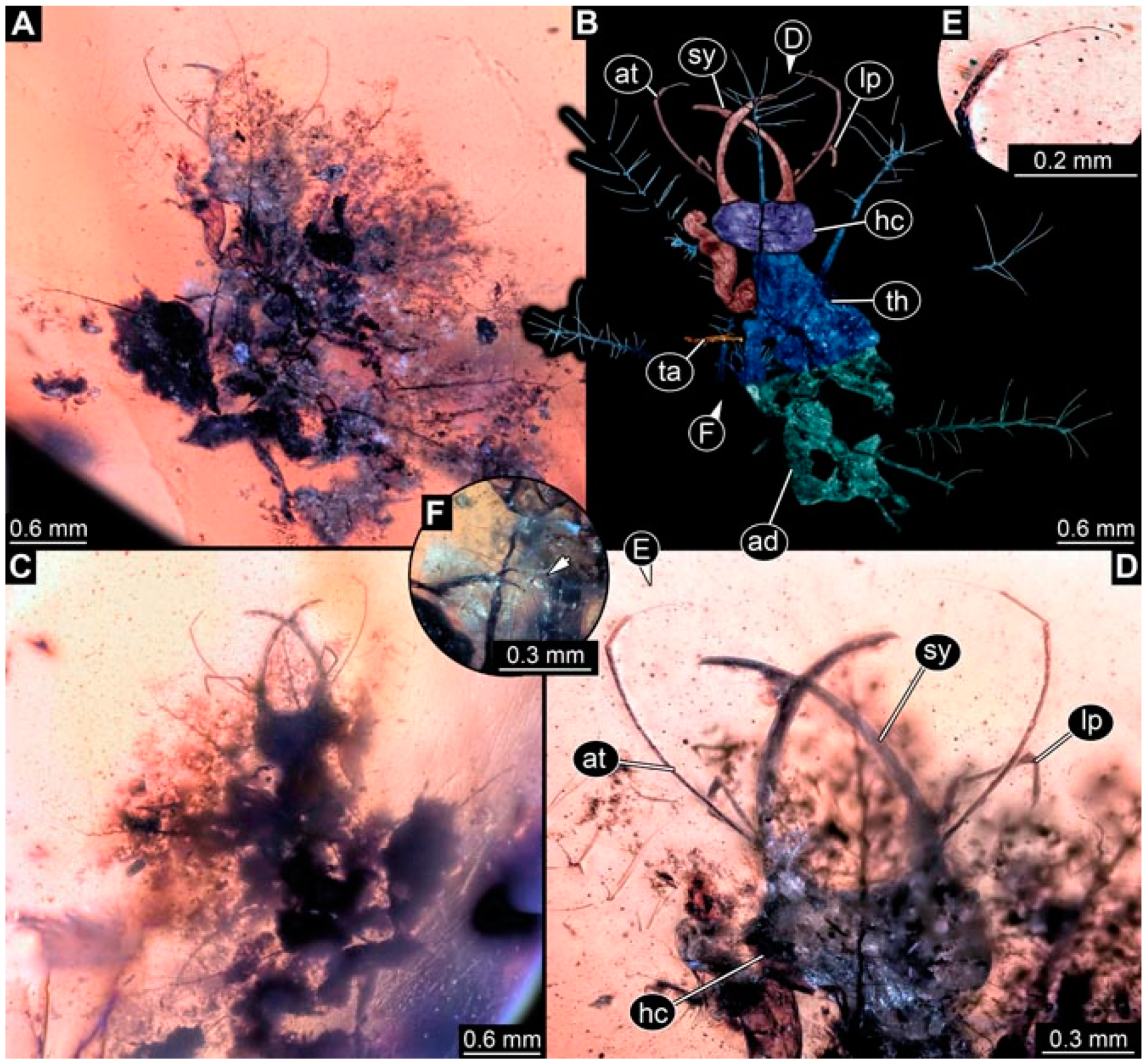
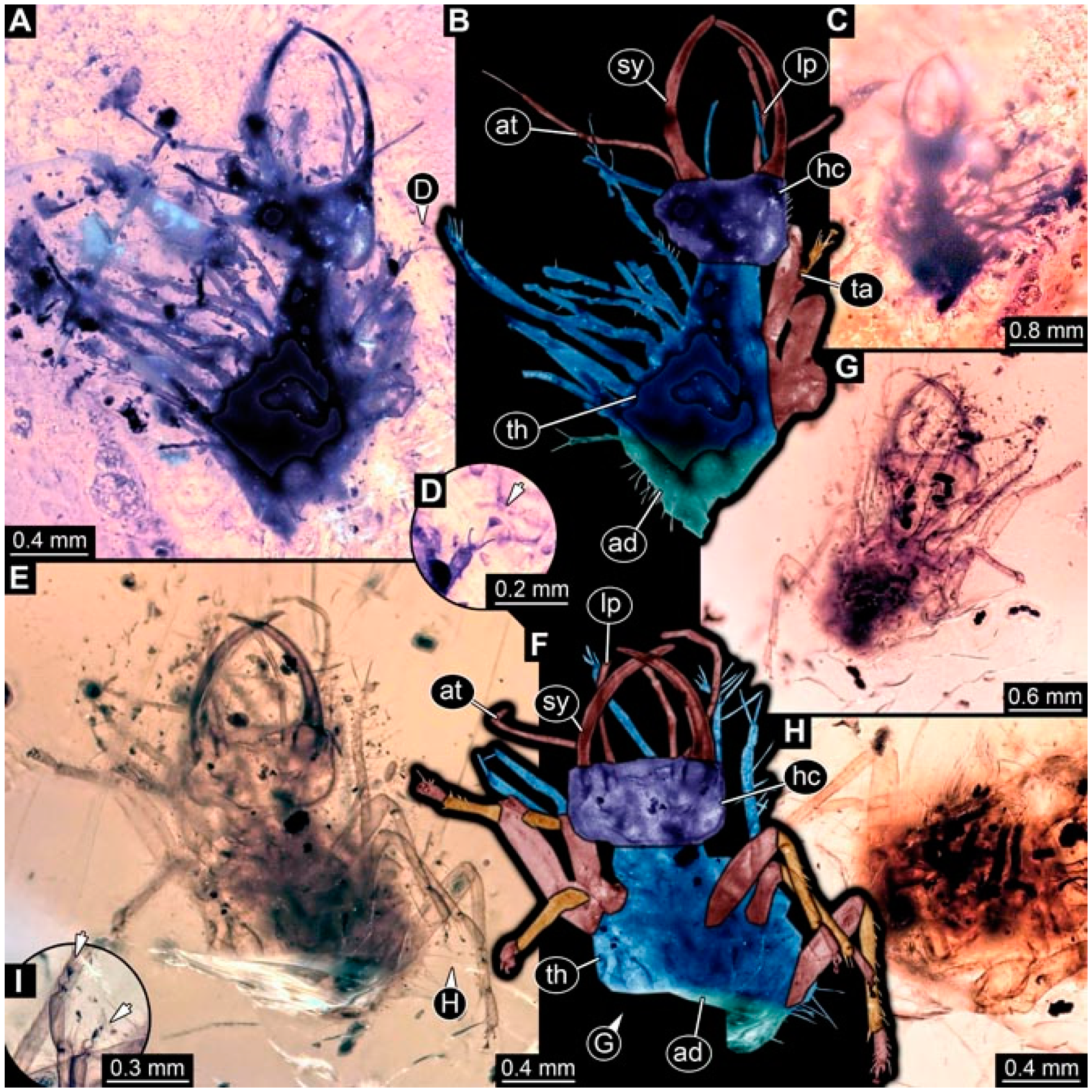
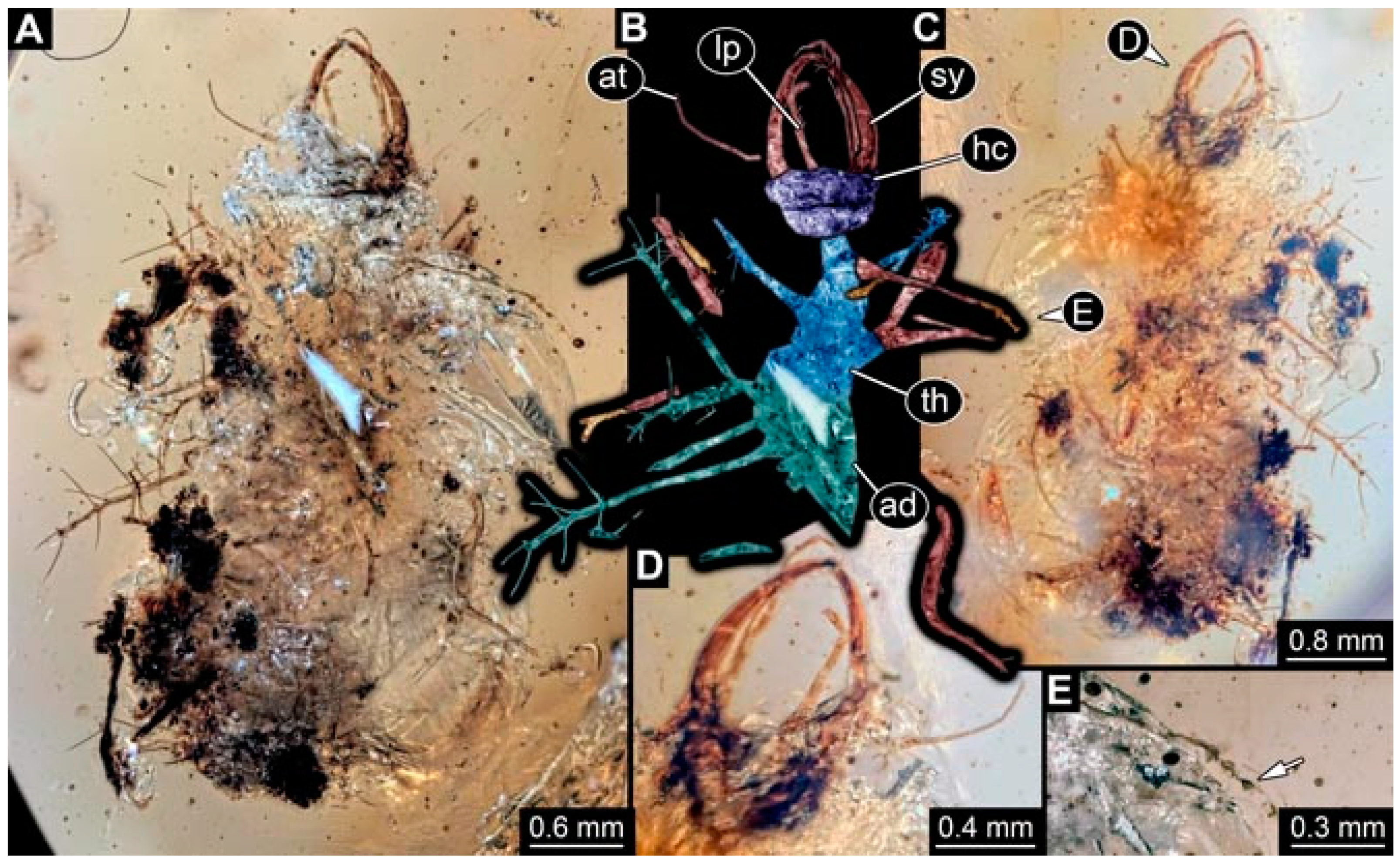
- Specimen 4824 (BUB 3359) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 5A,B) and ventral view (Figure 5C). No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 5D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 5F). Prominent protrusions on the back are well apparent (Figure 5E). The overall length of the larva is 1.05 mm.
- (6)
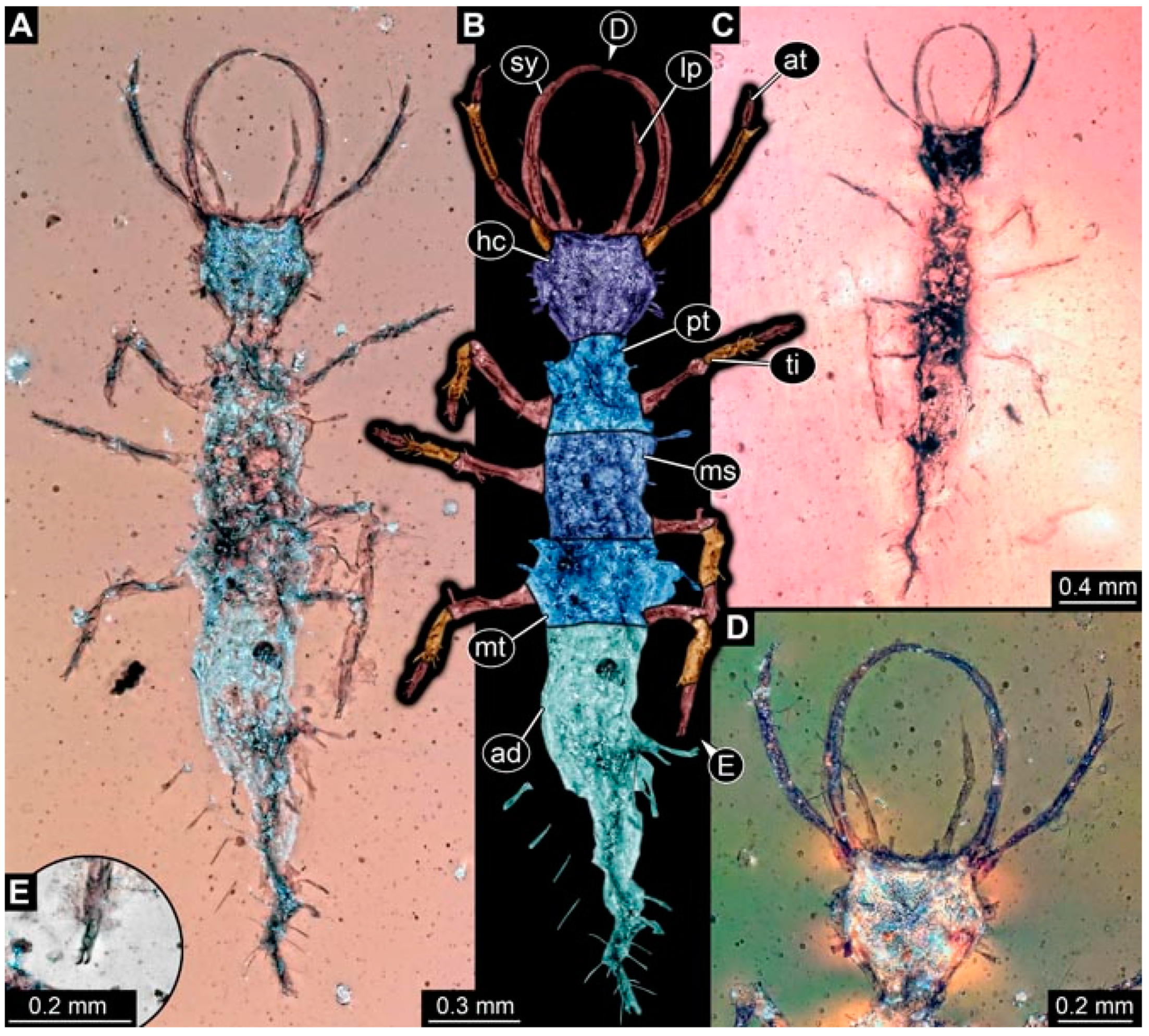
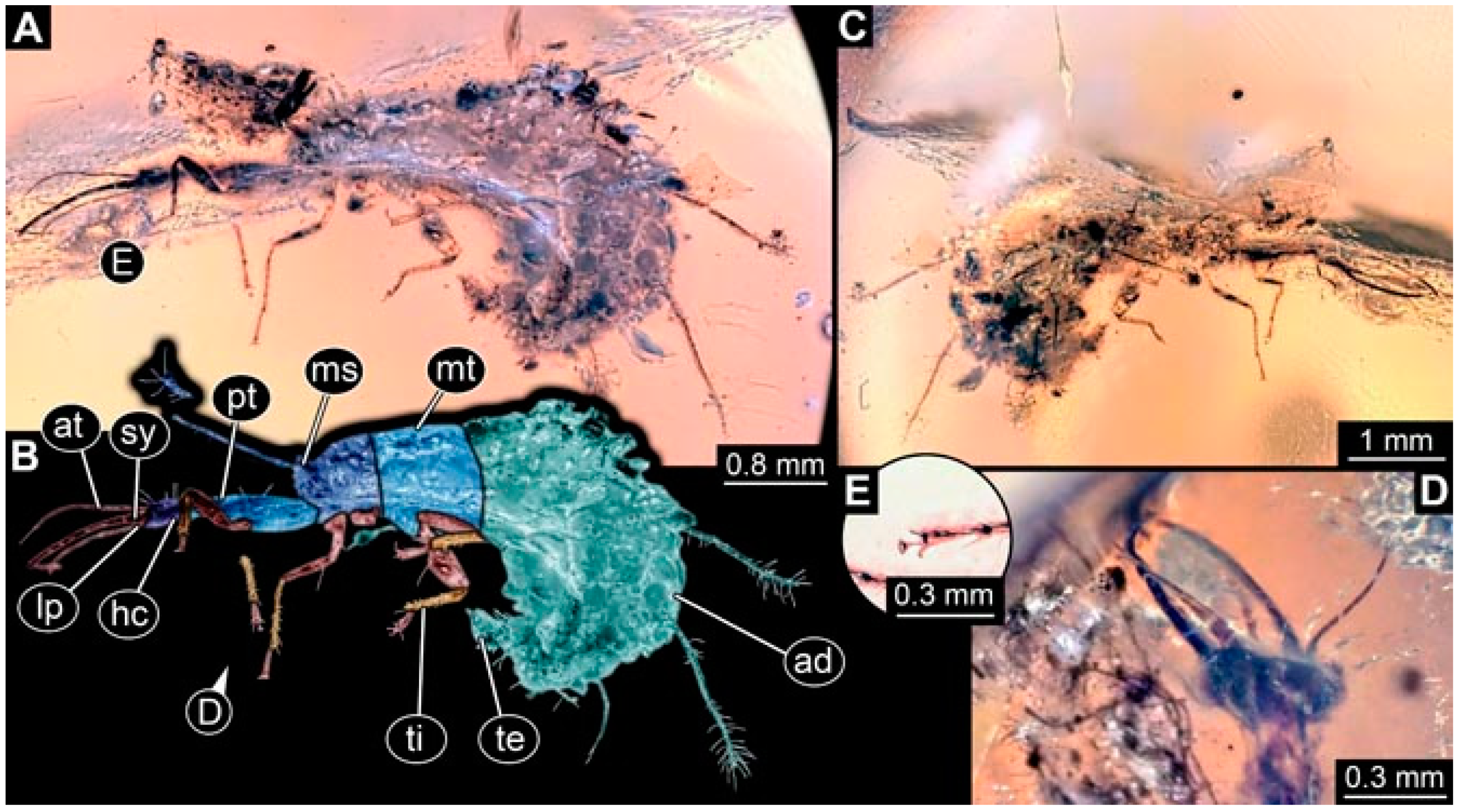
- Specimen 4820 (BUB 3361) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 6C) and ventral view (Figure 6A,B). Dorsally, the head is not well accessible, but it is ventrally (Figure 6D). No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 6D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 6A). Prominent protrusions on the back are apparent. The overall length of the larva is 2.56 mm.
- (7)
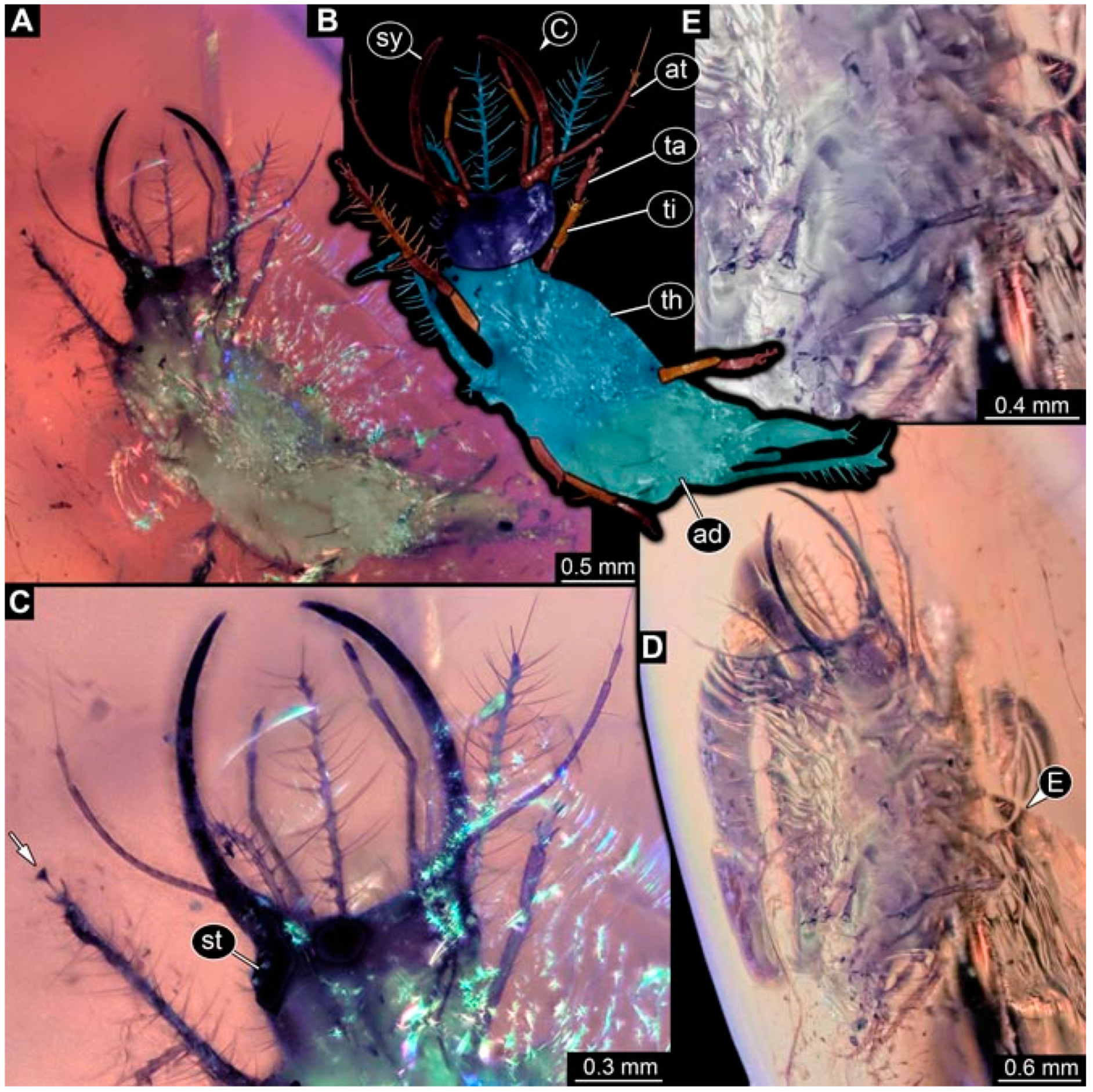
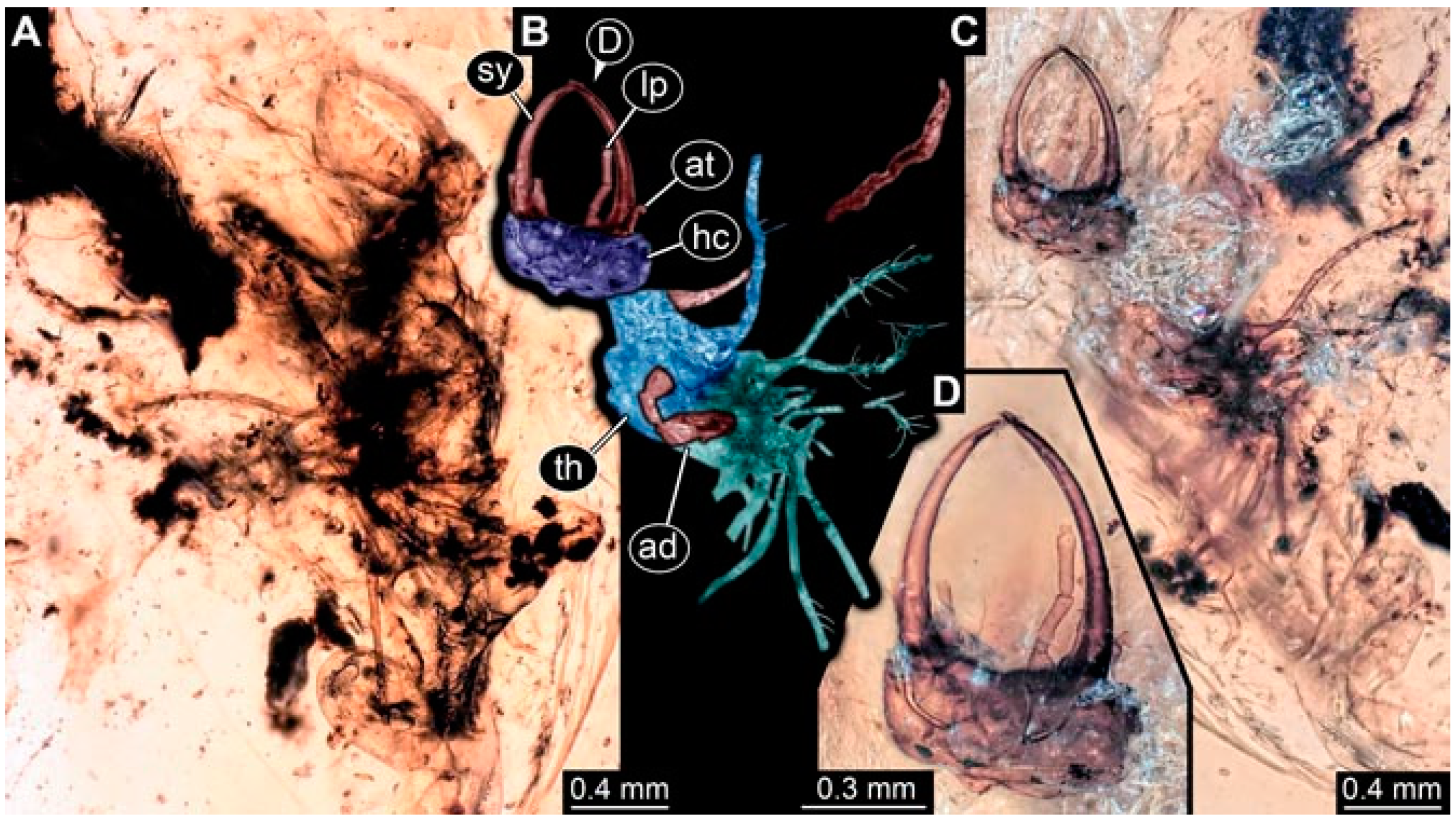
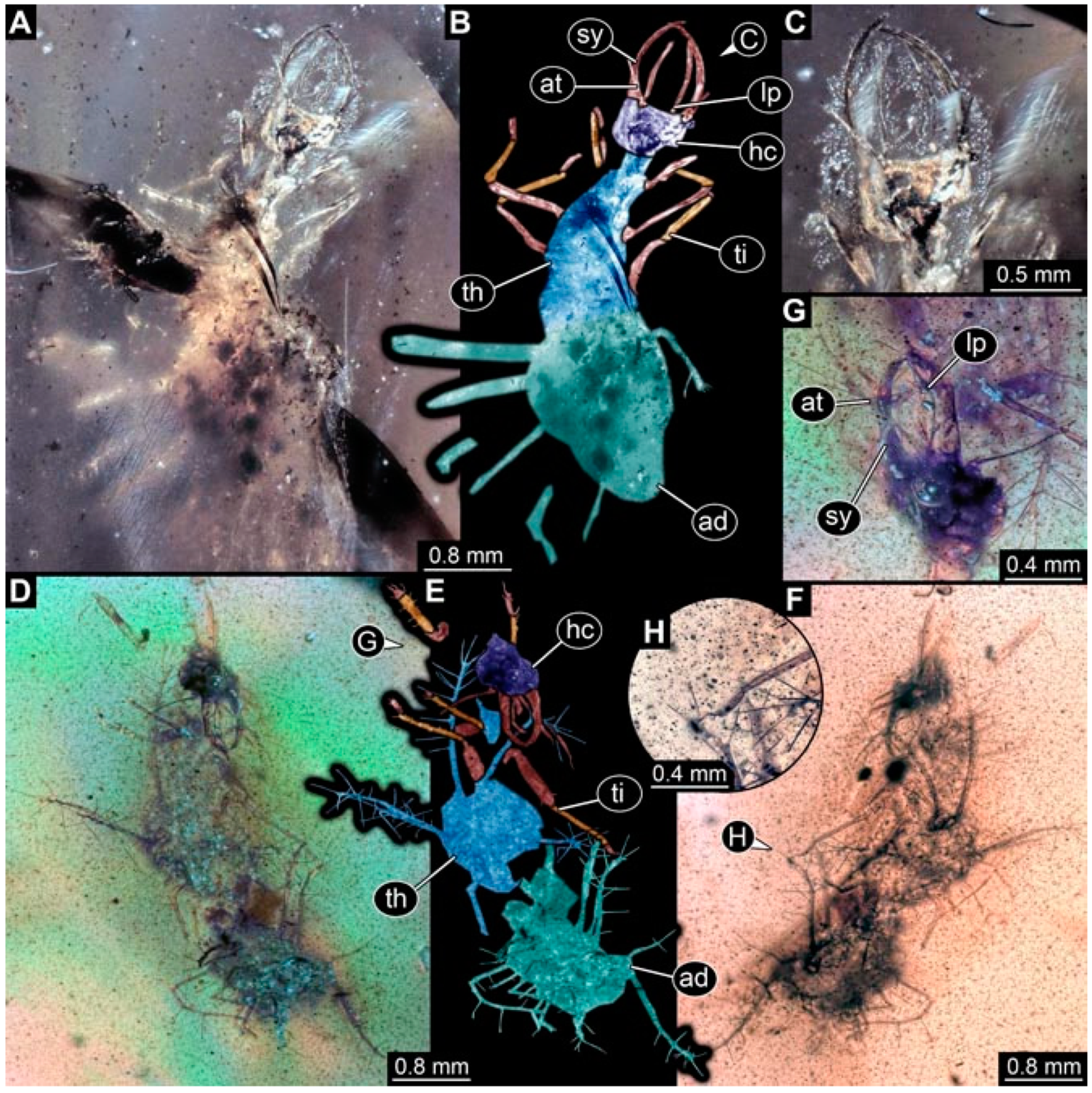
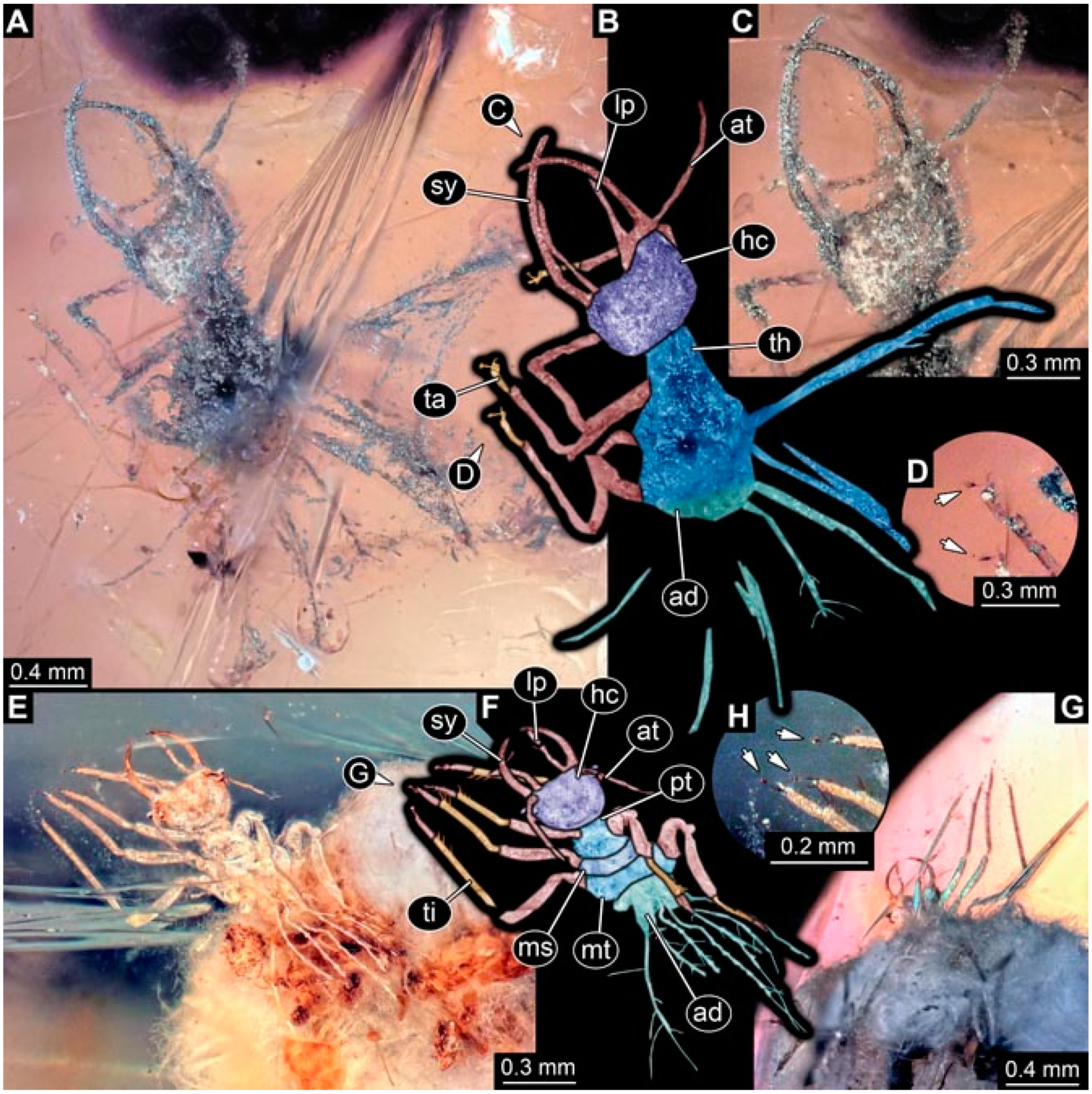
- Specimen 4825 (BUB 3379) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 7C) and ventral view (Figure 7A,B). No camouflaging cloak is apparent. The antenna bears a prominent, but short seta distally (Figure 7D). The trunk appendages lack empodia (Figure 7E). No prominent protrusions on the back are apparent. The overall length of the larva is 1.30 mm.
- (8)
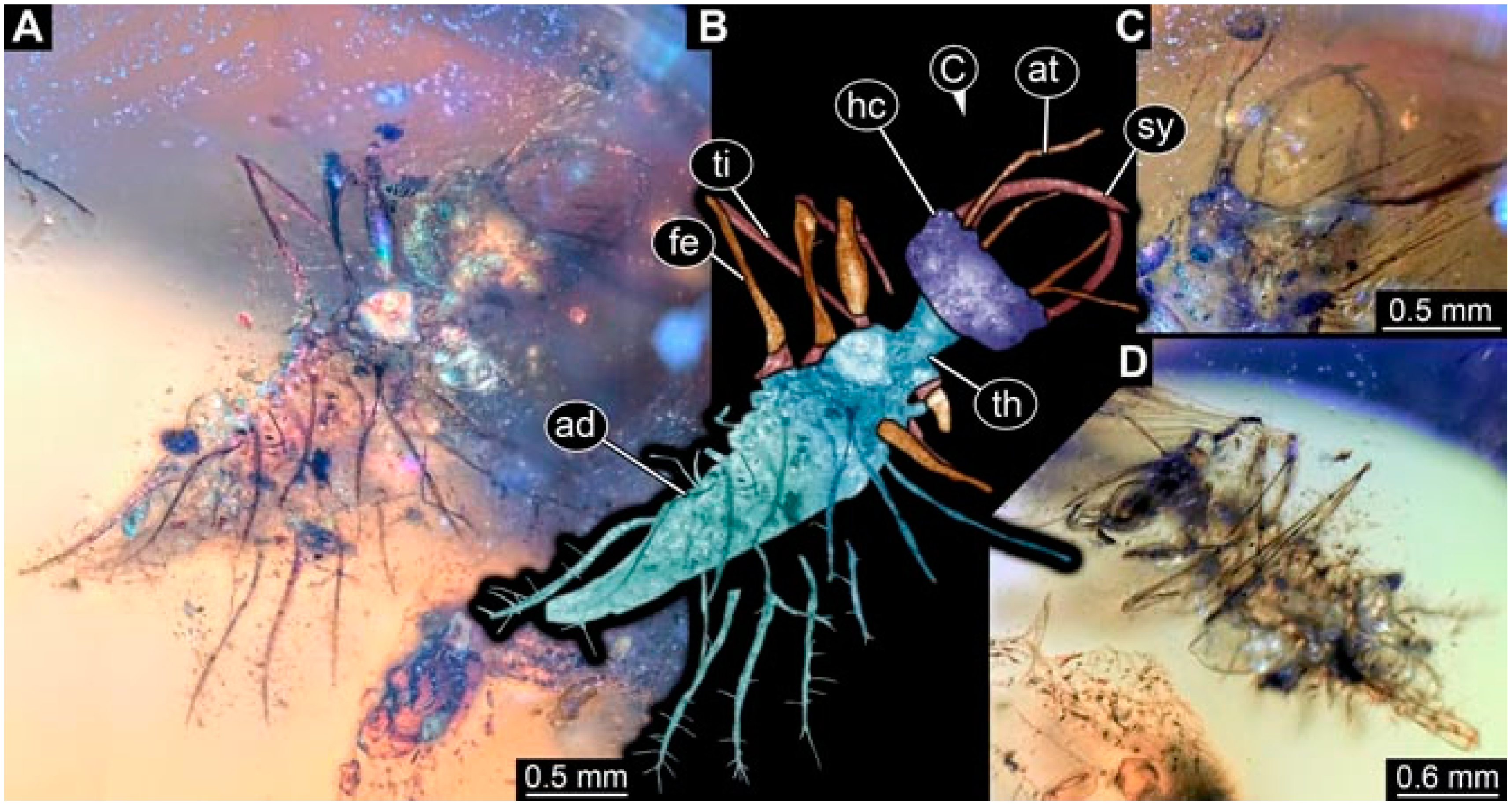
- Specimen 4826 (BUB 3393) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 8A,B) and ventral view (Figure 8C), but is partly concealed by bubbles. The head has a roughly rectangular shape (Figure 8D). No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 8D). Trunk appendages are not well accessible; however, the empodia are still apparent (Figure 8E). No prominent protrusions on the back are apparent. The trunk end is not well preserved (Figure 8F). The overall length of the larva is 6.00 mm.
- (9)
- Specimen 4827 (F 3196 BU CJW) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 9A,B) and lateral view (Figure 9C). No camouflaging cloak is apparent. The stylets are relatively longer than in other specimens. The antenna bears a prominent seta distally. The trunk appendages lack empodia (Figure 9D). No prominent protrusions on the back are apparent. The overall length of the larva is 1.22 mm.
- (10)
- Specimen 4829 (PED 0038) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 9E,F) and ventral view (Figure 9G). No camouflaging cloak is apparent. The specimen is incomplete and the posterior end is missing. The antenna bears a prominent seta distally. Labial palps appear short, element 3 appears to be the longest; also, part of the proximal part of the labium appears to be apparent (Figure 9H). The trunk appendages lack empodia. No prominent protrusions on the back are apparent. The preserved part is slightly longer than 1 mm. The specimen has some characters of larvae of Hemerobiidae.
- (11)
- Specimen 4828 (PED 0034) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 10C) and ventral view (Figure 10A,B), yet the ventral view is less distorted. The thorax region is rather slender, the abdomen short, and the head is relatively large. No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 10D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 10E). Prominent protrusions on the back are apparent. The overall length of the larva is 2.94 mm.
- (12)
- Specimen 4830 (PED 0065) is preserved in Myanmar amber. It is only accessible in a lateral view (Figure 11A–C), hence the head and stylet shape are not accessible. No camouflaging cloak is apparent (Figure 11A–C). The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 11D). Prominent protrusions on the back are apparent (Figure 11A–C). The trunk end is not well accessible; therefore, the total length of the specimen cannot be measured.
- (13)
- Specimen 4832 (PED 0248) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 11E,F) and ventral view (Figure 11G). No camouflaging cloak is apparent. The antennae and labial palps are rather short and broad (Figure 11H). The antenna does not bear a prominent seta distally. The trunk appendages lack empodia (Figure 11I). No prominent protrusions on the back are apparent. The overall length of the larva is 1.12 mm, but the trunk end is partly enrolled ventrally (Figure 11J); the true length should have been slightly longer. The specimen has some characters of larvae of Hemerobiidae.
- (14)
- Specimen 4831 (PED 0149) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 12A,B) and ventral view (Figure 12C). No camouflaging cloak is apparent. It has labial palps with three elements; the terminal one is the longest (Figure 12D,E). The antenna bears a prominent seta distally. No tarsi are accessible; hence, it remains unclear whether they bear empodia. No prominent protrusions on the back are apparent. The overall length of the larva is 6.19 mm. The specimen has some characters of larvae of Hemerobiidae.
- (15)
- Specimen 4833 (PED 0251) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 13A,B) and ventral view (Figure 13D). No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 13C). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 13C). Prominent protrusions on the back are apparent. The overall length of the larva is 3.37 mm.
- (16)
- Specimen 4834 (PED 0252) is preserved in Myanmar amber. It is accessible in a dorsal to dorso-lateral (Figure 14A,B) and ventral view (Figure 14C). No camouflaging cloak is apparent. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 14D). Prominent protrusions on the back are apparent and distally bear small objects. The overall length of the larva is 3.79 mm.
- (17)
- Specimen 4835 (PED 0253) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 15A,B) and ventral view (Figure 15C). No camouflaging cloak is apparent. Head and head appendages appear rather large in comparison to the trunk. The antenna bears a prominent seta distally. The labial palps have three elements; the second is the longest (Figure 15D). The trunk appendages lack empodia. No prominent protrusions on the back are apparent. The overall length of the larva is 1.05 mm.
- (18)
- Specimen 4836 (PED 0315) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 16A,B) and ventral view (Figure 16C). A prominent camouflaging cloak is present, partly concealing the dorsal side. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 16D). Prominent protrusions on the back are apparent. The overall length of the larva is 2.31 mm.
- (19)
- Specimen 4837 (PED 0323) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 16E,F) and ventral view (Figure 16G). No camouflaging cloak is apparent. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 16H). Prominent protrusions on the back are apparent. The abdomen is rather short. The overall length of the larva is 0.61 mm.
- (20)
- Specimen 4838 (PED 0330) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 17A,B) and ventral view, yet the ventral view is partly concealed by impurities of the amber (Figure 17D). No camouflaging cloak is apparent. The tips of the antennae are not accessible (Figure 17C). Each tarsus of the trunk appendages carries a trumpet-shaped empodium. Prominent protrusions on the back are apparent. The overall length of the larva is 2.69 mm.
- (21)
- Specimen 4839 (PED 0375) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 18D) and ventral view, yet the ventral view is partly concealed by impurities of the amber (Figure 18A,B). A prominent camouflaging cloak is present. The antenna bears a prominent seta distally (Figure 18C). Each tarsus of the trunk appendages carries a trumpet-shaped empodium. Prominent protrusions on the back are apparent. The overall length of the larva is 3.87 mm.
- (22)
- Specimen 4840 (PED 0427) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 19A,B) and ventral view (Figure 19D), yet the general view is partly concealed by impurities of the amber. A prominent camouflaging cloak is present. The antenna bears a prominent seta distally (Figure 19C). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 19E). Prominent protrusions on the back are apparent. The overall length of the larva is 1.65 mm.
- (23)
- Specimen 4841 (PED 0433) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 20A,B) and ventral view, yet the ventral view is partly concealed by impurities of the amber (Figure 20C). In general, the surface of the animal is not well accessible, and many details cannot be clearly recognised. No camouflaging cloak is apparent. The antenna bears a prominent seta distally; it is almost as long as the main antenna itself (Figure 20D). No tarsi are accessible; hence, it remains unclear if they bear empodia. Prominent protrusions on the back are apparent. The overall length of the larva is 3.73 mm.
- (24)
- Specimen 4842 (PED 0441) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 21C) and ventral view, yet ventrally several bubbles conceal many details (Figure 21A,B). The antenna bears a prominent, but short seta distally (Figure 21D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 21E). Thorax segments appear rather elongated in comparison to the abdomen. No prominent protrusions on the back are apparent. The overall length of the larva is 3.30 mm.
- (25)
- Specimen 4843 (PED 0455) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 22B,C) and ventral view (Figure 22A), but many details are concealed by impurities in the amber. No camouflaging cloak is apparent. The head is well accessible in the dorsal view, but the antennae and labial palps are incomplete (Figure 22D). No tarsi are accessible; hence, it remains unclear if they bear empodia. Prominent protrusions on the back are apparent. The overall length of the larva is 1.44 mm.
- (26)
- Specimen 4844 (PED 0518) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 23A,B) and ventral view (Figure 23C). No camouflaging cloak is apparent. The antenna bears a prominent seta distally. The trunk appendages lack empodia (Figure 23D). No prominent protrusions on the back are apparent. The overall length of the larva is 1.62 mm.
- (27)
- Specimen 4845 (PED 0541) is preserved in Myanmar amber. It is accessible in dorsal (Figure 24C) and ventral view (Figure 24A,B), but details are often concealed by impurities of the amber. No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 24D). No tarsi are accessible; hence, it remains unclear if they bear empodia. Prominent protrusions on the back are apparent. The overall length of the larva is 2.01 mm.
- (28)
- Specimen 4846 (PED 0580) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 25A,B) and ventral view (Figure 25E), but details are often concealed by impurities of the amber. A prominent camouflaging cloak is present. The head is roughly rectangular (Figure 25D). The antenna bears a prominent seta distally (Figure 25D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 25C). Prominent protrusions on the back are apparent. The overall length of the larva is 1.89 mm.
- (29)
- Specimen 4848 (PED 0642) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 26A,B), ventral (Figure 26D), and ventro-lateral view (Figure 26C). The head appears partly disarticulated. A prominent camouflaging cloak is present. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 26E). Prominent protrusions on the back are apparent. The exact length cannot be estimated due to the camouflaging cloak.
- (30)
- Specimen 4849 (PED 0666) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 27B,C) and ventral view (Figure 27A), the head only in dorsal view. No camouflaging cloak is apparent. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 27D). Prominent protrusions on the back are apparent. The trunk appears damaged; the length can therefore not be measured.
- (31)
- Specimen 4850 (PED 0667) is preserved in Myanmar amber. It is only accessible in dorsal view (Figure 28A,B). No camouflaging cloak is apparent. The head is roughly rectangular (Figure 28C). The antennae are not accessible. No tarsi are accessible; hence, it remains unclear if they bear empodia. Prominent protrusions on the back are apparent. The overall length of the larva is 3.61 mm.
- (32)
- Specimen 4851 (PED 0696) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 29A,B) and ventral view (Figure 29C). A prominent camouflaging cloak is present. The antenna bears a prominent seta distally. The labial palps appear rather broad (Figure 29D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 29E). Prominent protrusions on the back are apparent. The exact length cannot be measured.
- (33)
- Specimen 4852 (PED 0715) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 28F) and ventral view (Figure 28D,E). The head is separated from the trunk (Figure 28G). Many details of the trunk region are concealed by numerous bubbles. No clear camouflaging cloak is apparent. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 28H). Prominent protrusions on the back are apparent. The exact length cannot be measured.
- (34)
- Specimen 4854 (PED 0782) is preserved in Myanmar amber. It is only accessible in dorsal view (Figure 30A,B). No clear camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 30C,D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium. Prominent long protrusions on the back are apparent. The overall length of the larva is 0.95 mm.
- (35)
- Specimen 4855 (PED 0793) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 31C,D) and ventral view (Figure 31A,B). A prominent camouflaging cloak is present, concealing many details of the animal. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 31E). Prominent protrusions on the back are apparent. The overall length of the larva is 0.87 mm.
- (36)
- Specimen 4856 (PED 0807) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 31H) and ventral view (Figure 31F,G). A prominent camouflaging cloak is present. The head is roughly rectangular (Figure 31I). The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium. Prominent protrusions on the back are apparent. The overall length of the larva is 2.16 mm.
- (37)
- Specimen 4853 (PED 0754) is preserved in Myanmar amber. It is only accessible in dorsal view (Figure 32F,G). No clear camouflaging cloak is apparent. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 32H). Prominent long protrusions on the back are apparent. The overall length of the larva is 0.82 mm.
- (38)
- Specimen 4857 (PED 0837) is preserved in Myanmar amber. It is accessible in a ventral (Figure 32A,B) and anterior view (Figure 32C), yet the ventral view is partly concealed by impurities of the amber. The head is partly damaged (Figure 32E). No camouflaging cloak is apparent. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 32D). Prominent protrusions on the back are apparent. The overall length of the larva is 2.40 mm.
- (39)
- Specimen 4858 (PED 0901) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 33A,B) and ventral view (Figure 33C). A prominent camouflaging cloak is present. The head is rather short and broad (Figure 33D). The antenna bears a prominent seta distally (Figure 33E). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 33F). Prominent protrusions on the back are apparent. The exact length cannot be measured.
- (40)
- Specimen 4859 (PED 0952) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 34A,B) and ventral view (Figure 34C), but is partly concealed by bubbles. No camouflaging cloak is apparent. The antenna bears a prominent, but short seta distally (Figure 34D). The trunk appendages, bear claws, but lack empodia (Figure 34E). Prominent, but short protrusions on the back are apparent. The overall length of the larva is 2.53 mm.
- (41)
- Specimen 4860 (PED 0983) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 35A,B) and lateral to ventro-lateral view (Figure 35C). A prominent camouflaging cloak is present. The antenna bears a prominent seta distally (Figure 35D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 35E). Prominent long protrusions on the back are apparent. The overall length of the larva is 0.98 mm.
- (42)
- Specimen 4861 (PED 0989a) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 36A,B) and ventral view (Figure 36C), dorsally the posterior trunk is concealed (Figure 36E), ventrally only the head and part of the thorax are accessible. A prominent camouflaging cloak is present. The head is roughly rectangular (Figure 36C). The antenna has no prominent seta distally. No tarsi are accessible; hence, it remains unclear if they bear empodia. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 36D). Prominent protrusions on the back are apparent. The overall length of the larva is 3.32 mm.
- (43)
- Specimen 4862 (PED 0989b) is preserved in Myanmar amber, in the same amber piece as PED 0989a. It is accessible only in a dorso-lateral view (Figure 36F,G). No camouflaging cloak is apparent. The head bears stemmata. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium. Prominent protrusions on the back are apparent. The overall length of the larva is 1.23 mm.
- (44)
- Specimen 4863 (PED 1000) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 37A,B) and ventral view (Figure 37C), yet dorsally some structures conceal certain aspects. No camouflaging cloak is apparent. The head is rectangular (Figure 37D). The distal tips of the antennae are not well accessible. No tarsi are accessible; hence, it remains unclear if they bear empodia. Prominent protrusions on the back are apparent. The overall length of the larva is 1.29 mm.
- (45)
- Specimen 4864 (PED 1223) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 9K) and ventral view (Figure 9I,J). A whitish structure conceals part of the thorax in the ventral view (Figure 9I,J). No camouflaging cloak is apparent. The antenna bears no prominent seta distally (Figure 9L). The trunk appendages lack empodia (Figure 9M). No prominent protrusions on the back are apparent. The overall length of the larva is 0.88 mm.
- (46)
- Specimen 4865 (PED 1229a) is preserved in Myanmar amber. It is accessible only in a ventral view (Figure 38A,B). No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 38C). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 38D). Prominent long protrusions on the back are apparent. The overall length of the larva is 2.34 mm.
- (47)
- Specimen 4866 (PED 1229b) is preserved in Myanmar amber, in the same amber piece as PED 1229a. It is only accessible in ventral view (Figure 38E,F). The thorax and abdomen are partly deformed. It remains partly unclear whether a camouflaging cloak is present. The antenna bears a prominent seta distally (Figure 38G). Each tarsus of the trunk appendages carries a trumpet-shaped empodium. Prominent long protrusions on the back are apparent. The overall length of the larva is 1.24 mm.
- (48)
- Specimen 4867 (PED 1258a) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 39A,B) and ventral view (Figure 39C). A prominent camouflaging cloak is present. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 39D). Prominent protrusions on the back are apparent. The overall length of the larva is 1.78 mm.
- (49)
- Specimen 4868 (PED 1258b) is preserved in Myanmar amber, in the same amber piece as PED 1258a. It is accessible in a dorsal (Figure 39G) and ventral view (Figure 39E,F). A prominent camouflaging cloak is present, but more towards the posterior (Figure 39G,H). The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 39I). Prominent protrusions on the back are apparent. The overall length of the larva is 1.94 mm.
- (50)
- Specimen 4869 (PED 1287) is preserved in Myanmar amber. It is only accessible in a dorsal view (Figure 40A,B). Many bubbles surround the animal, making it unclear whether a camouflaging cloak is present. The distal tip of the antenna is not accessible (Figure 40C). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 40D). Prominent protrusions on the back are apparent. The overall length of the larva is 1.37 mm.
- (51)
- Specimen 4870 (PED 1301) is preserved in Myanmar amber. It is accessible in a dorso-lateral (Figure 41C) and ventral view (Figure 41A,B). The stylets are quite long, about as long as the trunk. No camouflaging cloak is apparent. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 41D). No prominent protrusions on the back are apparent, but there are setae along the trunk. The overall length of the larva is 1.45 mm.
- (52)
- Specimen 4871 (PED 1311) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 40E,F) and ventral view (Figure 40G), but is ventrally less well accessible. Some material at the posterior end may be the remains of a camouflaging cloak, yet this remains unclear. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 40H). Prominent protrusions on the back are apparent. The exact length cannot be measured.
- (53)
- Specimen 4872 (PED 1322) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 42A,B) and ventral view (Figure 42C). The trunk is separated into several pieces and spread through the amber piece. A prominent camouflaging cloak is present. The antenna bears a prominent seta distally (Figure 42D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 42E). Prominent protrusions on the back are apparent (Figure 42F). The exact length cannot be measured.
- (54)
- Specimen 4873 (PED 1323) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 43C,D) and ventral view (Figure 43A,B), but is ventrally partly concealed by impurities in the amber. A prominent camouflaging cloak is present. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 43E). Prominent protrusions on the back are apparent. The overall length of the larva is 2.37 mm.
- (55)
- Specimen 4874 (PED 1333) is preserved in Myanmar amber. It is accessible in a ventral (Figure 44A,B,D) and lateral view (Figure 44C). A prominent camouflaging cloak is present. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 44E). Prominent protrusions on the back are apparent. The overall length of the larva is 4.91 mm.
- (56)
- Specimen 4875 (PED 1335) is preserved in Myanmar amber. It is accessible in a lateral (Figure 45A–C) and partly in a dorsal view; the head, especially, is accessible in dorsal view (Figure 45D). A prominent camouflaging cloak is present. The antenna bears a prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 45E). Prominent protrusions on the back are apparent. The overall length of the larva is 4.73 mm.
- (57)
- Specimen 4876 (Weiterschan BuB 11) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 46A,B) and ventral view (Figure 46C). A darker object conceals part of the head appendages (Figure 46D). No camouflaging cloak is apparent. The antenna bears a prominent seta distally (Figure 46D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 46E). Prominent protrusions on the back are apparent. The overall length of the larva is 5.79 mm.
- (58)
- Specimen 4877 (Weiterschan BuB 31) is preserved in Myanmar amber. It is accessible in a dorsal (Figure 47A,B) and ventral view (Figure 47C). A prominent camouflaging cloak is present, largely concealing details of the animal. The antenna bears a prominent seta distally (Figure 47D). Each tarsus of the trunk appendages carries a trumpet-shaped empodium. Prominent protrusions on the back are apparent. The exact length cannot be measured.
- (59)
- Specimen 4703 (SMF Be 2021) is preserved in Baltic amber. It is accessible in a dorsal (Figure 48C), ventral (Figure 48A,B) and antero-ventral view (Figure 48D,E). Two round objects conceal the tips of the stylets ventrally. A prominent camouflaging cloak is present. The antenna bears a prominent seta distally (Figure 48F). Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 48G). Prominent protrusions on the back are apparent. The overall length of the larva is 1.66 mm.
- (60)
- Specimen 4702 (CCGG 7615) is preserved in Baltic amber. It is accessible in a dorsal view (Figure 49A,B). A prominent camouflaging cloak is present, largely concealing details of the animals. The head is rectangular. The antenna bears no prominent seta distally. No tarsi are accessible; hence, it remains unclear if they bear empodia. Prominent protrusions on the back are apparent. The exact length cannot be measured.
- (61)
- Specimen 4704 (SMF Be 1861) is preserved in Baltic amber. It is accessible in a dorsal view (Figure 49C,D). No clear camouflaging cloak is apparent. The antenna bears no prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium. No prominent protrusions on the back are apparent, but there are setae along the trunk. The overall length of the larva is 1.31 mm.
- (62)
- Specimen 4753 (CCHH 1786-3) is preserved in Baltic amber. It is accessible in dorsal (Figure 50A,B) and ventral view (Figure 50C). No camouflaging cloak is apparent. The head is well accessible in dorsal view (Figure 50D). The antenna bears no prominent seta distally. Each tarsus of the trunk appendages carries a trumpet-shaped empodium (Figure 50E). No prominent protrusions on the back are apparent. The overall length of the larva is 5.45 mm.
3.2. Shape Analysis
3.3. Size-Shape Correlation
4. Discussion
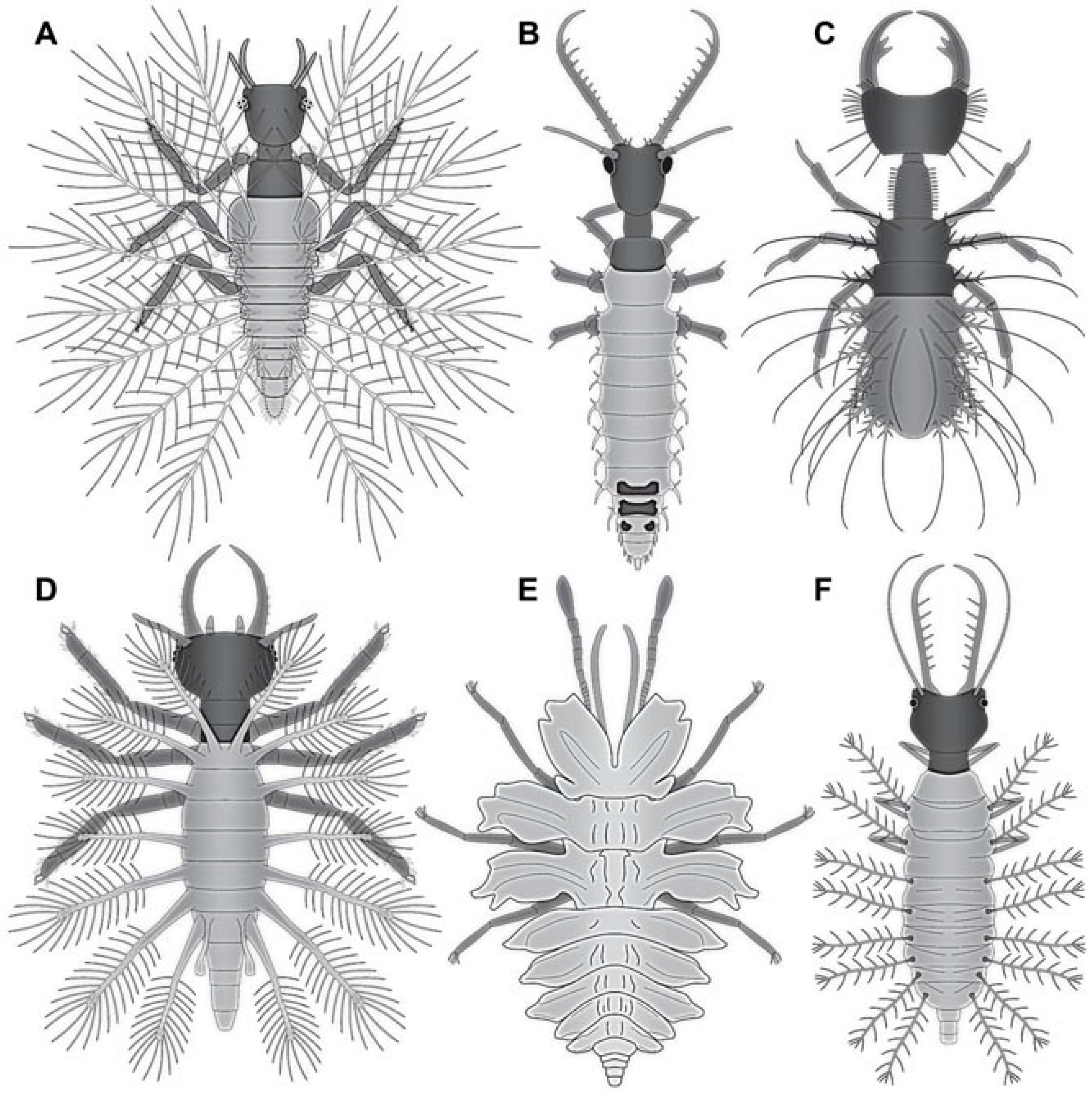
4.1. What Is an Aphidlion?
4.2. Identity of the Fossils
4.3. Differentiation of the New Cretaceous Material into Coarse Morphotypes
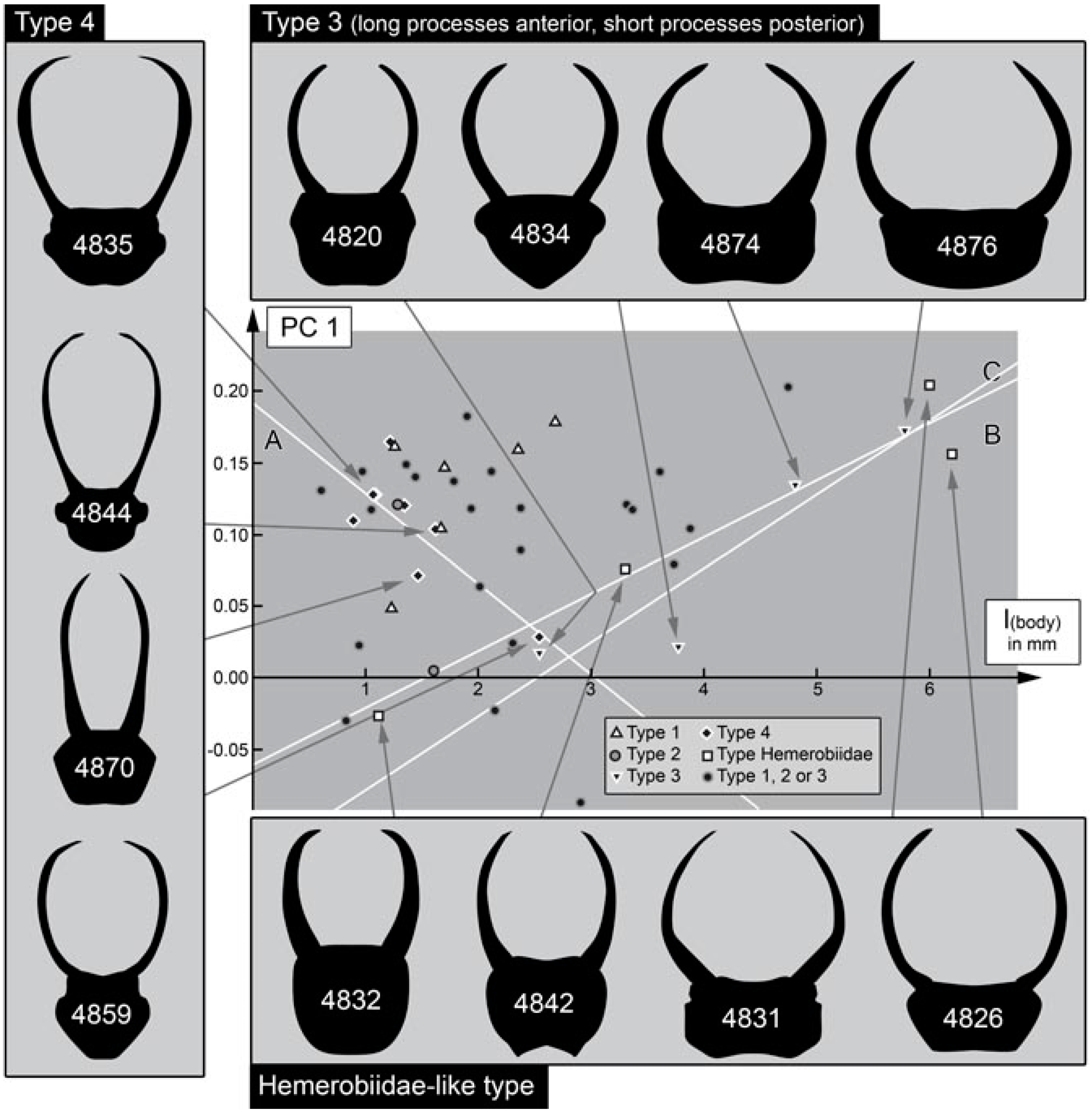
- (1)
- Larvae of type 1 have relatively long protrusions on the anterior and posterior trunk, which are all about the same length. Many of the not-that-well-preserved specimens may represent type 1 larvae, yet they cannot be reliably interpreted.
- (2)
- Larvae of type 2 have relatively long protrusions on the anterior trunk, but even longer ones on the posterior trunk.
- (3)
- Larvae of type 3 have relatively long protrusions on the anterior trunk, but rather short ones on the posterior trunk.
4.4. Identity of the Fossils: Stylet Shape and Head Shape
4.5. Identity of the Fossils: Other Head Appendages
4.6. Identity of the Fossils: Empodia and Claws
4.7. Identity of the Fossils: Processes on the Back and Camouflaging Cloak
4.8. Identity Summary
4.9. Ontogenetic Effects
4.10. Quantitative Comparison: Coarser Frame
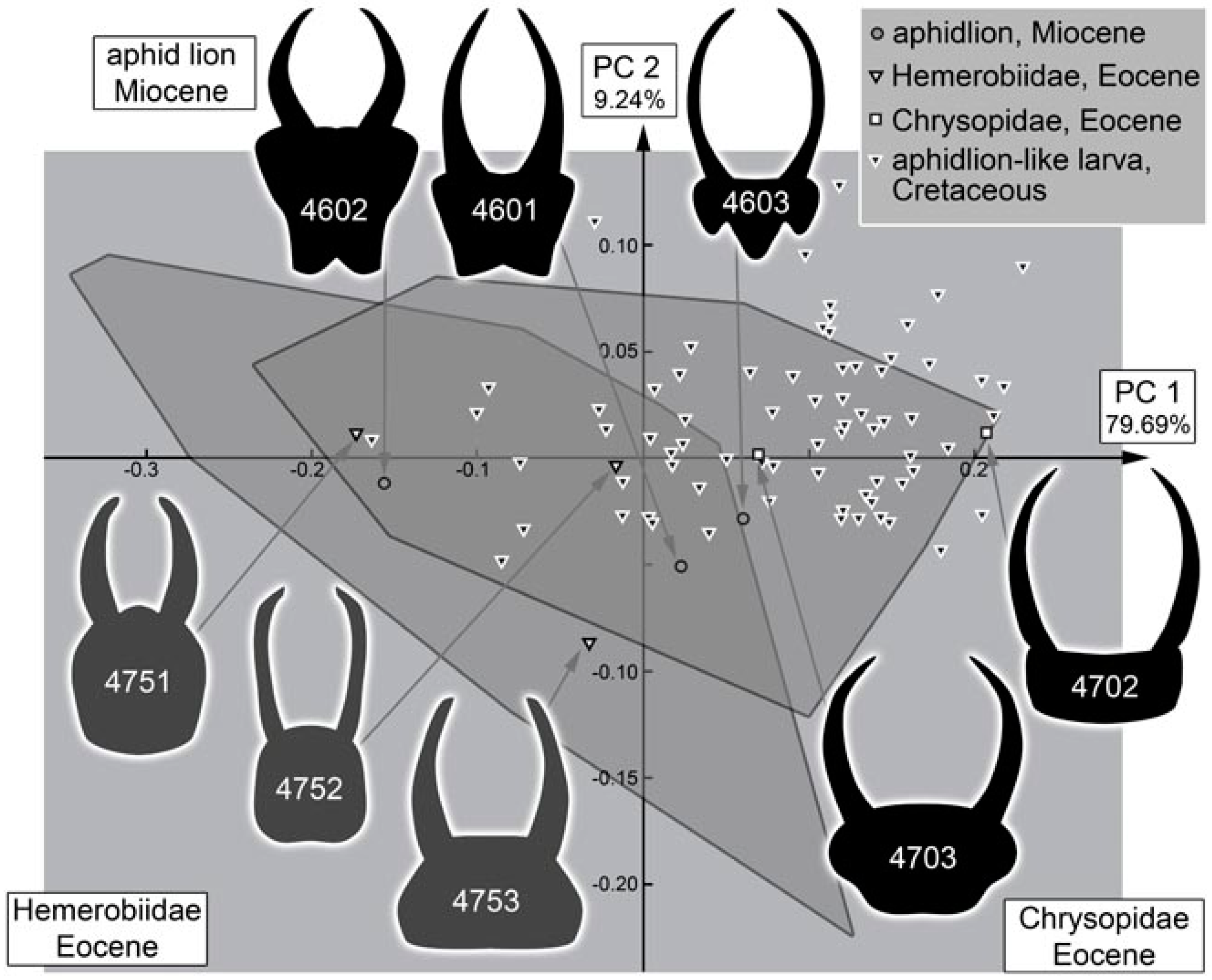
4.11. Quantitative Comparison: Morphospace of Changes of Green Lacewings through Time
4.12. Quantitative Comparison: Morphospace of Changes of Brown Lacewings through Time
4.13. Lacewing Diversity through Time: The Larval Side
4.14. Camouflaging Lacewing Larvae
5. Conclusions
- (1)
- (2)
- A loss of many further forms, especially of camouflaging larvae; yet this loss is factually compensated by:
- (3)
- A diversification of the modern lineages of green lacewings and their larval forms.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aspöck, U.; Aspöck, H. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia 2007, 20 (Kat. Oberöst. Landesmus. N. Ser. 66), 451–516. Available online: https://www.zobodat.at/pdf/DENISIA_0020_0451-0516.pdf (accessed on 6 February 2022).
- Labandeira, C.C. A compendium of fossil insect families. Milwaukee Public Mus. Contrib. Biol. Geol. 1994, 88, 11–16. [Google Scholar]
- Oswald, J.D.; Machado, R.J. Biodiversity of the Neuropterida (Insecta: Neuroptera, Megaloptera, and Raphidioptera). In Insect Biodiversity: Science and Society, 1st ed.; Foottit, R.G., Adler, P.H., Eds.; Wiley: Hoboken, NY, USA, 2018; Volume II, pp. 627–672. [Google Scholar] [CrossRef]
- Khramov, A.V.; Bashkuev, A.S.; Lukashevich, E.D. The fossil record of long-proboscid nectarivorous insects. Entomol. Rev. 2020, 100, 881–968. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Yang, Q.; Santiago-Blay, J.A.; Hotton, C.L.; Monteiro, A.; Wang, Y.-J.; Goreva, Y.; Shih, C.K.; Siljeström, S.; Rose, T.R.; et al. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152893. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, X.; Zhang, Q.; Chen, J.; Zheng, X.; Zhang, W.; Liu, X.; Wang, B. High niche diversity in Mesozoic pollinating lacewings. Nat. Commun. 2018, 9, 3793. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Speranza, M.; Wierzchos, J.; Ascaso, C.; Engel, M.S. Early evolution and ecology of camouflage in insects. Proc. Natl. Acad. Sci. USA 2012, 109, 21414–21419. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Engel, M.S. A defensive behavior and plant-insect interaction in Early Cretaceous amber—The case of the immature lacewing Hallucinochrysa diogenesi. Arthropod Struct. Dev. 2016, 45, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Fuente, R.; Peñalver, E.; Azar, D.; Engel, M.S. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Sci. Rep. 2018, 8, 16663. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Fuente, R.; Engel, M.S.; Azar, D.; Peñalver, E. The hatching mechanism of 130-million-year-old insects: An association of neonates, egg shells and egg bursters in Lebanese amber. Palaeontology 2019, 62, 547–559. [Google Scholar] [CrossRef]
- Wang, B.; Xia, F.; Engel, M.S.; Perrichot, V.; Shi, G.; Zhang, H.; Chen, J.; Jarzembowski, E.A.; Wappler, T.; Rust, J. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2016, 2, e1501918. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Winterton, S.L.; Breitkreuz, L.C.; Engel, M.S. Early morphological specialization for insect-spider associations in Mesozoic lacewings. Curr. Biol. 2016, 26, 1590–1594. [Google Scholar] [CrossRef]
- Liu, X.; Shi, G.; Xia, F.; Lu, X.; Wang, B.; Engel, M.S. Liverwort mimesis in a Cretaceous lacewing larva. Curr. Biol. 2018, 28, 1475–1481. [Google Scholar] [CrossRef]
- Badano, D.; Engel, M.S.; Basso, A.; Wang, B.; Cerretti, P. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nat. Commun. 2018, 9, 3257. [Google Scholar] [CrossRef] [PubMed]
- Badano, D.; Fratini, M.; Maugeri, L.; Palermo, F.; Pieroni, N.; Cedola, A.; Haug, J.T.; Weiterschan, T.; Velten, J.; Mei, M.; et al. X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Syst. Entomol. 2021, 46, 672–684. [Google Scholar] [CrossRef]
- Haug, C.; Herrera-Flórez, A.F.; Müller, P.; Haug, J.T. Cretaceous chimera—An unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution. Palaeodiversity 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Haug, J.T.; Müller, P.; Haug, C. A 100-million-year old predator: A fossil neuropteran larva with unusually elongated mouthparts. Zool. Lett. 2019, 5, 29. [Google Scholar] [CrossRef]
- Haug, J.T.; Müller, P.; Haug, C. A 100-million-year old slim insectan predator with massive venom-injecting stylets-a new type of neuropteran larva from Burmese amber. Bull. Geosci. 2019, 94, 431–440. [Google Scholar] [CrossRef]
- Haug, J.T.; Pazinato, P.G.; Haug, G.T.; Haug, C. Yet another unusual new type of lacewing larva preserved in 100-million-year old amber from Myanmar. Riv. Ital. Paleontol. Stratigr. 2020, 126, 821–832. [Google Scholar] [CrossRef]
- Haug, J.T.; Baranov, V.; Müller, P.; Haug, C. New extreme morphologies as exemplified by 100 million-year-old lacewing larvae. Sci. Rep. 2021, 11, 20432. [Google Scholar] [CrossRef] [PubMed]
- Haug, G.T.; Haug, C.; Pazinato, P.G.; Braig, F.; Perrichot, V.; Gröhn, C.; Müller, P.; Haug, J.T. The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time. Palaeontol. Electron. 2020, 23, a39. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; van der Wal, S.; Müller, P.; Haug, J.T. Split-footed lacewings declined over time: Indications from the morphological diversity of their antlion-like larvae. PalZ 2021. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; Haug, J.T. The morphological diversity of spoon-winged lacewing larvae and the first possible fossils from 99 million-year-old Kachin amber, Myanmar. Palaeodiversity 2021, 14, 133–152. [Google Scholar] [CrossRef]
- Haug, G.T.; Baranov, V.; Wizen, G.; Pazinato, P.G.; Müller, P.; Haug, C.; Haug, J.T. The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bull. Geosci. 2021, 96, 431–457. [Google Scholar] [CrossRef]
- Haug, J.T.; Haug, G.T.; Zippel, A.; van der Wal, S.; Müller, P.; Gröhn, C.; Wunderlich, J.; Hoffeins, C.; Hoffeins, H.-W.; Haug, C. Changes in the morphological diversity of larvae of lance lacewings, mantis lacewings and their closer relatives over 100 million years. Insects 2021, 12, 860. [Google Scholar] [CrossRef]
- Gepp, J. Erforschungsstand der Neuropteren-Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten). In Proceedings of the 1st International Symposium on Neuropterology, Graz, Austria, 22–26 September 1980; 1984; pp. 183–239. Available online: https://www.zobodat.at/pdf/MONO-ENT-NEURO_MEN1_0183-0239.pdf (accessed on 6 February 2022).
- Weed, C.M. The golden-eye or lace-wing fly. Am. Nat. 1897, 31, 500–502. [Google Scholar] [CrossRef]
- Townsend, L.H. Lacewings and their allies. Sci. Mon. 1939, 48, 350–357. [Google Scholar]
- Oswald, J.D.; Contreras-Ramos, A.; Penny, N.D. Neuroptera (Neuropterida). In Biodiversidad, Taxonomía y Biogeografía de Artrópodos de México: Hacia una Síntesis de su Conocimiento; Bousquets, J., Morone, J.J., Eds.; Universidad Nacional Autónoma de México: Distrito Federal, Mexico, 2002; Volume 3, pp. 559–581. [Google Scholar]
- Aspöck, U. Phylogeny of the Neuropterida (Insecta: Holometabola). Zool. Scr. 2002, 31, 51–55. [Google Scholar] [CrossRef]
- Aspöck, U.; Aspöck, H. Phylogenetic relevance of the genital sclerites of Neuropterida (Insecta: Holometabola). Syst. Entomol. 2008, 33, 97–127. [Google Scholar] [CrossRef]
- Garzón-Orduña, I.J.; Menchaca-Armenta, I.; Contreras-Ramos, A.; Liu, X.Y.; Winterton, S.L. The phylogeny of brown lacewings (Neuroptera: Hemerobiidae) reveals multiple reductions in wing venation. BMC Evol. Biol. 2016, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Jandausch, K.; Beutel, R.G.; Bellstedt, R. The larval morphology of the spongefly Sisyra nigra (Retzius, 1783) (Neuroptera: Sisyridae). J. Morphol. 2019, 280, 1742–1758. [Google Scholar] [CrossRef]
- Vasilikopoulos, A.; Misof, B.; Meusemann, K.; Lieberz, D.; Flouri, T.; Beutel, R.G.; Niehuis, O.; Wappler, T.; Rust, J.; Peters, R.S.; et al. An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evol. Biol. 2020, 20, 64. [Google Scholar] [CrossRef]
- Wang, Y.-y.; Liu, X.-y.; Garzón-Orduña, I.J.; Winterton, S.L.; Yan, Y.; Aspöck, U.; Aspöck, H.; Yang, D. Mitochondrial phylogenomics illuminates the evolutionary history of Neuropterida. Cladistics 2017, 33, 617–636. [Google Scholar] [CrossRef]
- Winterton, S.L.; Lemmon, A.R.; Gillung, J.P.; Garzón, I.J.; Badano, D.; Bakkes, D.K.; Breitkreuz, L.C.V.; Engel, M.S.; Lemmon, E.M.; Liu, X.-Y.; et al. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Syst. Entomol. 2017, 43, 330–354. [Google Scholar] [CrossRef]
- Weihrauch, F. The significance of Brown and Green Lacewings as aphid predators in the special crop hops (Neuroptera: Hemerobiidae, Chrysopidae). Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2012, 18, 587–590. [Google Scholar]
- Monserrat, V.J.; Oswald, J.D.; Tauber, C.A.; Díaz-Aranda, L.M. Recognition of larval Neuroptera. In Lacewings in the Crop Environment; McEwen, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 43–81. [Google Scholar] [CrossRef]
- Bänsch, R. Das Beutefangverhalten der aphidivoren Hemerobiidenlarven. Zool. Anz. 1964, 173, 278–281. [Google Scholar]
- Tauber, C.A. Systematics of North American chrysopid larvae: Chrysopa carnea group (Neuroptera). Can. Entomol. 1974, 106, 1133–1153. [Google Scholar] [CrossRef]
- Badano, D.; Aspöck, U.; Aspöck, H.; Cerretti, P. Phylogeny of Myrmeleontiformia based on larval morphology (Neuropterida: Neuroptera). Syst. Entomol. 2017, 42, 94–117. [Google Scholar] [CrossRef]
- Henry, C.S. Some aspects of the external morphology of larval owlflies (Neuroptera: Ascalaphidae), with particular reference to Ululodes and Ascaloptynx. Psyche J. Entomol. 1976, 83, 071439. [Google Scholar] [CrossRef]
- MacLeod, E.G. A Comparative Morphological Study of the Head Capsule and Cervix of Larval Neuroptera (Insecta). Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1964. [Google Scholar]
- Zimmermann, D.; Randolf, S.; Aspöck, U. From chewing to sucking via phylogeny–From sucking to chewing via ontogeny: Mouthparts of Neuroptera. In Insect Mouthparts, Zoological Monographs 5; Krenn, H.W., Ed.; Springer: Berlin, Germany, 2019; pp. 361–385. [Google Scholar]
- Tauber, C.A.; Tauber, M.J.; Albuquerque, G.S. Debris-carrying in larval Chrysopidae unraveling its evolutionary history. Ann. Entomol. Soc. Am. 2014, 107, 295–314. [Google Scholar] [CrossRef]
- Eisner, T.; Carrel, J.E.; Van Tassell, E.; Hoebeke, E.R.; Eisner, M. Construction of a defensive trash packet from sycamore leaf trichomes by a chrysopid larva (Neuroptera: Chrysopidae). Proc. Entomol. Soc. Wash. 2002, 104, 437–446. [Google Scholar]
- Eisner, T.; Hicks, K.; Eisner, M.; Robson, D.S. “Wolf-in-sheep’s-clothing” strategy of a predaceous insect larva. Science 1978, 199, 790–794. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Haug, C.; Haug, G.T.; Baranov, V.A.; Solórzano-Kraemer, M.M.; Haug, J.T. An owlfly larva preserved in Mexican amber and the Miocene record of lacewing larvae. Boletín Soc. Geológica Mex. 2021, 73, A271220. [Google Scholar] [CrossRef]
- Wu, R.J.C. Secrets of a Lost World: Dominican Amber and Its Inclusions; Privately Published: Santo Domingo, Dominican Republic, 1996. [Google Scholar]
- Haug, J.T.; Kiesmüller, C.; Haug, G.T.; Haug, C.; Hörnig, M.K. A fossil aphidlion preserved together with its prey in 40 million-year-old Baltic amber. Palaeobiodivers. Palaeoenviron. 2022. [Google Scholar] [CrossRef]
- Makarkin, V.N.; Wedmann, S.; Weiterschan, T. First record of a fossil larva of Hemerobiidae (Neuroptera) from Baltic amber. Zootaxa 2012, 3417, 53–63. [Google Scholar] [CrossRef]
- Weitschat, W.; Berning, B.; Podenas, S. Jäger, Gejagte, Parasiten, blinde Passagiere–Momentaufnahmen aus dem Bernsteinwald. Denisia 2009, 26, 243–256. [Google Scholar]
- Gröhn, C. Einschlüsse im Baltischen Bernstein; Wachholtz Murmann Publishers: Kiel, Germany, 2015. [Google Scholar]
- Xia, F.; Yang, G.; Zhang, Q.; Shi, G.; Wang, B. Amber: Life through Time and Space; Science Press: Beijing, China, 2015. [Google Scholar]
- Zhang, W.W. Frozen Dimensions of the Fossil Insects and Other Invertebrates in Amber; Chongqing University Press: Chonqing, China, 2017. [Google Scholar]
- Bengtson, S. Teasing fossils out of shales with cameras and computers. Palaeontol. Electron. 2000, 3, 14. [Google Scholar]
- Schaarschmidt, F. Pflanzenfossilien in ungewöhnlichem Licht. Nat. Mus. 1973, 103, 247–253. [Google Scholar]
- Kerp, H.; Bomfleur, B. Photography of plant fossils—New techniques, old tricks. Rev. Palaeobot. Palynol. 2011, 166, 117–151. [Google Scholar] [CrossRef]
- Iwata, H.; Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Büsse, S.; Büscher, T.H.; Heepe, L.; Gorb, S.N.; Stutz, H.H. Sand-throwing behaviour in pit-building antlion larvae: Insights from finite-element modelling. J. R. Soc. Interface 2021, 18, 20210539. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.R.; Worley, A.; Falkenberg, M.; Sendova-Franks, A.B.; Christensen, K. Digging the optimum pit: Antlions, spirals and spontaneous stratification. Proc. R. Soc. B 2019, 286, 20190365. [Google Scholar] [CrossRef] [PubMed]
- Badano, D.; Di Giulio, A.; Aspöck, H.; Aspöck, U.; Cerretti, P. Burrowing specializations in a lacewing larva (Neuroptera: Dilaridae). Zool. Anz. 2021, 293, 247–256. [Google Scholar] [CrossRef]
- Tillyard, R.J. The life-history of the Australian moth-lacewing, Ithone fusca, Newman (Order Neuroptera Planipennia). Bull. Entomol. Res. 1922, 13, 205–223. [Google Scholar] [CrossRef]
- Tröger, E.J. Die Larve von Nemoptera coa (Linnaeus, 1758).(Neuropteroidea, Planipennia). Dtsch. Entomol. Z. 1993, 40, 357–368. [Google Scholar] [CrossRef]
- Buys, S.C. Observations on the biology of Anchieta fumosella (Westwood 1867) (Neuroptera Mantispidae) from Brazil. Trop. Zool. 2008, 21, 91–95. [Google Scholar]
- Maia-Silva, C.; Hrncir, M.; Koedam, D.; Pires Machado, R.J.; Imperatriz Fonseca, V.L. Out with the garbage: The parasitic strategy of the mantisfly Plega hagenella mass-infesting colonies of the eusocial bee Melipona subnitida in northeastern Brazil. Naturwissenschaften 2013, 100, 101–105. [Google Scholar] [CrossRef] [PubMed]
- McKeown, K.C.; Mincham, V.H. The biology of an Australian mantispid (Mantispa vittata Guérin). Aust. Zool. 1948, 11, 207–224. [Google Scholar]
- Schremmer, F. Beitrag zur Entwicklungsgeschichte und zum Kokonbau von Mantispa styriaca. Z. Arb. Österr. Entomol. 1983, 35, 21–26. [Google Scholar]
- Snyman, L.P.; Ohl, M.; Pirk, C.W.W.; Sole, C.L. A review of the biology and biogeography of Mantispidae (Neuroptera). Insect Syst. Evol. 2020, 52, 125–166. [Google Scholar] [CrossRef]
- Engel, M.S.; Grimaldi, D.A. The neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). Am. Mus. Novit. 2007, 3587, 1–58. Available online: https://digitallibrary.amnh.org/handle/2246/5880 (accessed on 5 March 2022). [CrossRef]
- Haug, J.T.; Baranov, V.; Schädel, M.; Müller, P.; Gröhn, C.; Haug, C. Challenges for understanding lacewings: How to deal with the incomplete data from extant and fossil larvae of Nevrorthidae? (Neuroptera). Fragm. Entomol. 2020, 52, 137–168. [Google Scholar] [CrossRef]
- MacLeod, E.G. The immature stages of Boriomyia fidelis (Banks) with taxonomic notes on the affinities of the genus Boriomyia (Neuroptera: Hemerobiidae). Psyche J. Entomol. 1960, 67, 26–40. [Google Scholar] [CrossRef][Green Version]
- Dahl, F. Beiträge zur Kenntniss des Baues und der Funktionen der Insektenbeine. Arch. Nat. 1884, 50, 163–166. [Google Scholar]
- Zippel, A.; Kiesmüller, C.; Haug, G.T.; Müller, P.; Weiterschan, T.; Haug, C.; Hörnig, M.K.; Haug, J.T. Long-headed predators in Cretaceous amber—Fossil findings of an unusual type of lacewing larva. Palaeoentomology 2021, 4, 475–498. [Google Scholar] [CrossRef]
- Beutel, R.G.; Friedrich, F.; Aspöck, U. The larval head of Nevrorthidae and the phylogeny of Neuroptera (Insecta). Zool. J. Linn. Soc. 2010, 158, 533–562. [Google Scholar] [CrossRef]
- Smith, R.C. The trash-carrying habit of certain lace wing larvae. Sci. Mon. 1926, 23, 265–267. [Google Scholar]
- Withycombe, C.L. The life-history of Hemerobius stigma. Steph. Entomol. 1922, 55, 97–99. [Google Scholar]
- New, T.R. Neuroptera. In The Insects of Australia: A Textbook for Students and Research Workers; Commonwealth Scientific and Industrial Research Organisation, Ed.; Melbourne University Press: Victoria, Australia, 1991; Volume 2, pp. 525–542. [Google Scholar]
- Díaz-Aranda, L.; Monserrat, V.J. On the larval stages of genus Suarius Navás 1914 in Europe (Neuroptera: Chrysopidae). Dtsch. Entomol. Z. 1996, 43, 89–97. [Google Scholar] [CrossRef]
- Brauer, F.M. Larve von Hypochrysa nobilis Heyd. Verh. Kais. Königl. Zool.-Bot. Ges. Wien 1867, 17, 27–30. [Google Scholar]
- Monserrat, V.J. Sobre los Neurópteros de las Islas Canarias, III. Chrysopa flaviceps (Brullé, 1838) (Neur., Plan., Chrysopidae). Boletín Asoc. Española Entomol. 1982, 6, 113–119. [Google Scholar]
- Monserrat, V.J. Nuevos datos sobre algunas especies de crisópidos (Insecta: Neuroptera: Chrysopidae). Heteropterus Rev. Entomol. 2008, 8, 171–196. [Google Scholar]
- Principi, M.M. Contributi allo studio dei Neurotteri italiani. XIII. Studio morfologico etologico e sistematico di un gruppo omogeneo di specie del Gen. Chrysopa Leach (C flavifrons Brauer, prasina Burm. e clathrata Schn.). Boll. Dell’istituto Mus. Entomol. Univ. Degli Studi Bologna 1956, 21, 319–410. [Google Scholar]
- Makarkin, V.N.; Gröhn, C. The first unusual Hemerobiidae (Neuroptera) from mid-Cretaceous Burmese amber. Cretac. Res. 2020, 106, 104206. [Google Scholar] [CrossRef]
- Hörnig, M.K.; Kiesmüller, C.; Müller, P.; Haug, C.; Haug, J.T. A new glimpse on trophic interactions of 100-million-year old lacewing larvae. Acta Palaeontol. Pol. 2020, 65, 777–786. [Google Scholar] [CrossRef]
- Kiesmüller, C.; Haug, J.T.; Müller, P.; Hörnig, M.K. Debris-carrying behaviour of bark lice immatures preserved in 100 million years old amber. PalZ 2021. [Google Scholar] [CrossRef]
















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haug, J.T.; Linhart, S.; Haug, G.T.; Gröhn, C.; Hoffeins, C.; Hoffeins, H.-W.; Müller, P.; Weiterschan, T.; Wunderlich, J.; Haug, C. The Diversity of Aphidlion-like Larvae over the Last 130 Million Years. Insects 2022, 13, 336. https://doi.org/10.3390/insects13040336
Haug JT, Linhart S, Haug GT, Gröhn C, Hoffeins C, Hoffeins H-W, Müller P, Weiterschan T, Wunderlich J, Haug C. The Diversity of Aphidlion-like Larvae over the Last 130 Million Years. Insects. 2022; 13(4):336. https://doi.org/10.3390/insects13040336
Chicago/Turabian StyleHaug, Joachim T., Simon Linhart, Gideon T. Haug, Carsten Gröhn, Christel Hoffeins, Hans-Werner Hoffeins, Patrick Müller, Thomas Weiterschan, Jörg Wunderlich, and Carolin Haug. 2022. "The Diversity of Aphidlion-like Larvae over the Last 130 Million Years" Insects 13, no. 4: 336. https://doi.org/10.3390/insects13040336
APA StyleHaug, J. T., Linhart, S., Haug, G. T., Gröhn, C., Hoffeins, C., Hoffeins, H.-W., Müller, P., Weiterschan, T., Wunderlich, J., & Haug, C. (2022). The Diversity of Aphidlion-like Larvae over the Last 130 Million Years. Insects, 13(4), 336. https://doi.org/10.3390/insects13040336






