Succession of Ambrosia Beetles Colonizing the Logs of Fallen Alder and Birch Trees
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Bait Log Preparation and Beetle Sampling
2.3. Data Analysis
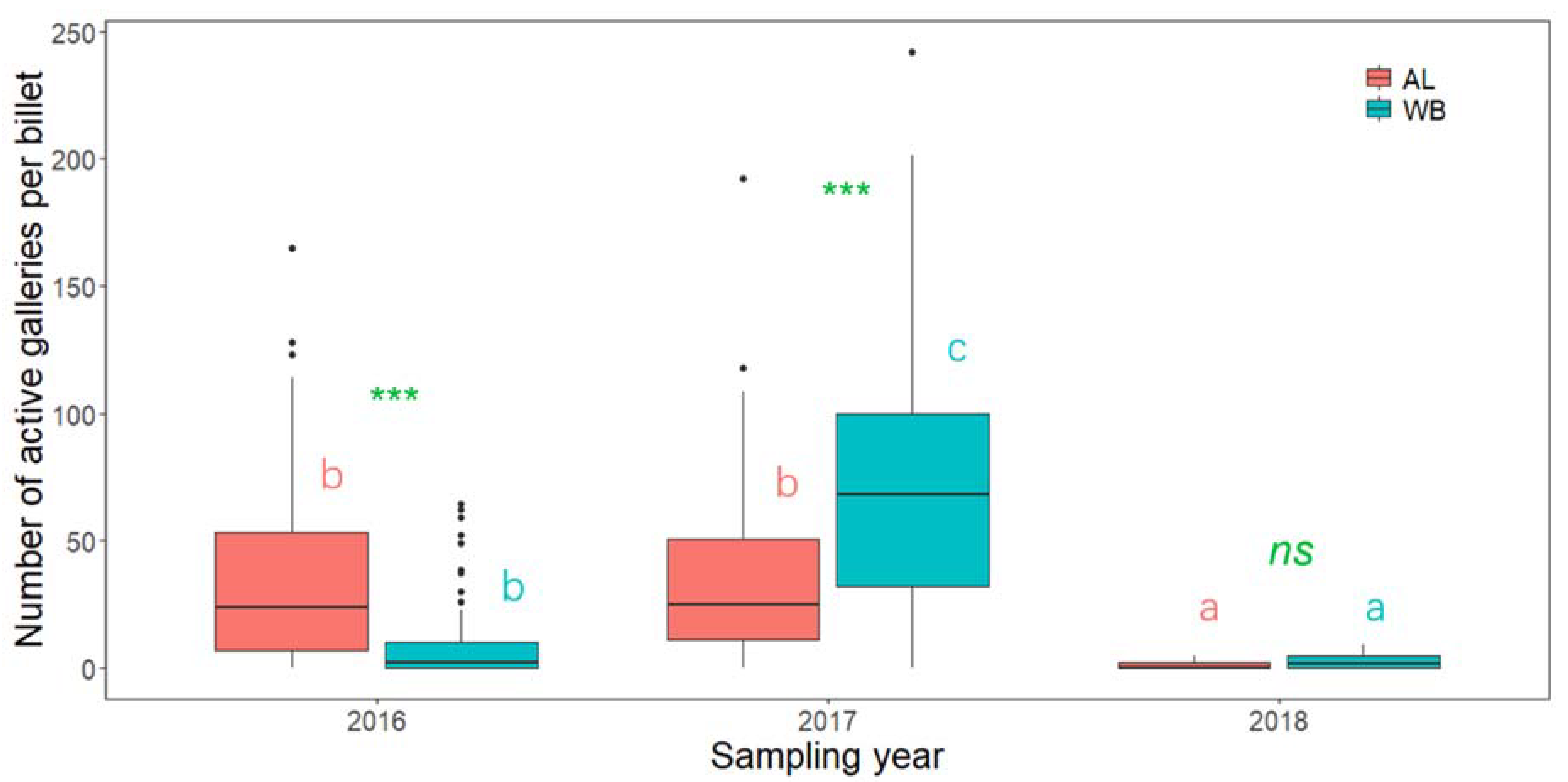
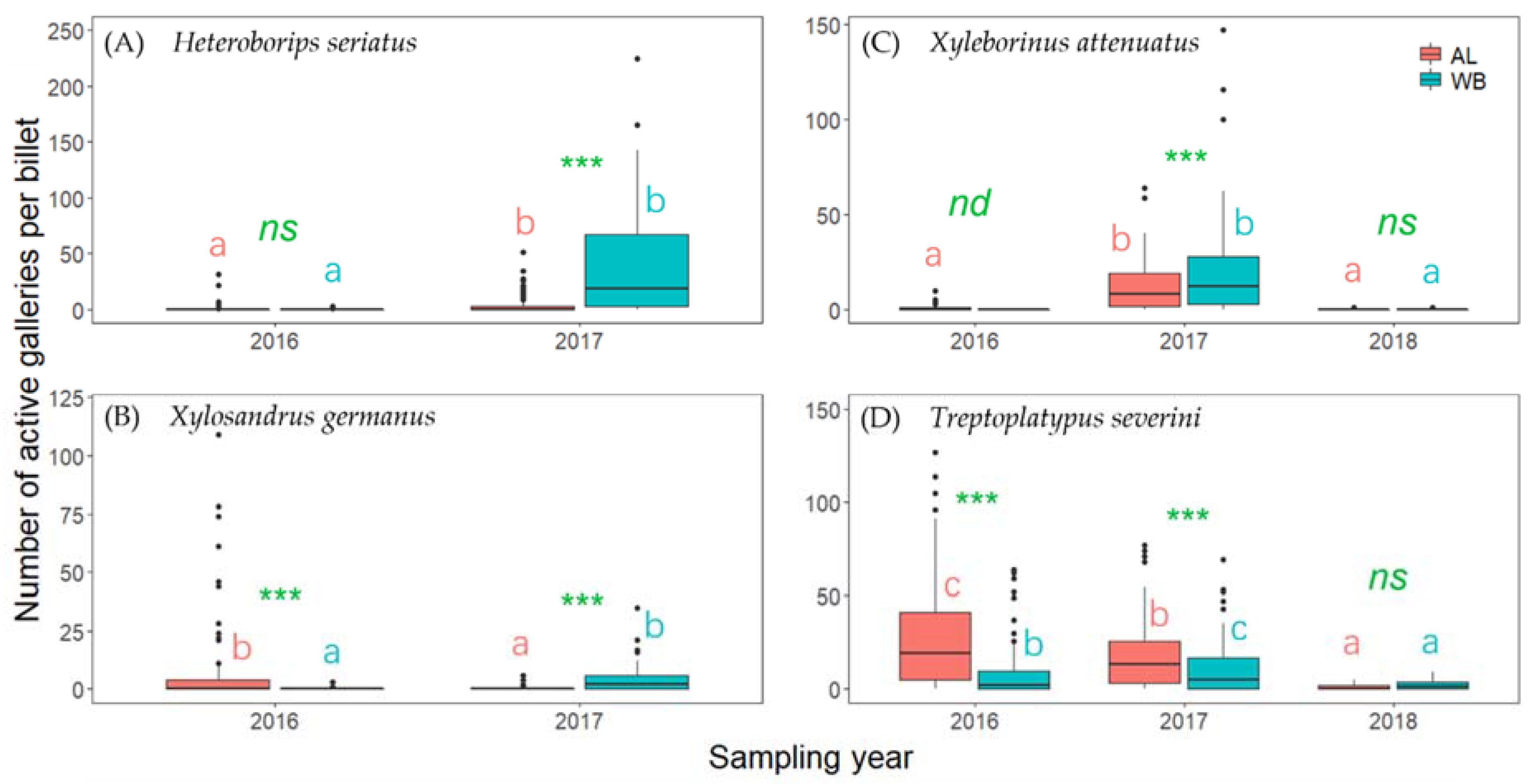
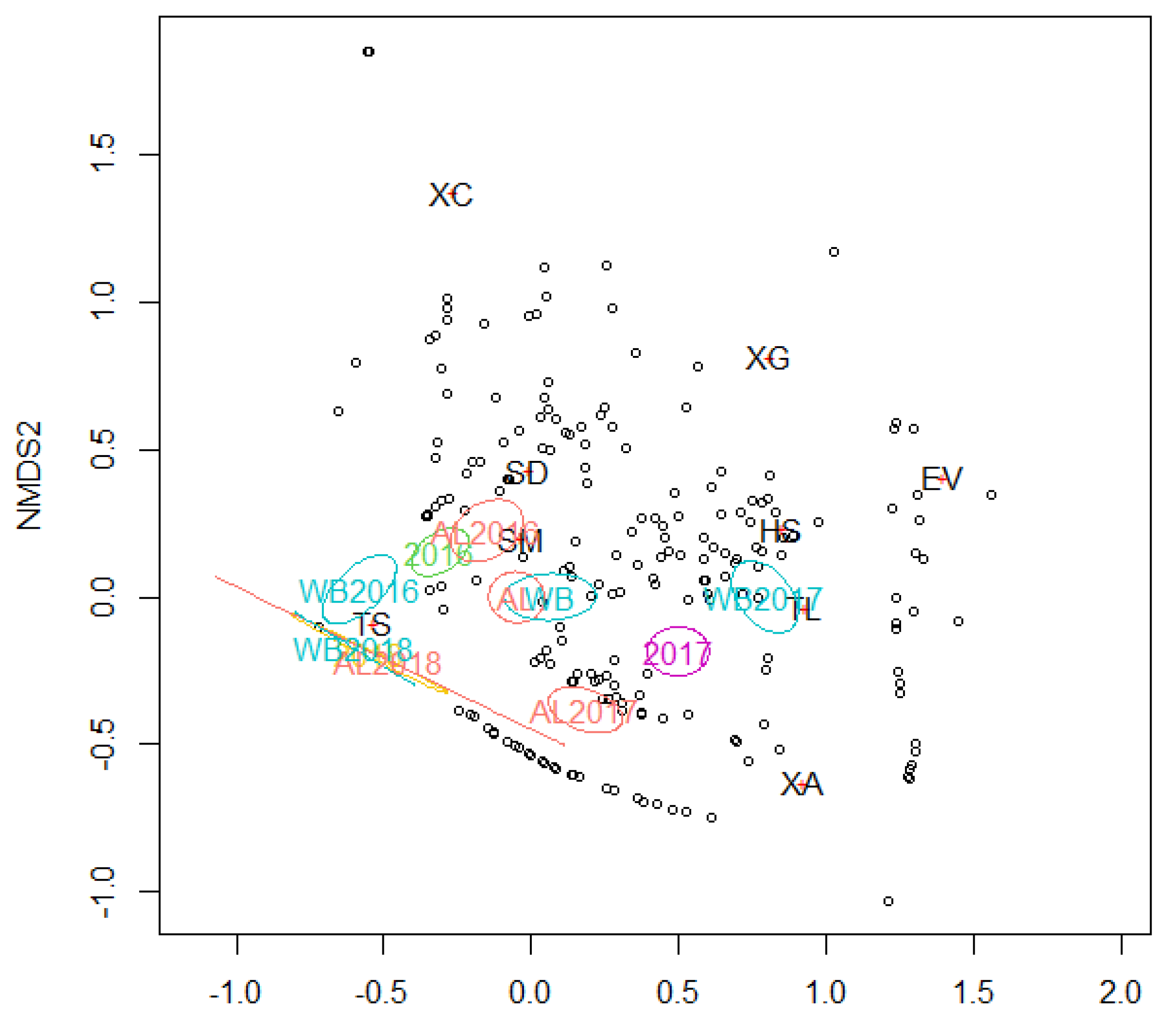
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega, F.E.; Hofstetter, R.W. Bark Beetles: Biology and Ecology of Native and Invasive Species; Academic Press: London, UK, 2015; p. 620. [Google Scholar]
- Kamata, N.; Esaki, K.; Kato, K.; Igeta, Y.; Wada, K. Potential impact of global warming on deciduous oak dieback caused by ambrosia fungus Raffaelea sp. carried by ambrosia beetle Platypus quercivorus (Coleoptera: Platypodidae) in Japan. Bull. Entomol. Res. 2002, 92, 119–126. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E., III; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Rabaglia, R.J. Exotic bark and ambrosia beetles in the USA: Potential and current invaders. In Potential Invasive Pests of Agricultural Crops; Peña, J.E., Ed.; CAB International: Boston, MA, USA, 2013; pp. 48–74. [Google Scholar]
- Wood, S.P. Bark and Ambrosia Beetles of South America (Coleoptera: Scolytidae); Monte L. Bean Life Science Museum, Brigham Young University: Provo, UT, USA, 2007; p. 900. [Google Scholar]
- Li, Y.; Simmons, D.R.; Bateman, C.C.; Short, D.P.G.; Kasson, M.T.; Rabaglia, R.J.; Hulcr, J. New fungus-insect symbiosis: Culturing, molecular, and histological methods determine saprophytic polyporales mutualists of Ambrosiodmus ambrosia beetles. PLoS ONE 2015, 10, e0137689. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.A.; Zhang, Q. Chemical ecology of bark beetles in regard to search and selection of host trees. In Recent Advances in Entomological Research: From Molecular Biology to Pest Management; Liu, T., Kang, L., Eds.; Springer: Berlin, Germany, 2011; pp. 150–190. [Google Scholar] [CrossRef]
- Clements, F.E. Plant Succession: An Analysis of the Development of Vegetation; Carnegie Institution of Washington: Washington, DC, USA, 1916; p. 512. [Google Scholar]
- McCullough, H.A. Plant succession on fallen logs in a virgin spruce-fir forest. Ecology 1948, 29, 508–513. [Google Scholar] [CrossRef]
- Nakamura, T.; Suga, H. Bryophyte succession on fallen logs in coniferous forests on Yaku-shima Island, southern Japan. Proc. Bryol. Soc. Jpn. 1995, 6, 135–140. [Google Scholar] [CrossRef]
- Tang, C.Q.; Zhao, M.H.; Li, X.S.; Ohsawa, M.; Ou, X.K. Secondary succession of plant communities in a subtropical mountainous region of SW China. Ecol. Res. 2010, 25, 149–161. [Google Scholar] [CrossRef]
- Walker, L.R.; Moral, R.D. Primary Succession and Ecosystem Rehabilitation; Cambridge University Press: Cambridge, UK, 2003; p. 442. [Google Scholar]
- Austin, M.P. Use of ordination and other multivariate descriptive methods to study succession. Vegetatio 1977, 35, 165–175. [Google Scholar] [CrossRef]
- Austin, M.P. Continuum concept, ordination methods, and niche theory. Annu. Rev. Ecol. Syst. 1985, 16, 39–61. [Google Scholar] [CrossRef]
- Kaufmann, R. Invertebrate succession on an alpine glacier foreland. Ecology 2001, 82, 2261–2278. [Google Scholar] [CrossRef]
- Vater, A.E. Insect and arachnid colonization on the Storbreen glacier foreland, Jotunheimen, Norway: Persistence of taxa sug-gests an alternative model of succession. Holocene 2012, 22, 1123–1133. [Google Scholar] [CrossRef]
- Vater, A.E.; Matthews, J.A. Testing the ‘addition and persistence model’ of invertebrate succession in a subalpine glacier-foreland chronosequence: Fåbergstølsbreen, southern Norway. Holocene 2013, 23, 1151–1162. [Google Scholar] [CrossRef]
- Brändle, M.; Brandl, R. Is the composition of phytophagous insects and parasitic fungi among trees predictable? Oikos 2006, 113, 296–304. [Google Scholar] [CrossRef]
- Novotny, V.; Miller, S.E.; Baje, L.; Balagawi, S.; Basset, Y.; Cizek, L.; Craft, K.J.; Dem, F.; Drew, R.A.; Hulcr, J. Guild-specific patterns of species richness and host specialization in plant-herbivore food webs from a tropical forest. J. Anim. Ecol. 2010, 79, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Murakami, M.; Hirao, T.; Kamata, N. Species diversity estimation of ambrosia and bark beetles in temperate mixed forests in Japan based on host phylogeny and specificity. Ecol. Res. 2014, 29, 299–307. [Google Scholar] [CrossRef]
- Fukuda, K.; Kuraji, K.; Owari, T.; Yasumura, N.; Kamata, N. The University of Tokyo Forests. In Developing a Network of Long-Term Research Field Stations to Monitor Environmental Changes and Ecosystem Responses in Asian Forests; Kamata, N., Kuraji, K., Owari, T., Guan, B.T., Eds.; The University of Tokyo Forests Press: Tokyo, Japan, 2019; pp. 3–23. [Google Scholar]
- Meteorology Division, Fundamental Data Development Committee, The University of Tokyo Forests. Annual Report of Meteorological Observations in the University of Tokyo Forests, The University of Tokyo (January 2016—December 2016). Misc. Info. Univ. Tokyo For. 2018, 60, 101–120. (In Japanese) [Google Scholar]
- Meteorology Division, Fundamental Data Development Committee, The University of Tokyo Forests. Annual Report of Meteorological Observations in the University of Tokyo Forests, The University of Tokyo (January 2017—December 2017). Misc. Info. Univ. Tokyo For. 2019, 61, 117–147. (In Japanese) [Google Scholar]
- Meteorology Division, Fundamental Data Development Committee, The University of Tokyo Forests. Annual Report of Meteorological Observations in the University of Tokyo Forests, The University of Tokyo (January 2018—December 2018). Misc. Info. Univ. Tokyo For. 2020, 62, 115–161. (In Japanese) [Google Scholar]
- Iidzuka, H.; Goto, H.; Yamasaki, M.; Osawa, N. Wood-boring beetles (Coleoptera: Scolytidae, Platypodidae) captured in ethanol-baited traps in a natural forest in Japan. Appl. Entomol. Zool. 2016, 51, 347–352. [Google Scholar] [CrossRef]
- Viloria, Z.; Villanueva, R.T.; Bessin, R.; O’Neal, P.; Ranger, C.M.; Dunwell, W. Scolytinae in nursery and fruit crops of Western Kentucky and seasonal population patterns of four invasive ambrosia beetles. J. Entomol. Sci. 2021, 56, 374–386. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; Khapugi, A.A. Seasonal activity of Coleoptera attracted by fermental crown traps in forest ecosystems of Central Russia. Ecol. Quest. 2021, 32, 1–21. [Google Scholar] [CrossRef]
- Netherer. S.; Panassiti, B.; Pennerstorfer, J.; Matthews, B. Acute drought is an important driver of bark beetle infestation in Austrian Norway spruce stands. Front. For. Glob. Chang. 2019, 2, 39. [Google Scholar] [CrossRef]
- Gandhi, K.J.; Cognato, A.I.; Lightle, D.M.; Mosley, B.J.; Nielsen, D.G.; Herms, D.A. Species composition, seasonal activity, and semiochemical response of native and exotic bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in northeastern Ohio. J. Econ. Entomol. 2010, 103, 1187–1195. [Google Scholar] [CrossRef]
- Jackman, S. Pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory; R Package Version 1.5.5; United States Studies Centre, University of Sydney: Sydney, NSW, Australia, 2020; Available online: https://github.com/atahk/pscl/ (accessed on 12 January 2022).
- Desmarais, B.A.; Harden, J.J. Testing for zero inflation in count models: Bias correction for the Vuong test. Stata J. 2013, 13, 810–835. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.X.; Orlóci, L. Species niche center: A useful ecological concept. Abstr. Bot. 1993, 17, 115–123. [Google Scholar]
- Colwell, R.K.; Futuyma, D.J. On the measurement of niche breadth and overlap. Ecology 1971, 52, 567–576. [Google Scholar] [CrossRef]
- Zhang, J.L. spaa: SPecies Association Analysis; R Package Version 0.2.2. 2016. Available online: https://CRAN.R-project.org/package=spaa (accessed on 12 January 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-152. 2021. Available online: https://CRAN.R-project.org/package=nlme (accessed on 12 January 2022).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef Stat. Ref. Online 2017, 1–15. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 January 2022).
- Beaver, R.A.; Shih, H.T. Checklist of Platypodidae (Coleoptera: Curculionoidea) from Taiwan. Plant Prot. Bull. 2003, 45, 75–90. [Google Scholar]
- Mandelshtam, M.Y.; Petrov, A.V.; Smith, S.M.; Cognato, A.I. Resurrection of Heteroborips Reitter, 1913 (Coleoptera: Curculionidae: Scolytinae) from Synonymy with Xyleborus Eichhoff, 1864. Coleopts. Bull. 2019, 73, 387–394. [Google Scholar] [CrossRef]
- Kappes, H.; Topp, W. Emergence of Coleoptera from deadwood in a managed broadleaved forest in central Europe. Biodivers. Conserv. 2004, 13, 1905–1924. [Google Scholar] [CrossRef]
- Ito, M.; Sato, S.; Kawasaki, Y.; Kajimura, H. Ambrosia beetles captured with ethanol traps in Irazu-yama National Forest, Kochi Prefecture. Bull. FFPRI 2008, 7, 183–185. (In Japanese) [Google Scholar]
- Kamata, N.; Sanguansub, S.; Beaver, R.A.; Saito, T.; Hirao, T. Investigating the factors influencing trap capture of bark and ambrosia beetles using long-term trapping data in a cool temperate forest in central Japan. J. Forest Res. 2020, 25, 163–173. [Google Scholar] [CrossRef]
- Saito, T.; Goto, H.; Hirao, T.; Kamata, N. Revision of a list of subfamily Scolytinae and Platypodinae captured by bait traps at the University of Tokyo Chichibu Forest in 1994–2003. Misc. Info. Univ. Tokyo For. 2013, 53, 169–193. (In Japanese) [Google Scholar]
- Van Driesche, R.G.; LaForest, J.H.; Bargeron, C.T.; Reardon, R.C.; Herlihy, M. Forest Pest Insects in North America: A Photographic Guide; USDA Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2013; p. 702.
- Beaver, R.A.; Gebhardt, H. A review of the Oriental species of Scolytoplatypus Schaufuss (Coleoptera, Curculionidae, Scolytinae). Dtsch. Entomol. Z. 2006, 53, 155–178. [Google Scholar] [CrossRef]
- Nakashima, T.; Otomo, T.; Owada, Y.; Iizuka, T. SEM observations on growing conditions of the fungi in the galleries of several ambrosia beetles: (Coleoptera Scolytidea and Platypodidae). J. Fac. Agric. Hokkaido Univ. 1992, 5, 239–273. [Google Scholar]
- Mandelshtam, M.Y. New synonymies and new combinations in Scolytidae from the Kuril Archipelago and continental territories of the Russian Far East (Coleoptera). Zoosyst. Ross. 2006, 15, 323–325. [Google Scholar] [CrossRef]
- Chang, C.; van Logtestijn, R.S.P.; Goudzwaard, L.; van Hal, J.; Zuo, J.; Hefting, M.; Sass-Klaassen, U.; Yang, S.; Sterck, F.J.; Poorter, L.; et al. Methodology matters for comparing coarse wood and bark decay rates across tree species. Methods Ecol. Evol. 2020, 11, 828–838. [Google Scholar] [CrossRef]
- Freschet, G.T.; Weedon, J.T.; Aerts, R.; van Hal, J.R.; Cornelissen, J.H. Interspecific differences in wood decay rates: Insights from a new short-term method to study long-term wood decomposition. J. Ecol. 2012, 100, 161–170. [Google Scholar] [CrossRef]
- Iidzuka, H.; Goto, H.; Yamasaki, M.; Osawa, N. Ambrosia beetles (Curculionidae: Scolytinae and Platypodinae) on Fagus crenata Blume: Community structure, seasonal population trends and resource utilization patterns. Entomol. Sci. 2014, 17, 167–180. [Google Scholar] [CrossRef]
- Atkinson, T.H. Ambrosia beetles, Platypus spp. (Insecta: Coleoptera: Platypodidae). DPI Entomol. Circ. Univ. Florida 2000, 321, 1–7. [Google Scholar] [CrossRef]
- Kavčič, A.; de Groot, M. Pest Risk Analysis for the Asian Ambrosia Beetle (Xylosandrus crassiusculus (Motschulsky, 1866)); Slovenian Forestry Institute, Gozdarski Inštitut Slovenije: Ljubljana, Slovenija, 2017. [Google Scholar]
- Atkinson, T.H.; Foltz, J.L.; Wilkinson, R.C.; Mizell, R.F. Granulate ambrosia beetle, Xylosandrus crassiusculus (Motschulsky) (Insecta: Coleoptera: Curculionidae: Scolytinae). DPI Entomol. Circ. Univ. Florida 2011, 310, 1–5. [Google Scholar]
- EPPO. EPPO Study on the Risk of Bark and Ambrosia Beetles Associated with Imported Non-Coniferous Wood. EPPO Paris. 2020. Available online: https://www.eppo.int/RESOURCES/eppo_publications (accessed on 15 May 2020).
- Björklund, N.; Boberg, J. Rapid Pest Risk Analysis Xyleborinus attenuates; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2017. [Google Scholar]
- Galko, J.; Dzurenko, M.; Ranger, C.M.; Kulfan, J.; Kula, E.; Nikolov, C.; Zúbrik, M.; Zach, P. Distribution, habitat preference, and management of the invasive ambrosia beetle Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) in European forests with an emphasis on the West Carpathians. Forests 2019, 10, 10. [Google Scholar] [CrossRef]
- Kamata, N.; Iguchi, K.; Sanguansub, S.; Maneerat, T. Infestation by insect borers on Betula maximowicziana following successive years of severe defoliation by Caligula japonica (Lepidoptera: Saturniidae) with special reference to Xyleborus seriatus Blandford (Coleoptera: Curculionidae: Scolytinae). Forest Pest. 2014, 65, 18–22. (In Japanese) [Google Scholar]
- Wood, S.L.; Bright, D. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2. Great Basin Nat. Mem. 1992, 3, 1–1553. [Google Scholar]
- Nikulina, T.; Mandelshtam, M.; Petrov, A.; Nazarenko, V.; Yunakov, N. A survey of the weevils of Ukraine. Bark and ambrosia beetles (Coleoptera: Curculionidae: Platypodinae and Scolytinae). Zootaxa 2015, 3912, 1–61. [Google Scholar] [CrossRef]
- Borowski, J.; Piętka, J.; Szczepkowski, A. Insects found on black alder Alnus glutinosa (L.) Gaertn. when stands are dying back. Forest Res. Pap. 2012, 73, 355–362. [Google Scholar] [CrossRef][Green Version]
- Dole, S.A.; Cognato, A.I. Phylogenetic revision of Xylosandrus Reitter (Coleoptera: Curculionidae: Scolytinae: Xyleborina). Proc. Calif. Acad. Sci. 2010, 61, 451–545. [Google Scholar]
- Weber, B.C.; McPherson, J.E. World list of host plants of Xylosandrus germanus (Blandford) (Coleoptera: Scolytidae). Coleopt. Bull. 1983, 37, 114–134. [Google Scholar] [CrossRef]
- Nobuchi, A. The ambrosia beetles of the subfamily Scolytoplatypinae (Coleoptera, Scolytidae) in Japan. Kontyû Tokyo 1980, 48, 42–52. [Google Scholar]
- Borden, J.H. The striped ambrosia beetle. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A.A., Ed.; Plenum Press: New York, NY, USA, 1988; pp. 579–596. [Google Scholar] [CrossRef]
- Murayama, J. On the Platypodidae of Formosa. J. Coll. Agric. Hokkaido Imperial Univ. 1925, 15, 197–228. [Google Scholar]
- Nobuchi, A. The Platypodidae of Japan (Coleoptera). Bull. Gov. For. Exp. Sta. 1973, 256, 1–22. [Google Scholar]

| Subfamily | Tribe | Species | Abbreviation | Alder | White Birch | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 (n = 129) 1 | 2017 (n = 75) | 2018 (n = 17) | 2016 (n= 129) | 2017 (n = 77) | 2018 (n = 18) | ||||
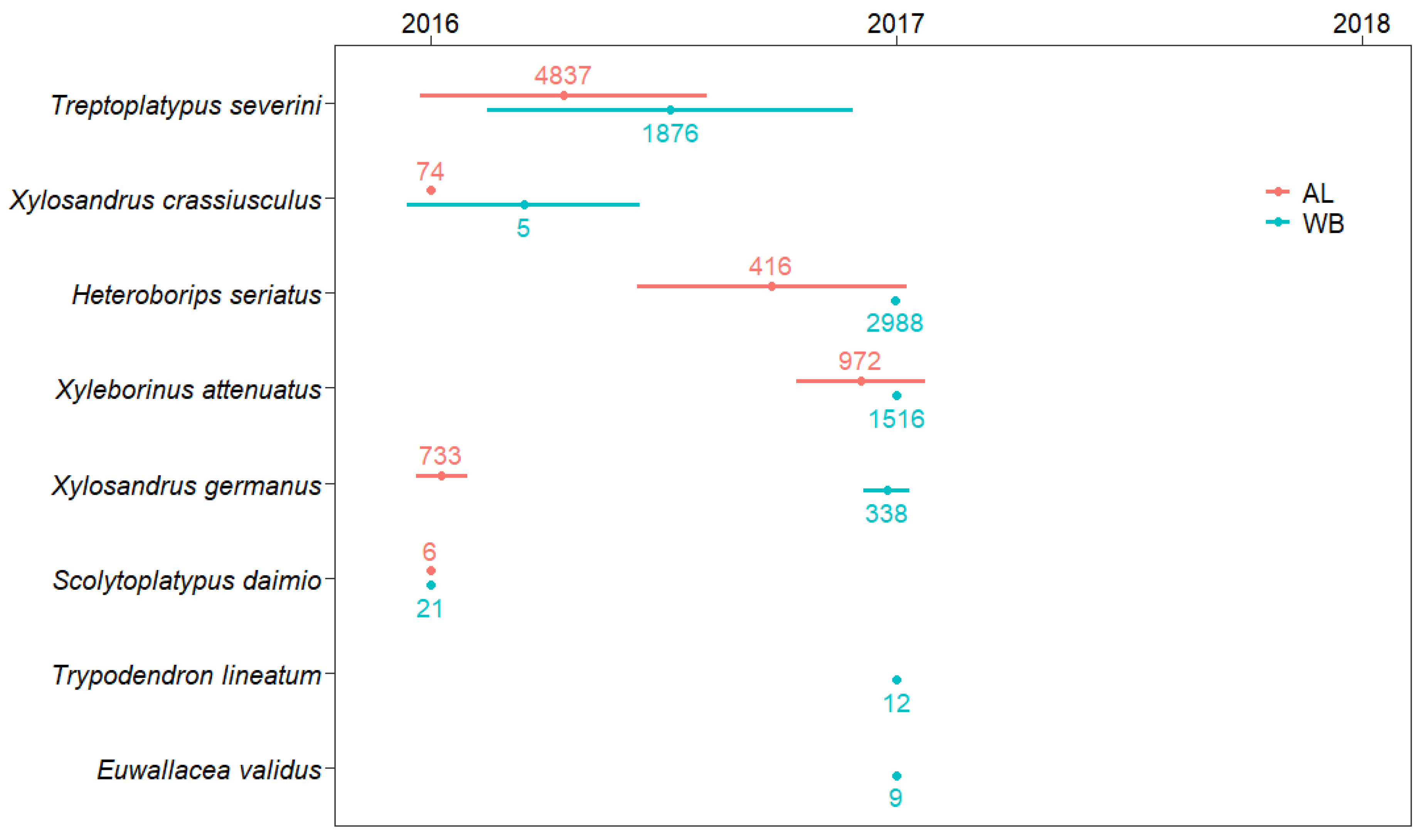
| Platypodinae | Platypodini | Treptoplatypus severini (Blandford) 2 | TS | 3476 (77.99) | 1342 (52.40) | 19 (95.00) | 953 (96.26) | 880 (15.36) | 43 (95.56) |
| Scolytinae | Xyleborini | Xyleborinus attenautus (Blandford) | XA | 74 (1.66) | 897 (35.03) | 1 (5.00) | 0 | 1514 (26.42) | 2 (4.44) |
| Xylosandrus germanus (Blandford) | XG | 716 (16.06) | 17 (0.66) | 0 | 7 (0.71) | 331 (5.78) | 0 | ||
| Heteroboripsseriatus (Blandford) 3 | HS | 111 (2.49) | 305 (11.91) | 0 | 4 (0.40) | 2984 (52.07) | 0 | ||
| Xylosandrus crassiusculus (Motschulsky) | XC | 74 (1.66) | 0 | 0 | 4 (0.40) | 1 (0.02) | 0 | ||
| Euwallacea validus (Eichhoff) | EV | 0 | 0 | 0 | 0 | 9 (0.16) | 0 | ||
| Xyloterini | Trypodendron lineatum (Olivier) | TL | 0 | 0 | 0 | 0 | 12 (0.21) | 0 | |
| Scolytoplatypodini | Scolytoplatypus daimio Blandford | SD | 6 (0.13) | 0 | 0 | 21 (2.12) | 0 | 0 | |
| Scolytoplatypus mikado Blandford | SM | 0 | 0 | 0 | 1 (0.10) | 0 | 0 | ||
| Total | 4457 | 2561 | 20 | 990 | 5731 | 45 | |||
| Number of species | 6 | 4 | 2 | 6 | 7 | 2 | |||
| Factors | Predictors | Niche Center | Niche Breadth | |||
|---|---|---|---|---|---|---|
| Estimates | p-Value | Estimates | p-Value | |||
| Tree species | (Intercept) | 1.39 | <0.001 | 0.249 | 0.072 | |
| White birch | 0.321 | 0.063 | −0.0726 | 0.620 | ||
| Insect species | (Intercept) | 1.963 | <0.001 | 0.144 | 0.431 | |
| Euwallacea validus | −0.073 | 0.818 | −0.144 | 0.642 | ||
| Treptoplatypus severini | −0.563 | 0.067 | 0.557 | 0.066 | ||
| Scolytoplatypus daimio | −0.963 | 0.010 | −0.144 | 0.571 | ||
| Trypodendron lineatum | −0.073 | 0.818 | −0.144 | 0.642 | ||
| Xyleborus seriatus | −0.097 | 0.705 | 0.151 | 0.551 | ||
| Xylosandrus crassiusculus | −0.863 | 0.016 | 0.107 | 0.672 | ||
| Xylosandrus germanus | −0.462 | 0.115 | −0.038 | 0.879 | ||
| Factors | R2 | p-Value |
|---|---|---|
| Tree species (SP) | 0.0248 | <0.001 |
| Sampling year (YR) | 0.3417 | <0.001 |
| SP * YR | 0.1126 | <0.001 |
| Insect Species | Tree Species | Alder | White Birch | |||||
|---|---|---|---|---|---|---|---|---|
| Alder | White birch | 2016 | 2017 | 2016 + 2017 | 2016 | 2017 | 2016 + 2017 | |
| Treptoplatypus severini | 0.323 *** | 0.465 *** | 0.264 * | |||||
| Xyleborinus attenautus | 0.582 *** | 0.536 *** | ||||||
| Xylosandrus germanus | 0.100 * | 0.276 ** | 0.502 *** | |||||
| Heteroborips seriatus | 0.235 *** | 0.294 ** | 0.561 *** | |||||
| Xylosandrus crassiusculus | 0.194 *** | 0.319 ** | ||||||
| Trypodendron lineatum | 0.128 ** | 0.254 ** | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Buranapanichpan, A.; Kamata, N. Succession of Ambrosia Beetles Colonizing the Logs of Fallen Alder and Birch Trees. Insects 2022, 13, 223. https://doi.org/10.3390/insects13030223
Peng Y, Buranapanichpan A, Kamata N. Succession of Ambrosia Beetles Colonizing the Logs of Fallen Alder and Birch Trees. Insects. 2022; 13(3):223. https://doi.org/10.3390/insects13030223
Chicago/Turabian StylePeng, Yong, Anut Buranapanichpan, and Naoto Kamata. 2022. "Succession of Ambrosia Beetles Colonizing the Logs of Fallen Alder and Birch Trees" Insects 13, no. 3: 223. https://doi.org/10.3390/insects13030223
APA StylePeng, Y., Buranapanichpan, A., & Kamata, N. (2022). Succession of Ambrosia Beetles Colonizing the Logs of Fallen Alder and Birch Trees. Insects, 13(3), 223. https://doi.org/10.3390/insects13030223







