Species Discrimination of Three Odontomachus (Formicidae: Ponerinae) Species in Thailand Using Outline Morphometrics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ant Specimens
2.2. Morphometric Analyses
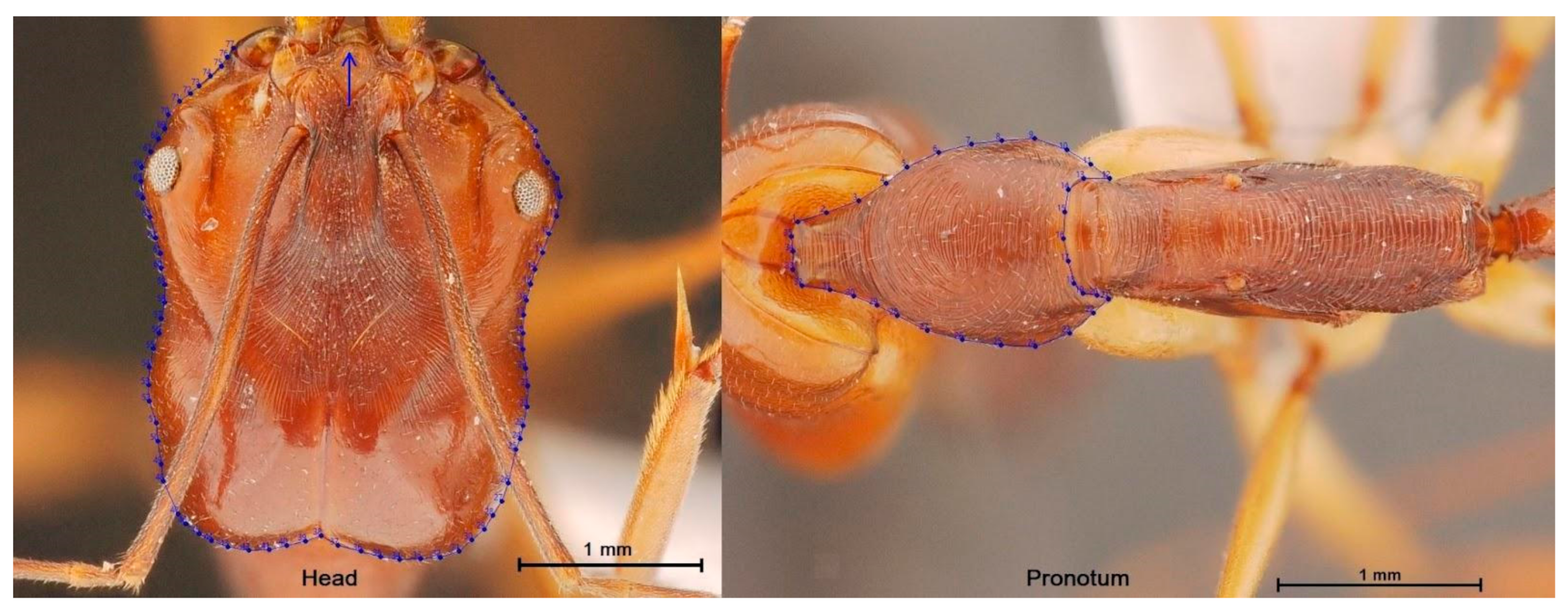
2.2.1. Digitization and Digitization Error
2.2.2. Size and Shape Data
2.2.3. Exploratory Data Analysis
2.2.4. Discriminant Space
2.2.5. Allometry
2.2.6. Mahalanobis-Based Classification
2.3. Software
3. Results
3.1. Repeatability
3.2. Allometry
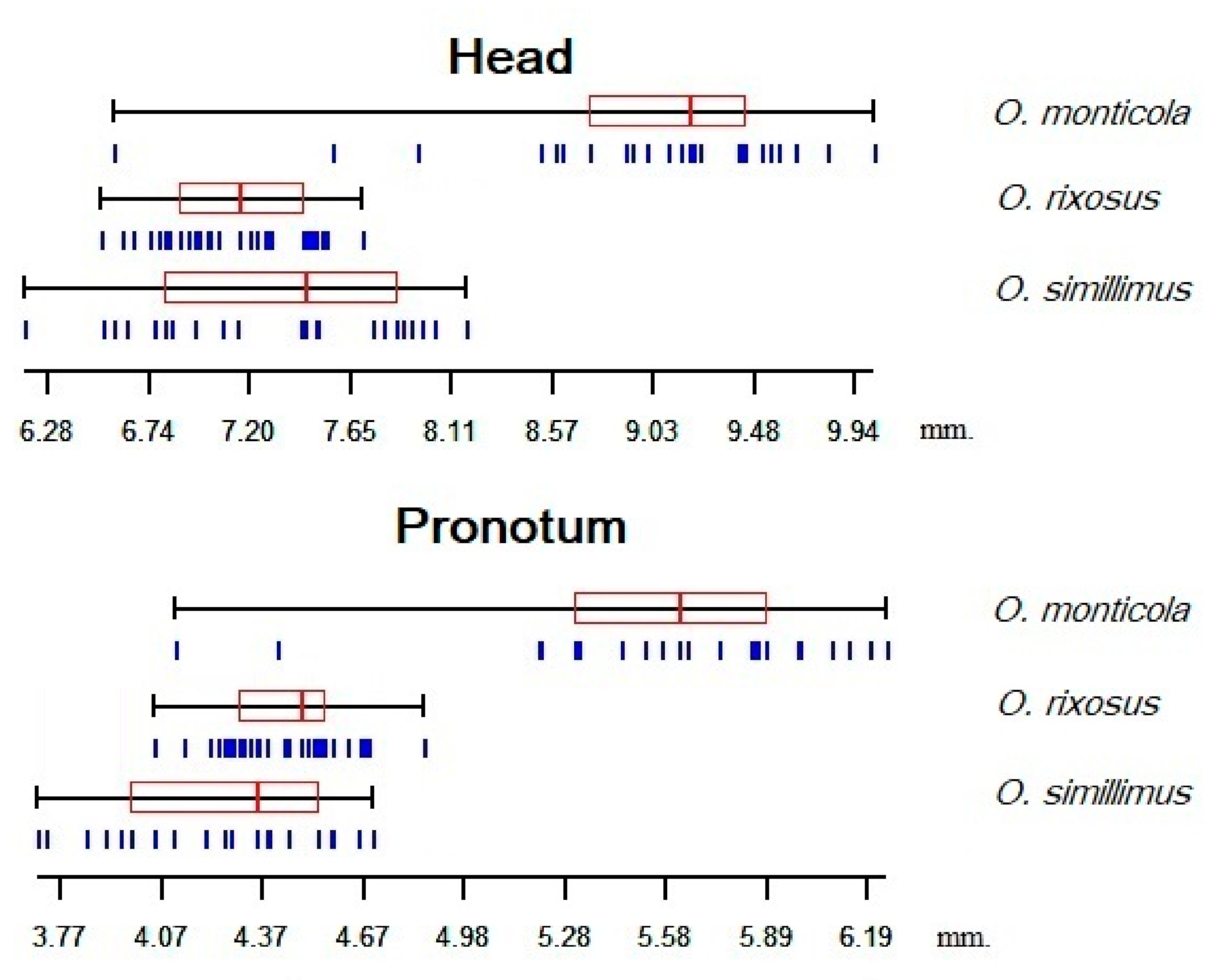
3.3. Size Variation among the Odontomachus Species
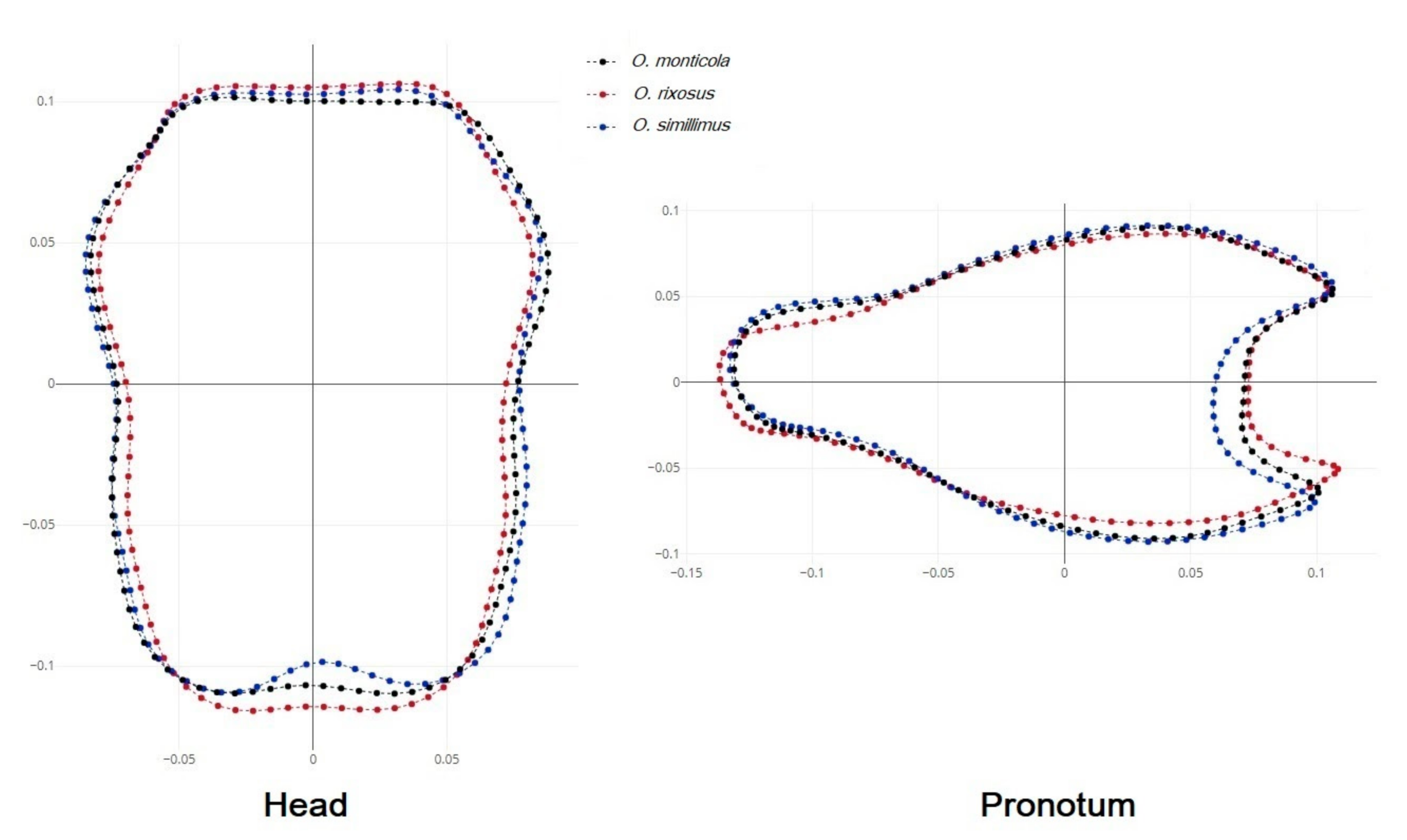
3.4. Shape Variation among the Odontomachus Species
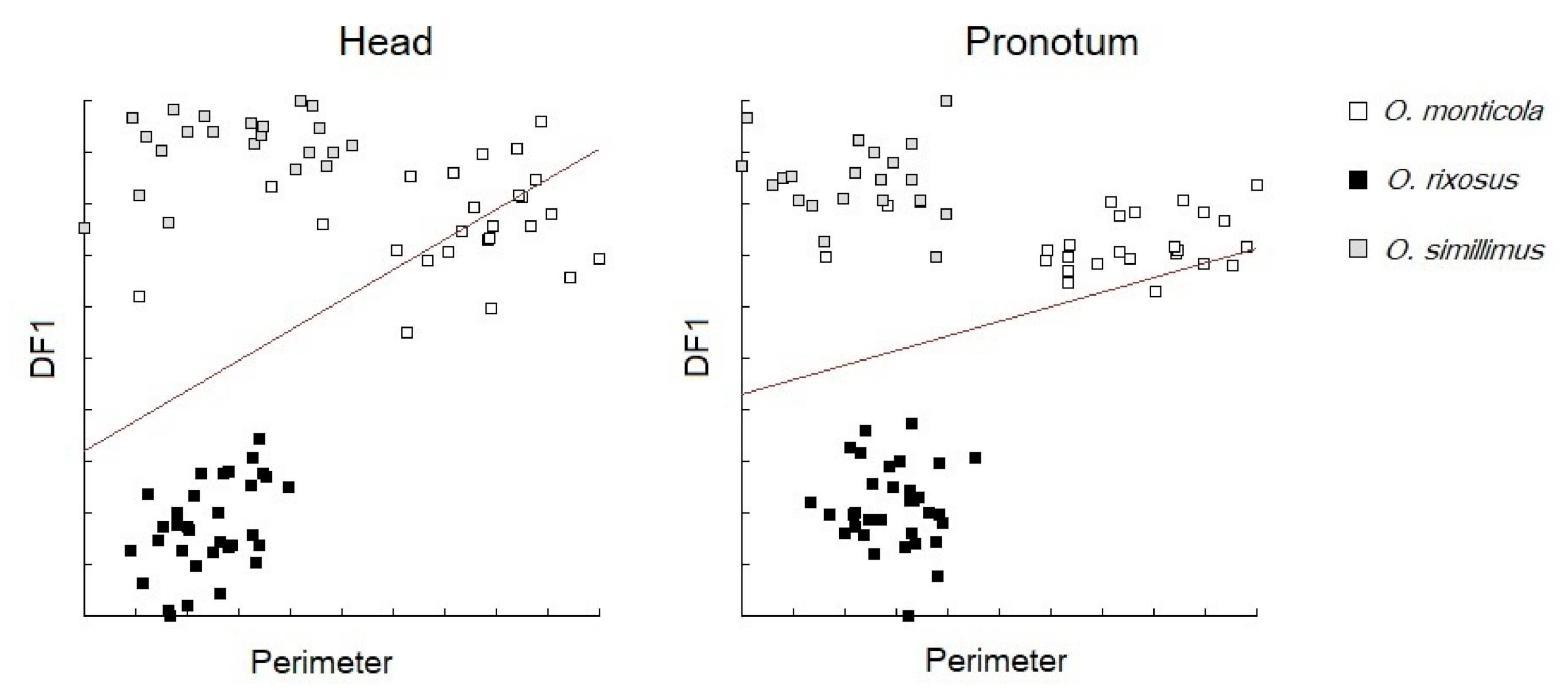
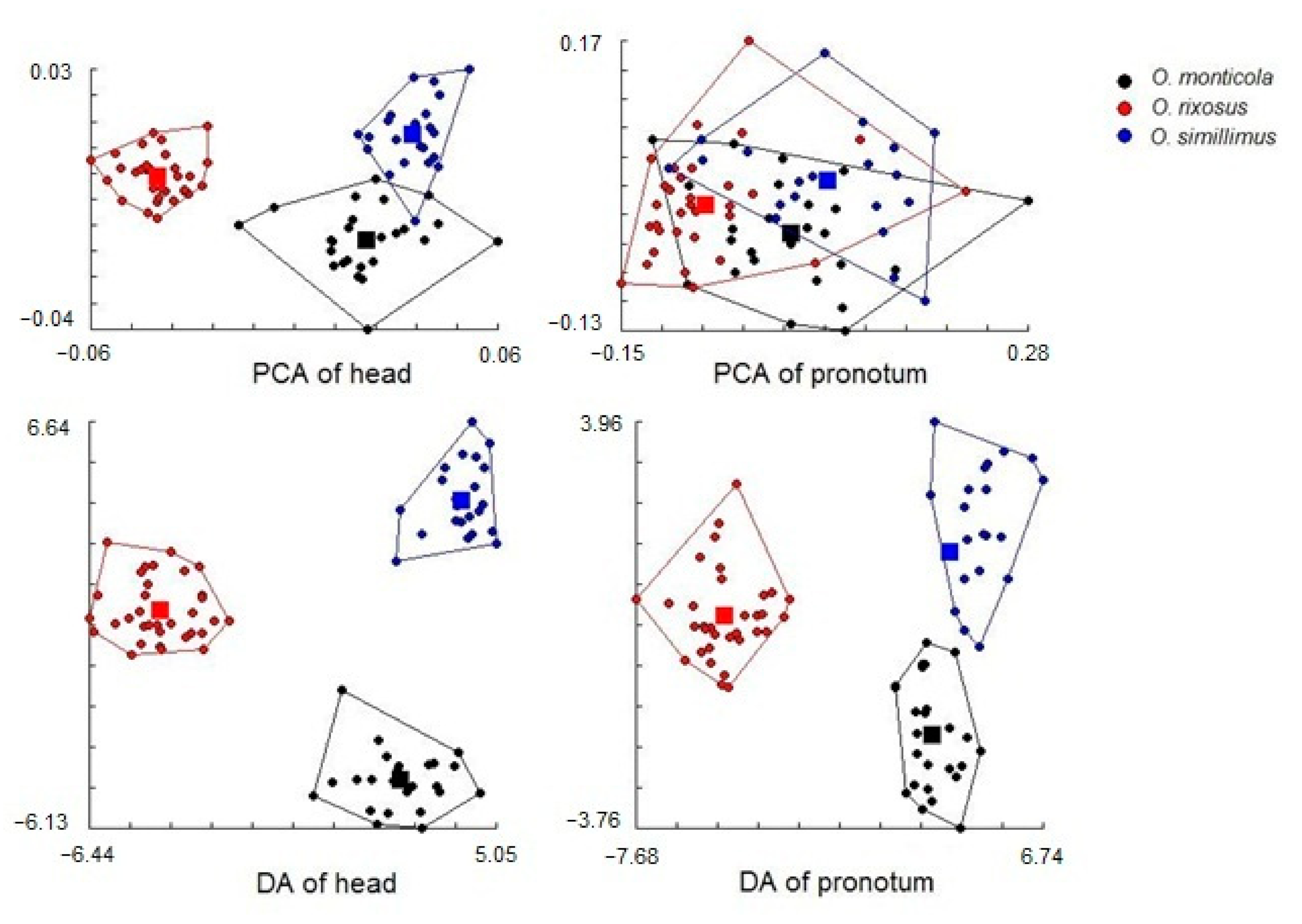
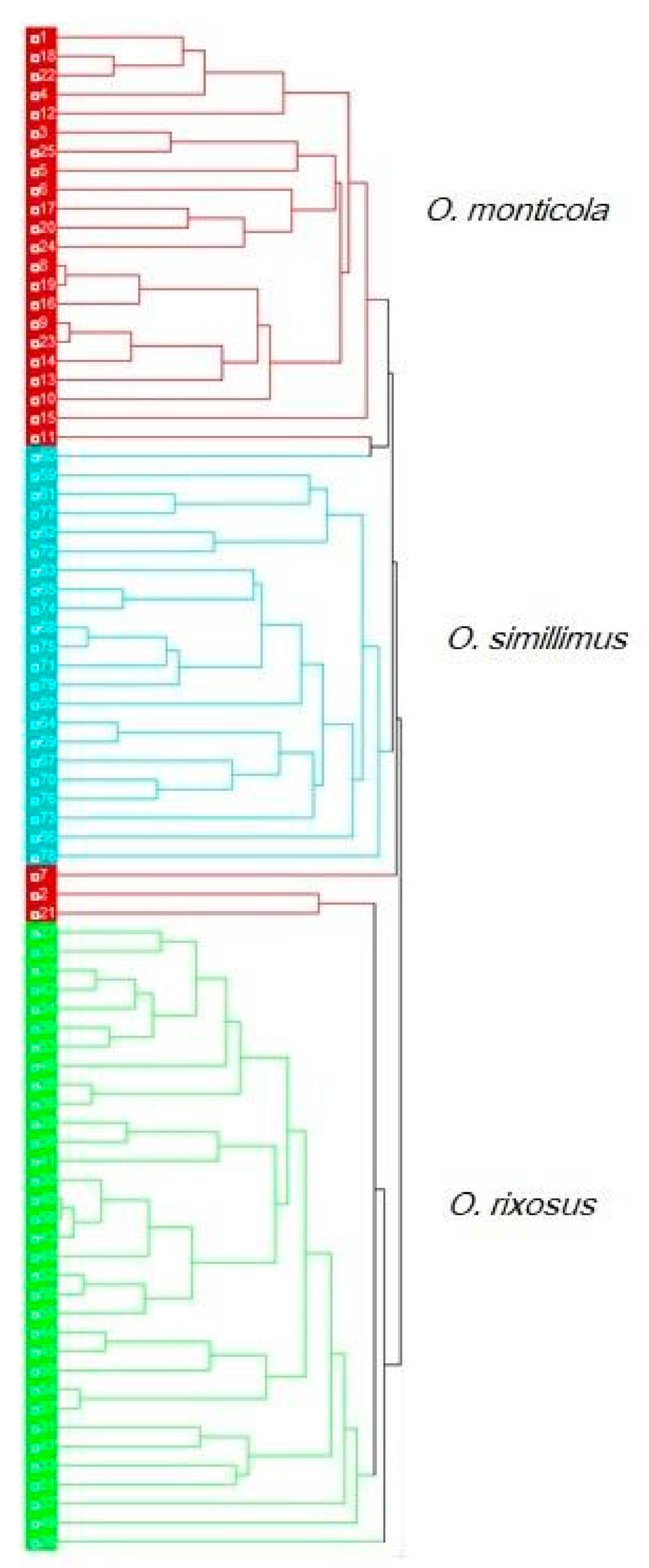
3.5. Morphospace and Exploratory Data Analyses
3.6. Discriminant Analysis
4. Discussion
4.1. Size Variation among the Odontomachus Species
4.2. Shape Variation among the Odontomachus Species
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Bolton, B. Book Review Identification Guide to the Ant Genera of the World. Psyche J. Entomol. 1994, 101, 203–208. [Google Scholar] [CrossRef][Green Version]
- Khachonpisitsak, S.; Yamane, S.; Sriwichai, P.; Jaitrong, W. An updated checklist of the ants of Thailand (Hymenoptera, Formicidae). Zookeys 2020, 998, 1–182. [Google Scholar] [CrossRef] [PubMed]
- Klotz, J.H.; de Shazo, R.D.; Pinnas, J.L.; Frishman, A.M.; Schmidt, J.O.; Suiter, D.R.; Price, G.W.; Klotz, S.A. Adverse reactions to ants other than imported fire ants. Ann. Allergy Asthma Immunol. 2005, 95, 418–425. [Google Scholar] [CrossRef]
- Touchard, A.; Koh, J.M.S.; Aili, S.R.; Dejean, A.; Nicholson, G.M.; Orivel, J.; Escoubas, P. The complexity and structural diversity of ant venom peptidomes is revealed by mass spectrometry profiling. Rapid Commun. Mass Spectrom. 2015, 29, 385–396. [Google Scholar] [CrossRef]
- Schmidt, J.O. Pain and lethality induced by insect Stings: An exploratory and correlational study. Toxins 2019, 21, 427. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Shattuck, S.O. The higher classification of the ant subfamily Ponerinae (Hymenoptera: Formicidae), with a review of Ponerine ecology and behavior. Zootaxa 2014, 3817, 1–242. [Google Scholar] [CrossRef]
- Satria, R.; Kurushima, H.; Herwina, H.; Yamane, S.; Eguchi, K. The trap-jaw ant genus Odontomachus Latreille (Hymenoptera: Formicidae) from Sumatra, with a new species description. Zootaxa 2015, 4048, 1–36. [Google Scholar] [CrossRef]
- Bolton, B. An Online Catalog of the Ants of the World. Available online: http://antcat.org (accessed on 7 July 2021).
- Larabee, F.J.; Fisher, B.L.; Schmidt, C.A.; Matos-Maravi, P.; Janda, M.; Suarez, A.V. Molecular phylogenetics and diversification of trap-jaw ants in the genera Anochetus and Odontomachus (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 2016, 103, 143–154. [Google Scholar] [CrossRef]
- Hoenle, P.O.; Lattke, J.E.; Donoso, D.A.; von Beeren, C.; Heethoff, M.; Schmelzle, S.; Argoti, A.; Camacho, L.; Ströbel, B.; Blüthgen, N. Odontomachus davidsoni sp. nov. (Hymenoptera, Formicidae), a new conspicuous trap-jaw ant from Ecuador. Zookeys 2020, 948, 75–105. [Google Scholar] [CrossRef]
- Fernandes, I.O.; Larabee, F.J.; Oliveira, M.L.; Delabie, J.H.C.; Schultz, T.R. A global phylogenetic analysis of trap-jaw ants, Anochetus Mayr and Odontomachus Latreille (Hymenoptera: Formicidae: Ponerinae). Syst. Entomol. 2021, 46, 685–703. [Google Scholar] [CrossRef]
- Sharaf, M.R.; Gotzek, D.; Guenard, B.; Fisher, B.L.; Aldawood, A.S.; Al Dhafer, H.M.; Mohamed, A.A. Molecular phylogenetic analysis and morphological reassessments of thief ants identify a new potential case of biological invasions. Sci. Rep. 2020, 10, 12040. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, J.P.; Kaba, D.; Solano, P.; Dupraz, M.; McCoy, K.D.; Jaramillo-O, N. Outline-based morphometrics, an overlooked method in arthropod studies? Infect. Genet. Evol. 2014, 28, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Katzke, J.; Barden, P.; Dehon, M.; Michez, D.; Wappler, T. Giant ants and their shape: Revealing relationships in the genus Titanomyrma with geometric morphometrics. PeerJ 2018, 6, e4242. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, A.; Münch, W.; Seifert, B. A first demonstration of interspecific hybridization in Myrmica ants by geometric morphometrics (Hymenoptera: Formicidae). Myrmecol. News 2012, 17, 121–131. [Google Scholar]
- Seifert, B.; Yazdi, A.B.; Schultz, R. Myrmica martini sp.n.—A cryptic species of the Myrmica scabrinodis species complex (Hymenoptera: Formicidae) revealed by geometric morphometrics and nest-centroid clustering. Myrmecol. News 2014, 19, 171–183. [Google Scholar]
- Casadei-Ferreira, A.; Friedman, N.R.; Economo, E.P.; Pie, M.R.; Feitosa, R.M. Head and mandible shapes are highly integrated yet represent two distinct modules within and among worker subcastes of the ant genus Pheidole. Ecol. Evol. 2021, 11, 6104–6118. [Google Scholar] [CrossRef]
- Brown, W.L., Jr. Contributions toward a reclassification of the Formicidae. Part VI. Ponerinae, tribe Ponerini, subtribe Odontomachiti. Section, A. Introduction, subtribal characters. Genus Odontomachus. Stud. Entomol. 1976, 19, 67–171. [Google Scholar]
- Sumruayphol, S.; Apiwathnasorn, C.; Ruangsittichai, J.; Sriwichai, P.; Attrapadung, S.; Samung, Y.; Dujardin, J.P. DNA barcoding and wing morphometrics to distinguish three Aedes vectors in Thailand. Acta Trop. 2016, 159, 1–10. [Google Scholar] [CrossRef]
- Chonephetsarath, S.; Raksakoon, C.; Sumruayphol, S.; Dujardin, J.P.; Potiwat, R. The unequal taxonomic signal of mosquito wing cells. Insects 2021, 12, 376. [Google Scholar] [CrossRef]
- Arnqvist, G.; Martensson, T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zool. Academ. Sci. Hung. 1998, 44, 73–96. [Google Scholar]
- Klingenberg, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef]
- Yezerinac, S.M.; Lougheed, S.C.; Handford, P. Measurement error and morphometric studies: Statistical power and observer experience. Syst. Biol. 1992, 41, 471–482. [Google Scholar] [CrossRef]
- Kuhl, F.P.; Giardina, C.R. Elliptic Fourier features of a closed contour. Comput. Graph. Image Process. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- Dujardin, J.P. Morphometrics applied to medical entomology. Infect. Genet. Evol. 2008, 8, 875–890. [Google Scholar] [CrossRef]
- Manly, B.F.J. Multivariate Statistical Methods: A Primer; Chapman & Hall: London, UK; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Dujardin, J.P.; Slice, D. Contributions of Morphometrics to Medical Entomology. In Encyclopedia of Infectious Diseases: Modern Methodologies; Tibayrenc, M., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2007; pp. 435–447. [Google Scholar]
- Dujardin, J.P.; Kaba, D.; Henry, A.B. The exchangeability of shape. BMC Res. Notes. 2010, 22, 266. [Google Scholar] [CrossRef]
- Dujardin, S.; Dujardin, J.P. Geometric morphometrics in the cloud. Infect. Genet. Evol. 2019, 70, 189–196. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The morphometry of Solenopsis fire ants. PLoS ONE 2013, 8, e79559. [Google Scholar]
- Csősz, S.; Fisher, B.L. Taxonomic revision of the Malagasy Nesomyrmex madecassus species-group using a quantitative morphometric approach. ZooKeys 2016, 603, 105–130. [Google Scholar] [CrossRef]
- Da Silva Camargo, R.; Hastenreiter, I.N.; Forti, L.; Lopes, J.F.S. Relationship between mandible morphology and leaf preference in leaf-cutting ants (Hymenoptera: Formicidae). Rev. Colomb. Entomol. 2015, 41, 241–244. [Google Scholar]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Rohlf, F.J. Rotational Fit (Procrustes) Methods. In Special Publication Number 2, Proceedings of the Michigan Morphometrics Workshop, Ann Arbor, MI, USA, 16–28 May 1988; Rohlf, F., Bookstein, F., Eds.; The University of Michigan Museum of Zoology: Ann Arbor, MI, USA, 1990; pp. 227–236, p. 380. [Google Scholar]
- Rohlf, F.J.; Archie, J.W. A comparison of Fourier methods for the description of wing shape in mosquitoes (Diptera: Culicidae). Syst. Biol. 1984, 33, 302–317. [Google Scholar] [CrossRef]
- Chaiphongpachara, T.; Sriwichai, P.; Samung, Y.; Ruangsittichai, J.; Vargas, R.E.M.; Cui, L.; Sattabongkot, J.; Dujardin, J.P.; Sumruayphol, S. Geometric morphometrics approach towards discrimination of three member species of Maculatus group in Thailand. Acta Trop. 2019, 192, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sumruayphol, S.; Chittsamart, B.; Polseela, R.; Sriwichai, P.; Samung, Y.; Apiwathnasorn, C.; Dujardin, J.P. Wing geometry of Phlebotomus stantoni and Sergentomyia hodgsoni from different geographical locations in Thailand. Comptes Rendus Biol. 2017, 340, 37–46. [Google Scholar] [CrossRef]
- Kitthawee, S.; Dujardin, J.P. The Diachasmimorpha longicaudata complex in Thailand discriminated by its wing venation. Zoomorphology 2016, 135, 323–332. [Google Scholar] [CrossRef]
- Friedman, N.R.; Lecroq Bennet, B.; Fischer, G.; Sarnat, E.M.; Huang, J.P.; Knowles, L.L.K.; Economo, E.P. Macroevolutionary integration of phenotypes within and across ant worker castes. Ecol. Evol. 2020, 18, 9371–9383. [Google Scholar] [CrossRef]

| Species | n | Perimeter of Head | Perimeter of Pronotum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (mm.) | Min (mm.) | Max (mm.) | SD | Mean (mm.) | Min (mm.) | Max (mm.) | SD | ||
| O. monticola | 25 | 8.96 b | 6.67 | 9.94 | 0.71 | 5.55 c | 4.16 | 6.19 | 0.48 |
| O. rixosus | 33 | 7.17 a | 6.61 | 7.73 | 0.29 | 4.48 b | 4.09 | 4.86 | 0.18 |
| O. simillimus | 22 | 7.36 a | 6.28 | 8.18 | 0.55 | 4.30 a | 3.77 | 4.72 | 0.30 |
| Species | Head | Pronotum | ||||
|---|---|---|---|---|---|---|
| O. monticola | O. rixosus | O. simillimus | O. monticola | O. rixosus | O. simillimus | |
| O. monticola | 0.00 | - | - | 0.00 | - | - |
| O. rixosus | 7.29 * | 0.00 | - | 3.62 * | 0.00 | - |
| O. simillimus | 6.89 * | 7.11 * | 0.00 | 1.78 * | 4.24 * | 0.00 |
| Species | Head (n = 80) | Pronotum (n = 78) |
|---|---|---|
| O. monticola | 96% (24/25) | 76% (19/25) |
| O. rixosus | 100% (33/33) | 100% (33/33) |
| O. simillimus | 100% (22/22) | 60% (12/20) |
| Total | 98.75% (79/80) | 82% (64/78) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samung, Y.; Chaiphongpachara, T.; Ruangsittichai, J.; Sriwichai, P.; Phayakkaphon, A.; Jaitrong, W.; Dujardin, J.-P.; Sumruayphol, S. Species Discrimination of Three Odontomachus (Formicidae: Ponerinae) Species in Thailand Using Outline Morphometrics. Insects 2022, 13, 287. https://doi.org/10.3390/insects13030287
Samung Y, Chaiphongpachara T, Ruangsittichai J, Sriwichai P, Phayakkaphon A, Jaitrong W, Dujardin J-P, Sumruayphol S. Species Discrimination of Three Odontomachus (Formicidae: Ponerinae) Species in Thailand Using Outline Morphometrics. Insects. 2022; 13(3):287. https://doi.org/10.3390/insects13030287
Chicago/Turabian StyleSamung, Yudthana, Tanawat Chaiphongpachara, Jiraporn Ruangsittichai, Patchara Sriwichai, Anon Phayakkaphon, Weeyawat Jaitrong, Jean-Pierre Dujardin, and Suchada Sumruayphol. 2022. "Species Discrimination of Three Odontomachus (Formicidae: Ponerinae) Species in Thailand Using Outline Morphometrics" Insects 13, no. 3: 287. https://doi.org/10.3390/insects13030287
APA StyleSamung, Y., Chaiphongpachara, T., Ruangsittichai, J., Sriwichai, P., Phayakkaphon, A., Jaitrong, W., Dujardin, J.-P., & Sumruayphol, S. (2022). Species Discrimination of Three Odontomachus (Formicidae: Ponerinae) Species in Thailand Using Outline Morphometrics. Insects, 13(3), 287. https://doi.org/10.3390/insects13030287





