Knockdown Resistance Mutations in the Voltage-Gated Sodium Channel of Aedes aegypti (Diptera: Culicidae) in Myanmar
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
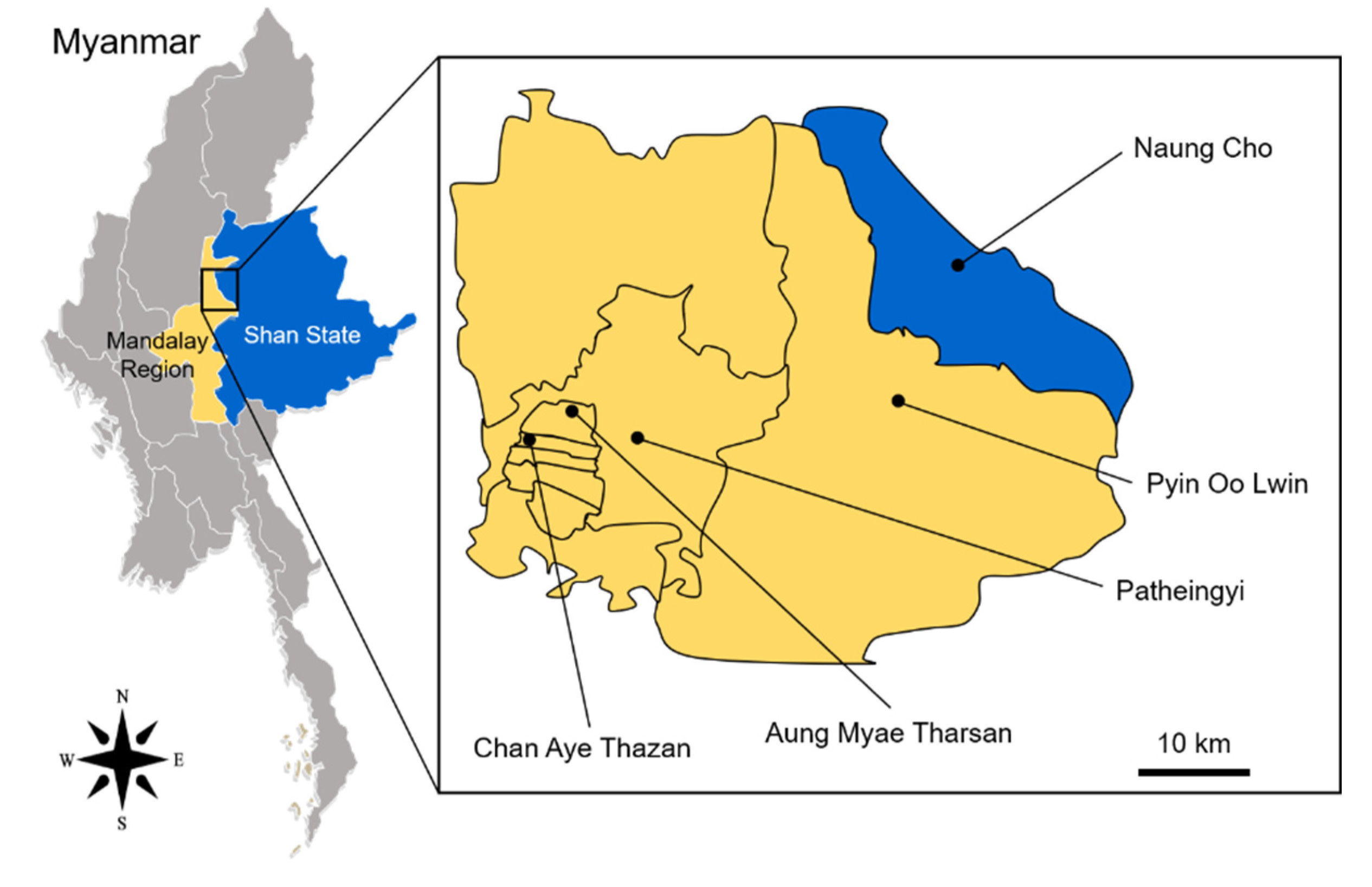
2.1. Mosquito Collection
2.2. DNA Extraction and Gene Amplification
2.3. Sequence Analysis
3. Results
3.1. kdr Mutations in vgsc of Myanmar Ae. aegypti
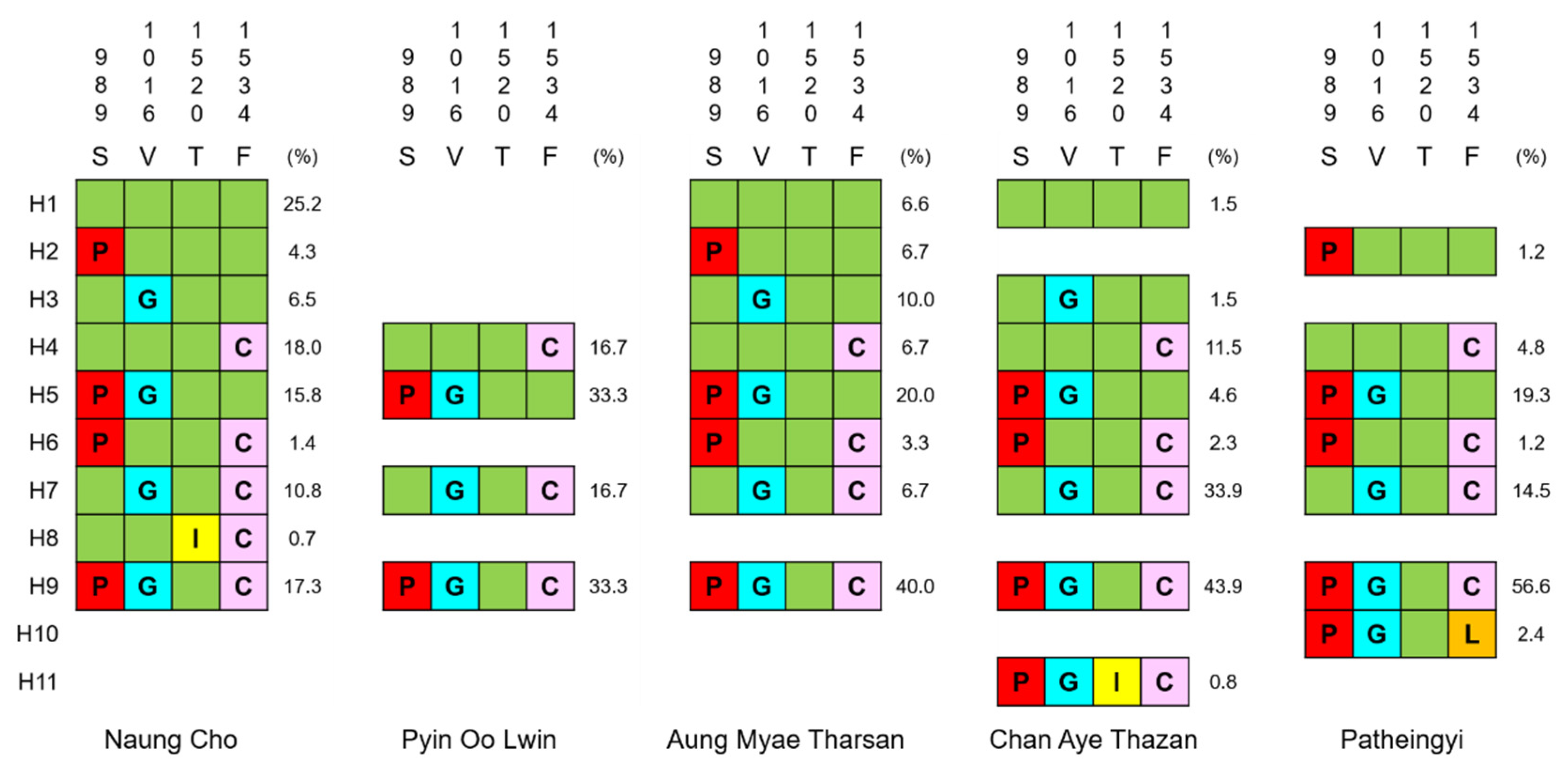
3.2. vgsc Haplotypes in Myanmar Ae. aegypti
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powell, J.R. Perspective piece mosquito-borne human viral diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg. 2018, 98, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Gloria-Soria, A.; Kotsakiozi, P. Recent history of Aedes aegypti: Vector genomics and epidemiology records. Bioscience 2018, 68, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Servadio, J.L.; Rosenthal, S.R.; Carlson, L.; Bauer, C. Climate patterns and mosquito-borne disease outbreaks in South and Southeast Asia. J. Infect. Public Health 2018, 11, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N. Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2019, 95, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, A.E.; Ponce, G.; Silva, B.G.; Gutierrez, S.M.; Bobadilla, C.; Lopez, B.; Mercado, R.; Black, W.C., IV. Wide spread cross resistance to pyrethroids in Aedes aegypti (Diptera: Culicidae) from Veracruz State Mexico. J. Econ. Entomol. 2013, 106, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amelia-Yap, Z.H.; Chen, C.D.; Sofian-Azirun, M.; Low, V.L. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasites Vectors 2018, 11, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 959–969. [Google Scholar] [CrossRef]
- Field, L.M.; Emyr Davies, T.G.; O’Reilly, A.O.; Williamson, M.S.; Wallace, B.A. Voltage-gated sodium channels as targets for pyrethroid insecticides. Eur. Biophys. J. 2017, 46, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Hemingway, J.; Hawkes, N.J.; McCarroll, L.; Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect. Biochem. Mol. Biol. 2004, 34, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M. Pyrethroids, knockdown resistance and sodium channels. Pest Manag. Sci. 2008, 64, 610–616. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Voltage-gated sodium channels as insecticide targets. Adv. Insect Phys. 2014, 46, 389–433. [Google Scholar] [CrossRef]
- Cosme, L.V.; Gloria-Soriaid, A.; Caccone, A.; Powell, J.R.; Martins, A.J. Evolution of kdr haplotypes in worldwide populations of Aedes aegypti: Independent origins of the F1534C kdr mutation. PLoS Negl. Trop. Dis. 2020, 14, e0008219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects 2016, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinkevich, F.D.; Du, Y.; Dong, K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013, 106, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyaw, A.K.; Ngwe Tun, M.M.; Moi, M.L.; Nabeshima, T.; Soe, K.T.; Thwe, S.M.; Myint, A.A.; Maung, K.T.T.; Aung, W.; Hayasaka, D.; et al. Clinical, virological and epidemiological characterization of dengue outbreak in Myanmar, 2015. Epidemiol. Infect. 2017, 145, 1886–1897. [Google Scholar] [CrossRef] [Green Version]
- Linn, N.N.; Kyaw, K.W.Y.; Shewade, H.D.; Kyaw, A.M.M.; Tun, M.M.; Khine, S.K.; Linn, N.Y.Y.; Thi, A.; Lin, Z. Notified dengue deaths in Myanmar (2017-18): Profile and diagnosis delays. F1000Research 2020, 9, 579. [Google Scholar] [CrossRef]
- Kawada, H.; Oo, S.Z.M.; Thaung, S.; Kawashima, E.; Maung, Y.N.M.; Thu, H.M.; Thant, K.Z.; Minakawa, N. Co-occurrence of Point Mutations in the Voltage-Gated Sodium Channel of Pyrethroid-Resistant Aedes aegypti Populations in Myanmar. PLoS Negl. Trop. Dis. 2014, 8, e3032. [Google Scholar] [CrossRef] [Green Version]
- Son-Un, P.; Choovattanapakorn, N.; Saingamsook, J.; Yanola, J.; Lumjuan, N.; Walton, C.; Somboon, P. Effect of relaxation of deltamethrin pressure on metabolic resistance in a pyrethroid-resistant Aedes aegypti (Diptera: Culicidae) strain harboring fixed P989P and G1016G kdr alleles. J. Med. Entomol. 2018, 55, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Naw, H.; Su, M.N.C.; Võ, T.C.; Lê, H.G.; Kang, J.M.; Jun, H.; Mya, Y.Y.; Myint, M.K.; Lee, J.; Sohn, W.M.; et al. Overall prevalence and distribution of knockdown resistance (Kdr) mutations in Aedes aegypti from Mandalay region, Myanmar. Korean J. Parasitol. 2020, 58, 709–714. [Google Scholar] [CrossRef]
- Huang, Y.-M. The subgenus Stegomyia of Aedes in the Afrotropical region I. The Africanus group of species (Diptera: Culicidae). Gainesville, USA. Am. Entomol. Inst. 1990, 26, 10–42. [Google Scholar]
- Sayono, S.; Hidayati, A.P.N.; Fahri, S.; Sumanto, D.; Dharmana, E.; Hadisaputro, S.; Asih, P.B.S.; Syafruddin, D. Distribution of voltage-gated sodium channel (NAV) alleles among the Aedes aegypti populations in central Java province and its aociation with resistance to pyrethroid insecticides. PLoS ONE 2016, 11, e0150577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, P.; Chatterjee, M.; Ballav, S.; Chowdhury, A.; Basu, N.; Maji, A.K. Prevalence of kdr mutations and insecticide susceptibility among natural population of Aedes aegypti in West Bengal. PLoS ONE 2019, 14, e0215541. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Higa, Y.; Komagata, O.; Kasai, S.; Tomita, T.; Nguyen, T.Y.; Luu, L.L.; Sánchez, R.A.P.; Takagi, M. Widespread distribution of a newly found point mutation in voltage-gated sodium channel in pyrethroid-resistant Aedes aegypti populations in Vietnam. PLoS Negl. Trop. Dis. 2009, 3, e527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanola, J.; Somboon, P.; Walton, C.; Nachaiwieng, W.; Somwang, P.; Prapanthadara, L. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop. Med. Int. Health 2011, 16, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ishak, I.H.; Jaal, Z.; Ranson, H.; Wondji, C.S. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit Vectors 2015, 8, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcombe, S.; Fustec, B.; Cattel, J.; Chonephetsarath, S.; Thammavong, P.; Phommavanh, N.; David, J.P.; Corbel, V.; Sutherland, I.W.; Hertz, J.C.; et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl. Trop. Dis. 2019, 13, e0007852. [Google Scholar] [CrossRef] [Green Version]
- Kushwah, R.B.S.; Dykes, C.L.; Kapoor, N.; Adak, T.; Singh, O.P. Pyrethroid-Resistance and Presence of Two Knockdown Resistance (kdr) Mutations, F1534C and a Novel Mutation T1520I, in Indian Aedes aegypti. PLoS Negl. Trop. Dis. 2015, 9, e3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Du, Y.; Wu, S.; Nomura, Y.; Zhu, G.; Zhorov, B.S.; Dong, K. Molecular evidence of sequential evolution of DDT-and pyrethroid-resistant sodium channel in Aedes aegypti. PLoS Negl. Trop. Dis. 2019, 13, e0007432. [Google Scholar] [CrossRef]
- Rahman, R.U.; Souza, B.; Uddin, I.; Carrara, L.; Brito, L.P.; Costa, M.M.; Mahmood, M.A.; Khan, S.; Lima, J.B.P.; Martins, A.J. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Sci. Rep. 2021, 11, 4555. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, R.B.S.; Kaur, T.; Dykes, C.L.; Ravi Kumar, H.; Kapoor, N.; Singh, O.P. A new knockdown resistance (kdr) mutation, F1534L, in the voltage-gated sodium channel of Aedes aegypti, co-occurring with F1534C, S989P and V1016G. Parasites Vectors 2020, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Marcombe, S.; Farajollahi, A.; Healy, S.P.; Clark, G.G.; Fonseca, D.M. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS ONE 2014, 9, e101992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xu, J.; Zhong, D.; Zhang, H.; Yang, W.; Zhou, G.; Su, X.; Wu, Y.; Wu, K.; Cai, S.; et al. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China. Parasites Vectors 2018, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Srisawat, R.; Komalamisra, N.; Apiwathnasorn, C.; Paeporn, P.; Roytrakul, S.; Rongsriyam, Y.; Eshita, Y. Field-collected permethrin-resistant Aedes aegypti from central Thailand contain point mutations in the domain IIS6 of the sodium channel gene (kdr). Southeast Asian J. Trop. Med. Public Health 2012, 43, 1380–1386. [Google Scholar]
- Stenhouse, S.A.; Plernsub, S.; Yanola, J.; Lumjuan, N.; Dantrakool, A.; Choochote, W.; Somboon, P. Detection of the V1016G mutation in the voltage-gated sodium channel gene of Aedes aegypti (Diptera: Culicidae) by allele-specific PCR assay, and its distribution and effect on deltamethrin resistance in Thailand. Parasites Vectors 2013, 6, 253. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Kaufman, P.E.; De Xue, R.; Zhao, M.H.; Wang, G.; Yan, T.; Guo, X.X.; Zhang, Y.M.; Dong, Y.D.; Xing, D.; et al. Relationship between insecticide resistance and kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasites Vectors 2015, 8, 325. [Google Scholar] [CrossRef] [Green Version]
- Wuliandari, J.R.; Lee, S.F.; White, V.L.; Tantowijoyo, W.; Hoffmann, A.A.; Endersby-Harshman, N.M. Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in Aedes aegypti from Yogyakarta, Indonesia. Insects 2015, 6, 658–685. [Google Scholar] [CrossRef] [PubMed]

| Domain | Mutation | Patheingyi (n = 83) | Chan Aye Thazan (n = 130) | Aung Myae Tharsan (n = 30) | Pyin Oo Lwin (n = 12) | Naung Cho (n = 139) | Total (n = 394) |
|---|---|---|---|---|---|---|---|
| DII-S6 | D960G | 1 | 1 | 2 | |||
| W966R | 2 | 2 | |||||
| N967D | 1 | 1 | 2 | ||||
| M972V | 2 | 2 | |||||
| M972I | 2 | 2 | |||||
| I977T | 1 | 2 | 3 | ||||
| E985G | 2 | 2 | |||||
| I987N | 2 | 2 | |||||
| W991R | 1 | 1 | 2 | ||||
| D992N | 2 | 2 | |||||
| M994V | 2 | 2 | |||||
| D998G | 2 | 2 | |||||
| P1003S | 2 | 2 | |||||
| F1004I | 2 | 2 | |||||
| F1020S | 4 | 20 | 2 | 2 | 22 | 50 | |
| DIII-S6 | V1512A | 2 | 2 | ||||
| K1514E | 5 | 5 | |||||
| M1524V | 1 | 2 | 3 | ||||
| L1526P | 1 | 1 | 2 | ||||
| Y1527F | 1 | 2 | 3 | ||||
| Y1527C | 4 | 4 | |||||
| F1528L | 2 | 2 | |||||
| I1533V | 2 | 2 | |||||
| I1533T | 2 | 2 | |||||
| F1543L | 1 | 1 | 2 | ||||
| I1544V | 2 | 1 | 3 | ||||
| I1548V | 1 | 1 | 2 | ||||
| E1553G | 9 | 1 | 10 | ||||
| G1581D | 1 | 1 | 2 | ||||
| K1584E | 1 | 1 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naw, H.; Võ, T.C.; Lê, H.G.; Kang, J.-M.; Mya, Y.Y.; Myint, M.K.; Kim, T.-S.; Shin, H.-J.; Na, B.-K. Knockdown Resistance Mutations in the Voltage-Gated Sodium Channel of Aedes aegypti (Diptera: Culicidae) in Myanmar. Insects 2022, 13, 322. https://doi.org/10.3390/insects13040322
Naw H, Võ TC, Lê HG, Kang J-M, Mya YY, Myint MK, Kim T-S, Shin H-J, Na B-K. Knockdown Resistance Mutations in the Voltage-Gated Sodium Channel of Aedes aegypti (Diptera: Culicidae) in Myanmar. Insects. 2022; 13(4):322. https://doi.org/10.3390/insects13040322
Chicago/Turabian StyleNaw, Haung, Tuấn Cường Võ, Hương Giang Lê, Jung-Mi Kang, Yi Yi Mya, Moe Kyaw Myint, Tong-Soo Kim, Ho-Joon Shin, and Byoung-Kuk Na. 2022. "Knockdown Resistance Mutations in the Voltage-Gated Sodium Channel of Aedes aegypti (Diptera: Culicidae) in Myanmar" Insects 13, no. 4: 322. https://doi.org/10.3390/insects13040322
APA StyleNaw, H., Võ, T. C., Lê, H. G., Kang, J.-M., Mya, Y. Y., Myint, M. K., Kim, T.-S., Shin, H.-J., & Na, B.-K. (2022). Knockdown Resistance Mutations in the Voltage-Gated Sodium Channel of Aedes aegypti (Diptera: Culicidae) in Myanmar. Insects, 13(4), 322. https://doi.org/10.3390/insects13040322








