Use of Age-Stage, Two-Sex Life Table to Compare the Fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Host Fruits
2.3. Life Table Study
2.4. Life Table Analysis
2.5. Population Projection
3. Results
3.1. Life History Statistics of B. dorsalis on Three Host Fruits
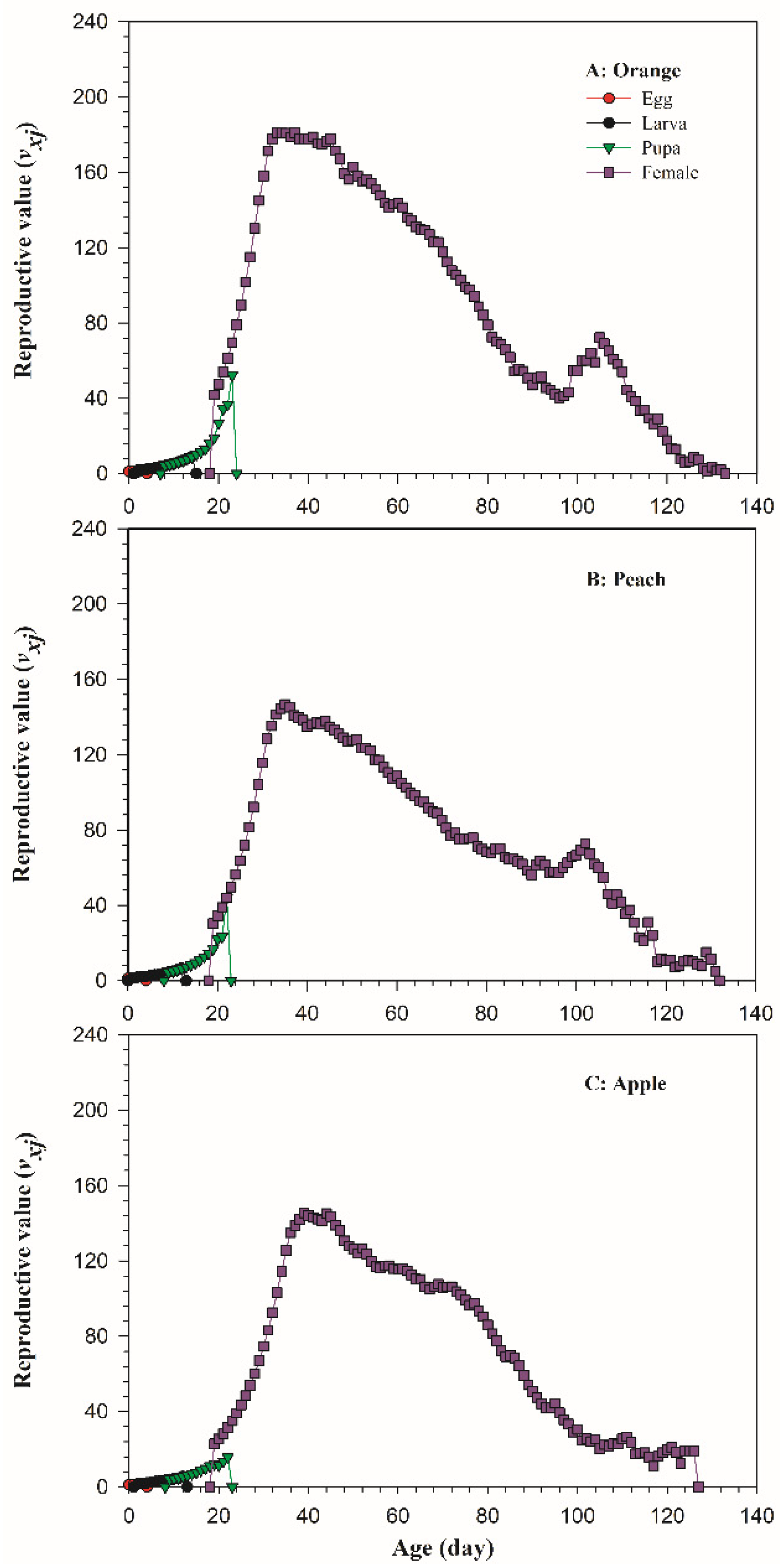
3.2. Life Table Parameters of B. dorsalis on Three Host Fruits
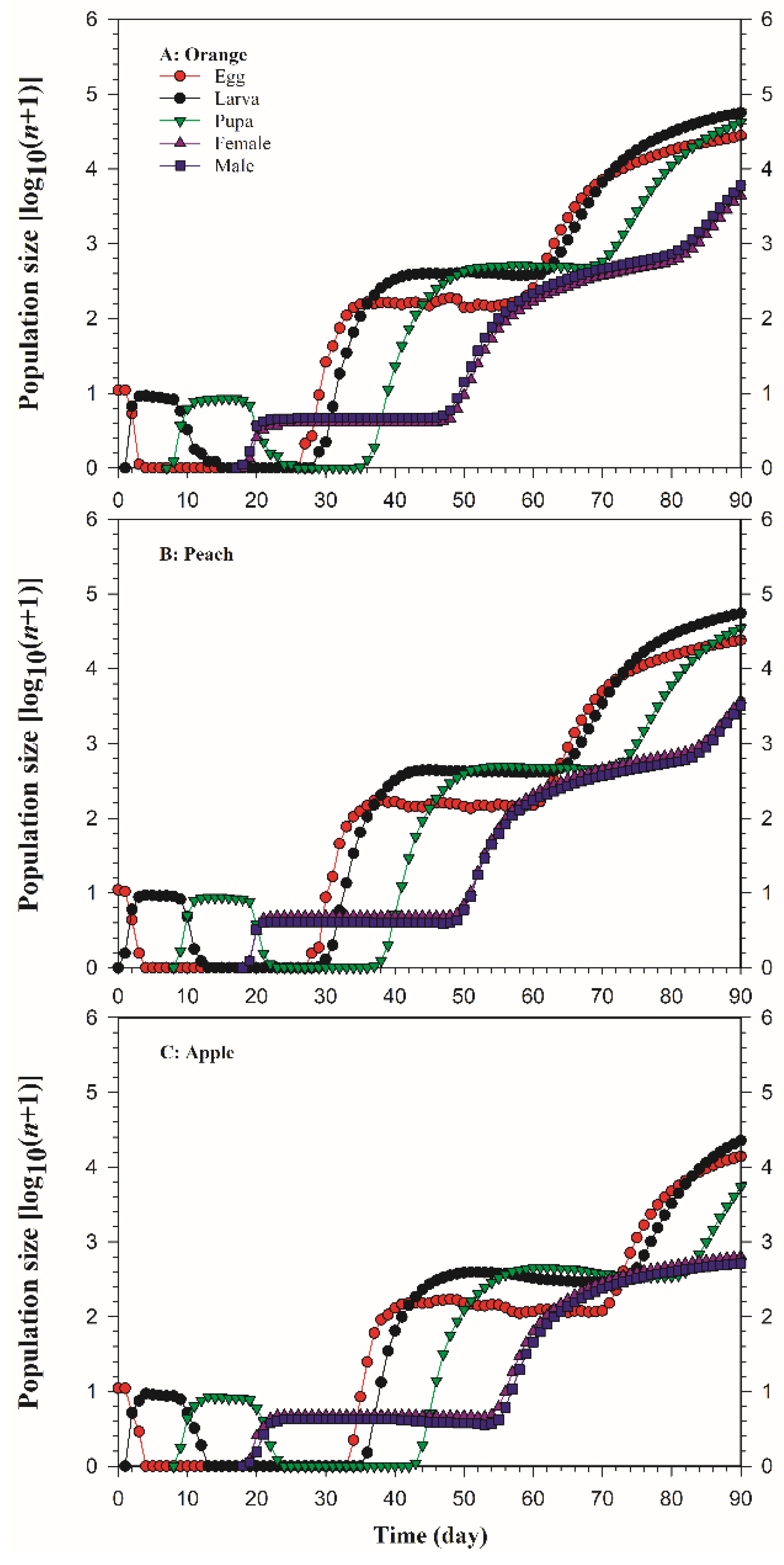
3.3. Population Prediction of B. dorsalis on Three Host Fruits
4. Discussions
4.1. Potential Fitness of B. dorsalis to Peaches and Apples
4.2. Fecundity as a Key Indicator of B. dorsalis Fitness to Its Hosts
4.3. Selected Parameters Taken into Consideration in the Use of Life Table Data
4.3.1. Longevity of Female and Male Adults Separately
4.3.2. Differentiating Oviposition Days and Oviposition Period
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.B.K.; Chi, H. Fitness of Bactrocera dorsalis (Hendel) on seven host plants and an artificial diet. Turk. Entomol. Derg-Tu 2014, 38, 401–414. [Google Scholar] [CrossRef]
- Schutze, M.K.; Bourtzis, K.; Cameron, S.L.; Clarke, A.R.; De Meyer, M.; Hee, A.K.; Hendrichs, J.; Krosch, M.N.; Mwatawala, M. Integrative taxonomy versus taxonomic authority without peer review: The case of the Oriental fruit fly, Bactrocera dorsalis (Tephritidae). Syst. Entomol. 2017, 42, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.P.; Lu, Y.Y.; Zeng, L.; Liang, G.W.; Schal, C. Life-history traits and population relative fitness of trichlorphon-resistant and -susceptible Bactrocera dorsalis (Diptera: Tephritidae). Psyche 2010, 3, 895935. [Google Scholar]
- Vargas, R.I.; Piñero, J.C.; Leblanc, L. An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 2015, 6, 297–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.H.; Reddy, G.V.P.; Chen, L.; Qin, Y.J.; Li, Z.H. The synergy between climate change and transportation activities drives the propagation of an invasive fruit fly. J. Pest Sci. 2020, 93, 615–625. [Google Scholar] [CrossRef]
- Ye, H.; Liu, J.H. Population dynamics of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) in the Kunming area, southwestern China. Insect Sci. 2005, 12, 387–392. [Google Scholar] [CrossRef]
- Yin, Y.C.; Wang, Q.Y. Beware of the new pest in northern orchard—Bactrocera dorsalis. Hebei Agricu. 2014, 11, 48–49. (In Chinese) [Google Scholar]
- Wang, X.M.; Sun, J.S.; Li, Z.Q.; Duan, Y.L.; Zhang, T.K.; Gao, Z.Y. Occurrences and control of Bactrocera dorsalis Hendel in Beijing. South China Fruit 2016, 45, 27–30. (In Chinese) [Google Scholar]
- Liang, L.; Li, Y.R. Risk Analysis of Bactrocera dorsalis (Hendel) in Shaanxi Province. Shaanxi For. Sci. Technol. 2017, 2, 46–48. (In Chinese) [Google Scholar]
- Mao, H.Y.; Zhao, Y.; Ding, H.F.; Jiao, Y.J.; Sun, G.Q.; Lu, C.X.; Han, S.P. Monitoring on the population dynamics of major fruit flies in Henan province. China Plant Prot. 2019, 39, 77–83. (In Chinese) [Google Scholar]
- Jaleel, W.; Lu, L.H.; He, Y.R. Biology, taxonomy, and IPM strategies of Bactrocera tau Walker and complex species (Diptera: Tephritidae) in Asia: A comprehensive review. Environ. Sci. Pollut. Res. 2018, 25, 19346–19361. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Chi, H.; Smith, C.L. Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. J. Econ. Entomol. 2018, 111, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Chi, H. Assessing the application of the Jackknife and Bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: A case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J. Agric. For. 2012, 61, 37–45. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Bussaman, P.; Sa-uth, C.; Chandrapatya, A.; Atlihan, R.; Gökçe, A.; Saska, P.; Chi, H. Fast population growth in physogastry reproduction of Luciaphorus perniciosus (Acari: Pygmephoridae) at different temperatures. J. Econ. Entomol. 2017, 110, 1397–1403. [Google Scholar] [CrossRef]
- Li, J.; Ding, T.B.; Chi, H.; Chu, D. Effects of tomato chlorosis virus on the performance of its key vector, Bemisia tabaci, in China. J. Appl. Entomol. 2018, 142, 296–304. [Google Scholar] [CrossRef]
- Jaleel, W.; Tao, X.B.; Wang, D.S.; Lu, L.H.; He, Y.R. Using two-sex life table traits to assess the fruit preference and fitness of Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2018, 111, 2936–2945. [Google Scholar] [CrossRef]
- Cheng, D.F.; Guo, Z.J.; Riegler, M.; Xi, Z.Y.; Liang, G.W.; Xu, Y.J. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2021; Available online: http://140.120.197.173/Ecology/Download/TWOSEX-MSChart.rar (accessed on 8 March 2021).
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Tuan, S.J.; Lee, C.C.; Chi, H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manag. Sci. 2014, 70, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2021; Available online: http://140.120.197.173/Ecology/Download/TIMING-MSChart-exe.rar (accessed on 8 March 2021).
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.Y.; Lin, Y.Y.; Tuan, S.J.; Tang, L.C.; Chi, H.; Atlihan, R.; Ozgokce, M.S.; Guncan, A. Integrating demography, predation rate, and computer simulation for evaluation of Orius strigicollis as biological control agent against Frankliniella intonsa. Entomol. Gen. 2021, 41, 179–196. [Google Scholar] [CrossRef]
- Goundoudaki, S.; Tsitsipis, J.A.; Margaritopoulos, J.T.; Zarpas, K.D.; Divanidis, S. Performance of the tobacco aphid Myzus persicae (Hemiptera: Aphididae) on Oriental and Virginia tobacco varieties. Agric. For. Entomol. 2003, 5, 285–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Atlihan, R.; Chi, H.; Chu, D. Demographic analysis of progeny fitness and timing of resurgence of Laodelphax striatellus after insecticides exposure. Entomol. Gen. 2019, 39, 221–230. [Google Scholar] [CrossRef]
- Balagawi, S.; Vijaysegaran, S.; Drew, R.A.; Raghu, S. Influence of fruit traits on oviposition preference and offspring performance of Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) on three tomato (Lycopersicon lycopersicum) cultivars. Aust. Entomol. 2005, 44, 97–103. [Google Scholar] [CrossRef]
- May, M.L.; Ahmad, S. Host location in the Colorado potato beetle: Searching mechanisms in relation to oligophagy. In Herbivorous Insects: Host Seeking Behavior and Mechanisms; Ahmad, S., Ed.; Stony Brook University: New York, NY, USA, 1983; Volume 59, pp. 173–199. [Google Scholar]
- McCormick, A.C.; Arrigo, L.; Eggenberger, H.; Mescher, M.C.; De Moraes, C.M. Divergent behavioral responses of gypsy moth (Lymantria dispar) caterpillars from three different subspecies to potential host trees. Sci. Rep. 2019, 9, 8953. [Google Scholar] [CrossRef] [Green Version]
- Rattanapun, W.; Amornsak, W.; Clarke, A.R. Bactrocera dorsalis preference for and performance on two mango varieties at three stages of ripeness. Entomol. Exp. Appl. 2009, 131, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kong, W.N.; Zhao, L.L.; Xiang, H.M.; Zhang, L.J.; Li, J.; Ridsdill-Smith, J.; Ma, R.Y. Methods to measure performance of Grapholitha molesta on apples of five varieties. Entomol. Exp. Appl. 2018, 166, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Vargas, R.I.; Carey, J.R. Comparative survival and demographic statistics for wild oriental fruit fly, Mediterranean fruit fly, and melon fly (Diptera: Tephritidae) on papaya. J. Econ. Entomol. 1990, 83, 1344–1349. [Google Scholar] [CrossRef]




| Statistics | Mean ± SE 1 | ||
|---|---|---|---|
| Orange | Peach | Apple | |
| Hatching rate (%) | 82.22 ± 4.14 a | 83.33 ± 0.00 a | 83.33 ± 4.60 a |
| Pupation rate (%) | 90.22 ± 2.95 a | 92.00 ± 2.62 a | 89.29 ± 4.48 a |
| Eclosion rate (%) | 90.78 ± 3.31 a | 91.67 ± 4.36 a | 93.89 ± 3.68 a |
| Pupal weight (mg) | 16.33 ± 0.25 a | 15.06 ± 0.19 b | 14.38 ± 0.22 c |
| Statistics | Orange | Peach | Apple |
|---|---|---|---|
| Egg duration (d) | 2.32 ± 0.06 b | 2.40 ± 0.08 b | 2.72 ± 0.09 a |
| Larval duration (d) | 7.84 ± 0.15 a | 8.13 ± 0.08 a | 8.12 ± 0.11 a |
| Pupal duration (d) | 10.62 ± 0.18 a | 9.90 ± 0.08 b | 10.30 ± 0.06 a |
| Pre-adult survival rate (%) | 66.67 ± 4.97 a | 70.01 ± 4.84 a | 70.03 ± 4.83 a |
| Adult duration (d) | 75.42 ± 2.33 a | 72.63 ± 2.67 ab | 65.35 ± 2.86 b |
| Female adult longevity (d) | 83.78 ± 3.70 aA | 82.89 ± 2.84 aA | 75.53 ± 3.45 aA |
| Male adult longevity (d) | 68.58 ± 2.42 aB | 59.82 ± 3.63 bB | 53.41 ± 3.65 cB |
| Total longevity (d) | 66.46 ± 4.69 a | 67.21 ± 4.58 a | 62.24 ± 4.40 a |
| Oviposition days (d) | 66.00 ± 3.43 a | 63.86 ± 2.88 a | 52.50 ± 3.00 b |
| Oviposition period (d) | 73.48 ± 4.01 a | 71.26 ± 3.13 a | 59.35 ± 3.48 b |
| Fecundity (F) (eggs) | 1157.33 ± 54.07 a | 910.57 ± 49.19 b | 723.21 ± 46.57 c |
| TPOP (d) | 29.30 ± 0.33 c | 30.14 ± 0.26 b | 35.85 ± 0.35 a |
| Proportion of female adult (Nf/N) | 0.30 ± 0.05 a | 0.39 ± 0.05 a | 0.38 ± 0.05 a |
| Proportion of male adult (Nm/N) | 0.37 ± 0.05 a | 0.31 ± 0.05 a | 0.32 ± 0.05 a |
| N, Nf | 90, 27 | 90, 35 | 90, 34 |
| Statistics | Mean ± SE 1 | ||
|---|---|---|---|
| Orange | Peach | Apple | |
| R0 (offspring/individual) | 347.20 ± 58.24 a | 354.11 ± 50.45 a | 273.21 ± 40.90 a |
| r (d−1) | 0.1266 ± 0.0044 a | 0.1235 ± 0.0035 a | 0.1077 ± 0.0033 b |
| λ (d−1) | 1.1349 ± 0.0050 a | 1.1314 ± 0.0039 a | 1.1137 ± 0.0036 b |
| T (d) | 46.21 ± 0.48 c | 47.54 ± 0.44 b | 52.08 ± 0.62 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Qi, F.; Tan, X.; Zhang, T.; Teng, Z.; Fan, Y.; Wan, F.; Zhou, H. Use of Age-Stage, Two-Sex Life Table to Compare the Fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China. Insects 2022, 13, 258. https://doi.org/10.3390/insects13030258
Zhu Y, Qi F, Tan X, Zhang T, Teng Z, Fan Y, Wan F, Zhou H. Use of Age-Stage, Two-Sex Life Table to Compare the Fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China. Insects. 2022; 13(3):258. https://doi.org/10.3390/insects13030258
Chicago/Turabian StyleZhu, Yanfei, Fangjian Qi, Xiumei Tan, Tong Zhang, Ziwen Teng, Yinjun Fan, Fanghao Wan, and Hongxu Zhou. 2022. "Use of Age-Stage, Two-Sex Life Table to Compare the Fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China" Insects 13, no. 3: 258. https://doi.org/10.3390/insects13030258
APA StyleZhu, Y., Qi, F., Tan, X., Zhang, T., Teng, Z., Fan, Y., Wan, F., & Zhou, H. (2022). Use of Age-Stage, Two-Sex Life Table to Compare the Fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China. Insects, 13(3), 258. https://doi.org/10.3390/insects13030258







