Nematodes in the Pine Forests of Northern and Central Greece
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
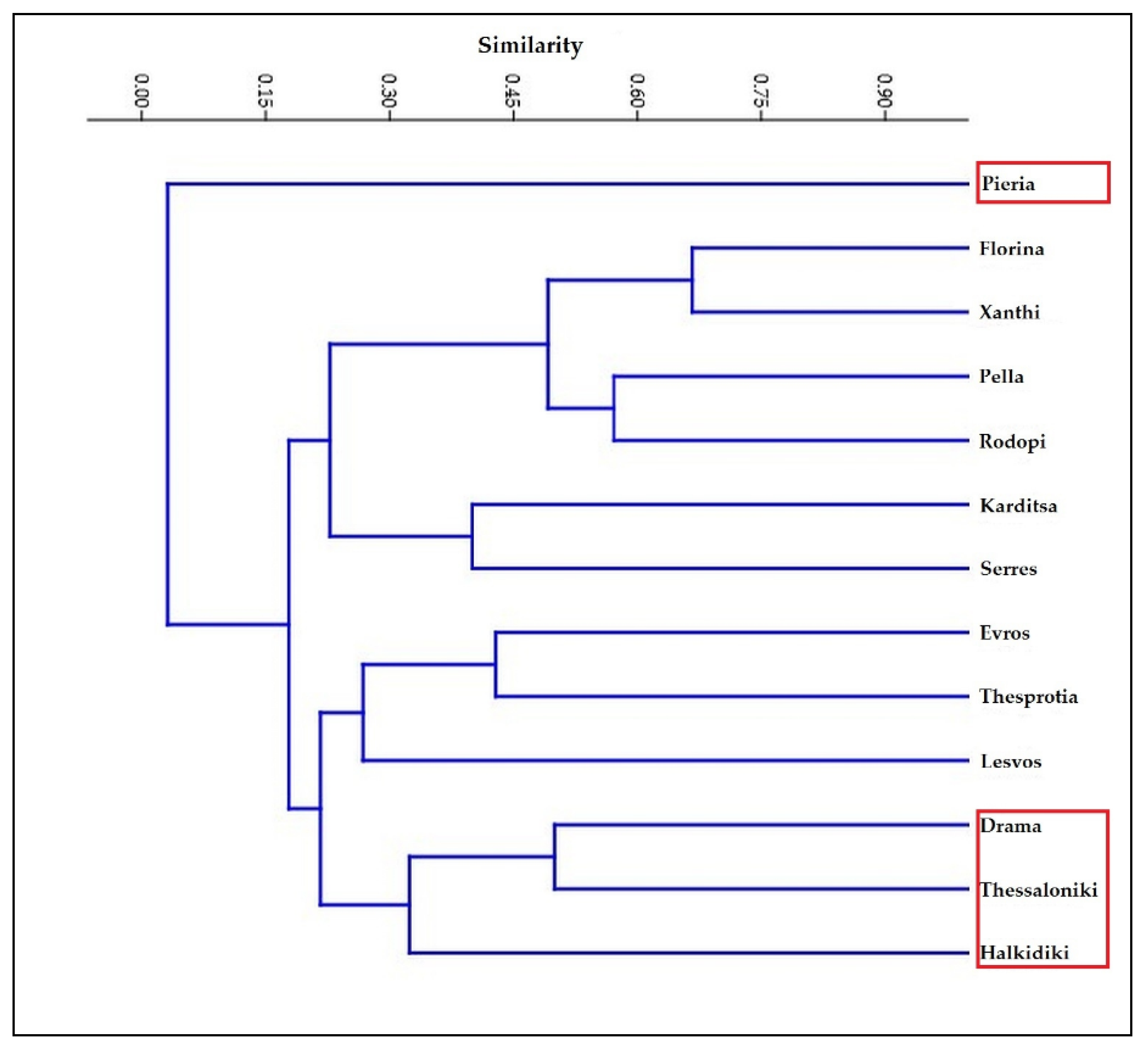
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inácio, M.; Nobrega, F.; Vieira, P.; Bonifacio, L.; Naves, P.; Sousa, E.; Mota, M. First detection of Bursaphelenchus xylophilus associated with Pinus nigra in Portugal and in Europe. For. Pathol. 2015, 45, 235–238. [Google Scholar] [CrossRef]
- Back, M. Pine Wilt Disease a Global Threat to Forestry. Plant Pandemic Study 4. 2020 British Society for Plant Pathology. Available online: https://www.bspp.org.uk/ (accessed on 15 June 2021).
- Naves, P.; Bonifácio, L.; de Sousa, E. The pine wood nematode and its local vectors in the Mediterranean Basin. In Insects and Diseases of Mediterranean Forest Systems; Paine, T., Lieutier, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 329–378. [Google Scholar] [CrossRef]
- Mota, M.M.; Futai, K.; Vieira, P. Pine wilt disease and the pinewood nematode, Bursaphelenchus xylophilus. In Integrated Management of Fruit Crops Nematodes; Ciancio, A., Mukerji, K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 253–274. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Evans, H.; Kulinich, O.; Magnusson, C.; Robinet, C.; Schroeder, T. Report of a Pest Risk Analysis for Bursaphelenchus xylophilus. 09/15450; EPPO: Paris, France, 2009; pp. 1–17. [Google Scholar]
- Sousa, E.; Naves, P.; Bonifácio, L.; Inácio, L.; Henriques, J.; Evans, H. Survival of Bursaphelenchus xylophilus and Monochamus galloprovincialis in pine branches and wood packaging material. Bull. OEPP/EPPO Bull. 2011, 41, 203–207. [Google Scholar] [CrossRef]
- Ryss, A.; Vieira, P.; Mota, M.; Kulinich, O. A synopsis of the genus Bursaphelenchus Fuchs, 1937 (Aphelenchida: Parasitaphelenchidae) with keys to species. Nematology 2005, 7, 393–458. [Google Scholar] [CrossRef]
- Nickle, W.; Golden, A.; Mamiya, Y.; Wergin, W. On the taxonomy and morphology of the pine wood nematode, Bursaphelenchus xylophilus (Steiner & Buhrer 1934) Nickle 1970. J. Nematol. 1981, 13, 385. [Google Scholar]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Malek, R.B.; Appleby, J.E. Epidemiology of pine wilt in Illinois. Disease distribution. Plant Dis. 1984, 68, 180–186. [Google Scholar] [CrossRef]
- Liebhold, A.M.; MacDonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by exotic forest pests: A threat to forest ecosystems. For. Sci. 1995, 41 (Suppl. S1), a0001–z0001. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Sun, J.; Liu, J.; Fei, K.; Zhang, C.; Xu, M.; Sun, J.; Ma, X.; Lai, R. Molecular phylogeny of geographical isolates of Bursaphelenchus xylophilus: Implications on the origin and spread of this species in China and worldwide. J. Nematol. 2008, 40, 127–137. [Google Scholar]
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219. [Google Scholar]
- Futai, K. Pine wilt in Japan: From first incidence to the present. In Pine wilt disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 5–12. [Google Scholar] [CrossRef]
- Li, G.; Shao, G.; Huo, Y.; Xu, F. Discovery of and preliminary investigations on pine wood nematodes in China. For. Sci. Technol. 1983, 7, 25–28. [Google Scholar]
- Dwinell, L.D. The pinewood nematode: Regulation and mitigation. Annu. Rev. Phytopathol. 1997, 35, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.G. Pine wilt disease in China. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 18–25. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-C. Pine wilt disease in Korea. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 26–32. [Google Scholar] [CrossRef]
- An, H.; Lee, S.; Cho, S.J. The effects of climate change on pine wilt disease in South Korea: Challenges and Prospects. Forests 2019, 10, 486. [Google Scholar] [CrossRef]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Fonseca, L.; Cardoso, J.; Lopes, A.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 2012, 49, 96–103. [Google Scholar] [CrossRef]
- Tóth, Á.; Elekes, M.; Kiss, J. Monitoring quarantine pine wood nematode, Bursaphelenchus xylophilus in Hungary. Acta Phytopathol. Entomol. Hung. 2012, 47, 55–59. [Google Scholar] [CrossRef]
- Soliman, T.; Mourits, M.C.; Van Der Werf, W.; Hengeveld, G.M.; Robinet, C.; Lansink, A.G.O. Framework for modelling economic impacts of invasive species, applied to pine wood nematode in Europe. PLoS ONE 2012, 7, e45505. [Google Scholar] [CrossRef]
- Braasch, H. Influence of temperature and water supply on mortality of 3-year-old pines inoculated with Bursaphelenchus xylophilus and B. mucronatus. Nachr. Deut. Pflanzenschutzd. 2000, 52, 244–249. [Google Scholar]
- Ichihara, Y.; Fukuda, K.; Suzuki, K. Early symptom development and histological changes associated with migration of Bursaphelenchus xylophilus in seedling tissues of Pinus thunbergii. Plant Dis. 2000, 84, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Matsunaga, K.; Watanabe, A. Influence of temperature on pine wilt disease progression in Pinus thunbergii seedlings. Eur. J. Plant Pathol. 2020, 156, 581–590. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef] [PubMed]
- Skordilis, A.; Thanos, C. Comparative ecophysiology of seed germination strategies in the seven pine species naturally growing in Greece. In Basic and Applied Aspects of Seed Biology; Ellis, R.H., Black, M., Murdoch, A.J., Hong, T.D., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 623–632. [Google Scholar] [CrossRef]
- Skarmoutsos, G.; Skarmoutsou, H. First record of Bursaphelenchus nematodes from pine forests in Greece. Plant Dis. 1999, 83, 879. [Google Scholar] [CrossRef]
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R.; Alpert, P.; Artale, V.; Li, L.; Luterbacher, J.; May, W.; Trigo, R.; Tsimplis, M. The Mediterranean climate: An overview of the main characteristics and issues. Dev. Earth Environ. Sci. 2006, 4, 1–26. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Rutherford, T.; Mamiya, Y.; Webster, J. Nematode-induced pine wilt disease: Factors influencing its occurrence and distribution. For. Sci. 1990, 36, 145–155. [Google Scholar] [CrossRef]
- Skarmoutsos, G.; Michalopoulos-Skarmoutsos, H. Pathogenicity of Bursaphelenchus sexdentati, Bursaphelenchus leoni and Bursaphelenchus hellenicus on European pine seedlings. For. Pathol. 2000, 30, 149–156. [Google Scholar] [CrossRef]
- Van Bezooijen, J. Methods and Techniques for Nematology; Wageningen University: Wageningen, The Netherlands, 2006; pp. 19–20. [Google Scholar]
- Braasch, H.; Burgermeister, W.; Gu, J. Revised intra-generic grouping of Bursaphelenchus Fuchs, 1937 (Nematoda: Aphelenchoididae). J. Nematode Morphol. Syst. 2009, 12, 65–88. [Google Scholar]
- Bongers, T. Identification Key: De Nematoden van Nederland, 2nd ed.; KNNV Bibliotheekuitgave: Utrecht, The Netherland, 1994; Volume 46, 408p. [Google Scholar]
- Ryss, A.Y.; Subbotin, S. Coevolution of wood-inhabiting nematodes of the genus Bursaphelenchus Fuchs, 1937 with their insect vectors and plant hosts. Zhurnal Obs. Biol. 2017, 78, 13–42. [Google Scholar]
- Kanzaki, N.; Giblin-Davis, R.M. Diversity and plant pathogenicity of Bursaphelenchus and related nematodes in relation to their vector bionomics. Curr. For. Rep. 2018, 4, 85–100. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—an outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar] [PubMed]
- Scholze, V.; Sudhaus, W. A pictorial key to current genus groups of ‘Rhabditidae’. J. Nematode Morphol. Syst. 2011, 14, 105–112. [Google Scholar]
- Nemaplex—The “Nematode-Plant Expert Information System” A Virtual Encyclopedia on Soil and Plant Nematodes. Available online: http://nemaplex.ucdavis.edu/ (accessed on 29 December 2021).
- Hammer, O.; Harper, A.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- GEODATA.gov.gr. Available online: www.geodata.gov.gr (accessed on 2 December 2020).
- Gu, J.; Wang, J.; Zheng, J. Devibursaphelenchus wangi sp. n. (Nematoda: Ektaphelenchinae) feeding on Aphelenchoides sp. Russ. J. Nematol. 2010, 18, 49–57. [Google Scholar]
- Aliramaji, F.; Pourjam, E.; Atighi, M.R.; Karegar, A.; Pedram, M. Devibursaphelenchus kheirii sp. n. (Nematoda: Ektaphelenchinae) from Iran with remarks on Devibursaphelenchus Kakuliya, 1967. Nematology 2014, 16, 1069–1078. [Google Scholar] [CrossRef]
- Akbulut, S.; Stamps, W. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Douma, J.; van der Werf, W.; Hemerik, L.; Magnusson, C.; Robinet, C. Development of a pathway model to assess the exposure of European pine trees to pine wood nematode via the trade of wood. Ecol. Appl. 2017, 27, 769–785. [Google Scholar] [CrossRef]
- EPPO Datasheet: Bursaphelenchus xylophilus. Available online: https://gd.eppo.int/taxon/BURSXY/datasheet (accessed on 30 December 2021).
- Webster, J.; Mota, M. Pine wilt disease: Global issues, trade and economic impact. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems, 1st ed.; Mota, M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–3. [Google Scholar]
- Gu, J.; Braasch, H.; Burgermeister, W.; Zhang, J. Records of Bursaphelenchus spp. intercepted in imported packaging wood at Ningbo, China. For. Pathol. 2006, 36, 323–333. [Google Scholar] [CrossRef]
- Robinet, C.; Van Opstal, N.; Baker, R.; Roques, A. Applying a spread model to identify the entry points from which the pine wood nematode, the vector of pine wilt disease, would spread most rapidly across Europe. Biol. Invasions 2011, 13, 2981–2995. [Google Scholar] [CrossRef]
- PM, E. 9/1 Bursaphelenchus xylophilus and its vectors: Procedures for official control. EPPO Bull. 2018, 48, 503–505. [Google Scholar]
- Evans, H.; McNamara, D.; Braasch, H.; Chadoeuf, J.; Magnusson, C. Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bull. 1996, 26, 199–249. [Google Scholar] [CrossRef]
- Meteo. Available online: https://meteo.gr/ (accessed on 31 December 2021).
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Naves, P.; de Sousa, E. Threshold temperatures and degree-day estimates for development of post-dormancy larvae of Monochamus galloprovincialis (Coleoptera: Cerambycidae). J. Pest Sci. 2009, 82, 1–6. [Google Scholar] [CrossRef]
- Sousa, E.; Bravo, M.A.; Pires, J.; Naves, P.; Penas, A.C.; Bonifácio, L.; Mota, M.M. Bursaphelenchus xylophilus (Nematoda; Aphelenchoididae) associated with Monochamus galloprovincialis (Coleoptera; Cerambycidae) in Portugal. Nematology 2001, 3, 89–91. [Google Scholar] [CrossRef]
- Koutroumpa, F.A.; Rougon, D.; Bertheau, C.; Lieutier, F.; Roux-Morabito, G. Evolutionary relationships within European Monochamus (Coleoptera: Cerambycidae) highlight the role of altitude in species delineation. Biol. J. Linn. Soc. 2013, 109, 354–376. [Google Scholar] [CrossRef][Green Version]
- Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.A.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; Navas-Cortes, J.A. Pest categorisation of non-EU Monochamus spp. EFSA J. 2018, 16, e05435. [Google Scholar] [CrossRef]
- European Food Safety Authority; Schenk, M.; Loomans, A.; den Nijs, L.; Hoppe, B.; Kinkar, M.; Vos, S. Pest survey card on Bursaphelenchus xylophilus. EFSA Support. Publ. 2020, 17, 1782E. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Sheng, R.-C.; Sun, H.; Sun, S.-H.; Chen, F.-M. The first record of Monochamus saltuarius (Coleoptera; Cerambycidae) as vector of Bursaphelenchus xylophilus and its new potential hosts in China. Insects 2020, 11, 636. [Google Scholar] [CrossRef]
- Pimentel, C.S.; Ayres, M.P.; Vallery, E.; Young, C.; Streett, D.A. Geographical variation in seasonality and life history of pine sawyer beetles Monochamus spp: Its relationship with phoresy by the pinewood nematode Bursaphelenchus xylophilus. Agric. For. Entomol. 2014, 16, 196–206. [Google Scholar] [CrossRef]
- Gavrikov, V.L.; Vetrova, V.P. Effects of fir sawyer beetle on spatial structure of Siberian fir stands. In Forest Insect Guilds: Patterns of Interaction with Host Trees; Baranchikov, Y.N., Mattson, W.J., Hain, F.P., Payne, T.L., Eds.; Gen. Tech. Rep. NE-153; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1991. [Google Scholar]
- Togashi, K. Vector-nematode relationships and epidemiology in pine wilt disease. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 162–183. [Google Scholar]
- Michalopoulos-Skarmoutsos, H.; Skarmoutsos, G.; Kalapanida, M.; Karageorgos, A. Surveying and recording of nematodes of the genus Bursaphelenchus in conifer forests in Greece and pathogenicity of the most important species. In The Pinewood Nematode, Bursaphelenchus xylophilus, Proceedings of the International Workshop, Évora, Portugal, 20–22 August 2001; Mota, M., Vieira, P., Eds.; Brill Academic Publishers: Leiden, The Netherlands, 2004; pp. 113–126. [Google Scholar]
- Lange, C.; Burgermeister, W.; Metge, K.; Braasch, H. Molecular Characterization of Isolates of the Bursaphelenchus sexdentati Group Using Ribosomal DNA Sequences and ITS-RFLP. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems, 1st ed.; Mota, M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 165–173. [Google Scholar] [CrossRef]
- Braasch, H. Bursaphelenchus species in conifers in Europe: Distribution and morphological relationships. Bull. OEPP/EPPO Bull. 2001, 31, 127–142. [Google Scholar] [CrossRef]
- D’Errico, G.; Carletti, B.; Schröder, T.; Mota, M.; Vieira, P.; Roversi, P.F. An update on the occurrence of nematodes belonging to the genus Bursaphelenchus in the Mediterranean area. Forestry 2015, 88, 509–520. [Google Scholar] [CrossRef]
- Torrini, G.; Paoli, F.; Mazza, G.; Simoncini, S.; Strangi, A.; Guidotti, A.; Mori, E.; Roversi, P.F.; Marianelli, L. First detection of Bursaphelenchus abietinus and B. andrassyi in Italy. For. Pathol. 2020, 50, e12627. [Google Scholar] [CrossRef]
- Akbulut, S.; Braasch, H.; Cebeci, H. First report of Bursaphelenchus hellenicus Skarmoutsos, Braasch, Michalopoulou (Nematoda: Aphelenchoididae) from Turkey. For. Pathol. 2013, 43, 402–406. [Google Scholar] [CrossRef]
- Skarmoutsos, G.; Braasch, H.; Michalopoulou, H. Bursaphelenchus hellenicus sp. n. (Nematoda, Aphelenchoididae) from Greek pine wood. Nematologica 1998, 44, 623–629. [Google Scholar] [CrossRef]
- Polomski, J.; Rigling, D. Effect of watering regime on disease development in Pinus sylvestris seedlings inoculated with Bursaphelenchus vallesianus and B. mucronatus. Plant Dis. 2010, 94, 1055–1061. [Google Scholar] [CrossRef]
- Akbulut, S.; Yüksel, B.; Serin, M.; Erdem, M. Comparison of pathogenic potential of Bursaphelenchus species on conifer seedlings between greenhouse and outdoor conditions. Phytoparasitica 2015, 43, 209–214. [Google Scholar] [CrossRef]
- Öztürk, N.; Akbulut, S.; Baysal, İ. Determination of pathogenicity of Bursaphelenchus species on different pine species under natural conditions in Düzce. Phytoparasitica 2019, 47, 89–97. [Google Scholar] [CrossRef]
- Caroppo, S.; Cavalli, M.; Coniglio, D.; Ambrogioni, L. Pathogenicity studies with various Bursaphelenchus populations on conifer seedlings under controlled and open air conditions. Redia 2000, 83, 61–75. [Google Scholar]
- Philis, J.; Braasch, H. Occurrence of Bursaphelenchus leoni (Nematoda, Aphelenchoididae) in Cyprus and its extraction from pine wood. Nematol. Mediterr. 1996, 24, 119–123. [Google Scholar]
- Braasch, H.; Philis, J. New records of Bursaphelenchus spp. in Cyprus. Nematol. Mediterr. 2002, 30, 55–57. [Google Scholar]
- Akbulut, S.; Vieira, P.; Ryss, A.; Yuksel, B.; Keten, A.; Mota, M.; Valadas, V. Preliminary survey of the pinewood nematode in Turkey. Bull. OEPP/EPPO Bull. 2006, 36, 538–542. [Google Scholar] [CrossRef]
- Akbulut, S.; Braasch, H.; Baysal, İ.; Brandstetter, M.; Burgermeister, W. Description of Bursaphelenchus anamurius sp. n. (Nematoda: Parasitaphelenchidae) from Pinus brutia in Turkey. Nematology 2007, 9, 859–867. [Google Scholar] [CrossRef]
- Akbulut, S.; Vieira, P.; Ryss, A.; Valadas, V.; Keten, A.; Mota, M. Bursaphelenchus Fuchs, 1937 (Nematoda: Parasitaphelenchidae) species associated with Pinus species in northern Turkey. Helminthologia 2008, 45, 89–95. [Google Scholar] [CrossRef]
- Akbulut, S.; Elekçioğlu, I.H.; Keten, A. First record of Bursaphelenchus vallesianus Braasch, Schönfeld, Polomski, and Burgermeister in Turkey. Turk. J. Agric. For. 2008, 32, 273–279. [Google Scholar]
- Ambrogioni, L.; Palmisano, A.M. Description of Bursaphelenchus tusciae sp. n. from Pinus pinea in Italy. Nematol. Mediterr. 1998, 26, 243–254. [Google Scholar]
- Carletti, B.; Irdani, T.; Cosi, E.; Brandstetter, M.; Pennacchio, F.; Roversi, P.F.; Ambrogioni, L. First record of Bursaphelenchus fraudulentus Rühm (Goodey) (Nematoda Aphelenchoididae) in Italy. Redia 2006, 88, 27–35. [Google Scholar]
- Carletti, B.; Ambrogioni, L.; Irdani, T.; Brandstetter, M.; Puleri, F.; Surico, F.; Pennacchio, F.; Roversi, P. Morphometrics and molecular identification of some Italian populations of Bursaphelenchus eremus Rühm (Goodey) associated with Quercus spp. Redia 2007, 90, 3–21. [Google Scholar]
- Torrini, G.; Strangi, A.; Simoncini, S.; Luppino, M.; Roversi, P.F.; Marianelli, L. First report of Bursaphelenchus fungivorus (Nematoda: Aphelenchida) in Italy and an overview of nematodes associated with Crocus sativus L. J. Nematol. 2020, 52, e2020-23. [Google Scholar] [CrossRef]
- Caroppo, S.; Ambrogioni, L.; Cavalli, M.; Coniglio, D. Occurrence of the pine wood nematodes, Bursaphelenchus spp., and their possible vectors in Italy. Nematol. Mediter.r 1998, 26, 87–92. [Google Scholar]
- Penas, A.C.; Bravo, M.A.; Naves, P.; Bonifácio, L.; Sousa, E.; Mota, M. Species of Bursaphelenchus Fuchs, 1937 (Nematoda: Parasitaphelenchidae) and other nematode genera associated with insects from Pinus pinaster in Portugal. Ann. Appl. Biol. 2006, 148, 121–131. [Google Scholar] [CrossRef]
- Robertson, L.; García-Álvarez, A.; Arcos, S.C.; Díez-Rojo, M.; Mansilla, J.P.; Sanz, R.; Martínez, C.; Escuer, M.; Castresana, L.; Notario, A. Potential insect vectors of Bursaphelenchus spp. (Nematoda: Parasitaphelenchidae) in Spanish pine forests. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems, 1st ed.; Mota, M., Vieira, P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 221–234. [Google Scholar]
- Polyanina, K.; Mandelshtam, M.Y.; Ryss, A.Y. Brief review of the associations of xylobiont nematodes with bark beetles (Coleoptera, Curculionidae: Scolytinae). Entomol. Rev. 2019, 99, 598–614. [Google Scholar] [CrossRef]
- Dayi, M.; Akbulut, S. Survey for the detection of Bursaphelenchus insect-vector species in the western part of Turkey. Kast. Univ. J. For. Fac. 2018, 18, 215–224. [Google Scholar] [CrossRef]
- Sousa, E.; Naves, P.; Bonifácio, L.; Bravo, M.; Penas, A.; Pires, J.; Serrao, M. Preliminary survey for insects associated with Bursaphelenchus xylophilus in Portugal. EPPO Bull. 2002, 32, 499–502. [Google Scholar] [CrossRef]
- Andreieva, O.; Korma, O.; Zhytova, O.; Martynchuk, I.; Vyshnevskyi, A. Beetles and nematodes associated with wither Scots pines. Cent. Eur. For. J. 2020, 66, 49–59. [Google Scholar] [CrossRef]
- Polomski, J.; Schönfeld, U.; Braasch, H.; Dobbertin, M.; Burgermeister, W.; Rigling, D. Occurrence of Bursaphelenchus species in declining Pinus sylvestris in a dry Alpine valley in Switzerland. For. Pathol. 2006, 36, 110–118. [Google Scholar] [CrossRef]
- Mamiya, Y. Initial pathological changes and disease development in pine trees induced by the pine wood nematode, Bursaphelenchus xylophilus. Jpn. J. Phytopathol. 1985, 51, 546–555. [Google Scholar] [CrossRef]
- Kuroda, K.; Yamada, T.; Mineo, K.; Tamura, H. Effects of cavitation on the development of pine wilt disease caused by Bursaphelenchus xylophilus. Jpn. J. Phytopathol. 1988, 54, 606–615. [Google Scholar] [CrossRef]
- Kiyohara, T.; Suzuki, K. Nematode population growth and disease development in the pine wilting disease. Eur. J. For. Pathol. 1978, 8, 285–292. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, P.; Humble, L.M.; Sun, J. Within-tree distribution and attractant sampling of propagative pinewood nematode, Bursaphelenchus xylophilus: An early diagnosis approach. For. Ecol. Manag. 2009, 258, 1932–1937. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Aikawa, T.; Matsushita, M.; Hoshizaki, K. Sampling design for efficient detection of pine wood nematode, Bursaphelenchus xylophilus, in diseased trees using a DNA detection kit: Variation across branch, trunk and tree. Nematology 2018, 20, 641–652. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Moll, J.; Roy, F.; Bässler, C.; Heilmann-Clausen, J.; Hofrichter, M.; Kellner, H.; Krabel, D.; Schmidt, J.H.; Buscot, F.; Hoppe, B. First Evidence That Nematode Communities in Deadwood Are Related to Tree Species Identity and to Co-Occurring Fungi and Prokaryotes. Microorganisms 2021, 9, 1454. [Google Scholar] [CrossRef] [PubMed]
- Welch, H. Entomophilic nematodes. Annu. Rev. Entomol. 1965, 10, 275–302. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr. Nematodes as facultative parasites of insects. Annu. Rev. Entomol. 1972, 17, 103–122. [Google Scholar] [CrossRef]
- Adams, B.; Nguyen, K. Nematode parasites of insects. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2577–2584. [Google Scholar] [CrossRef]
- Grucmanová, Š.; Holuša, J. Nematodes associated with bark beetles, with focus on the genus Ips (Coleoptera: Scolytinae) in Central Europe. Acta Zool. Bulg. 2013, 65, 547–556. [Google Scholar]
- Prospero, S.; Polomski, J.; Rigling, D. Occurrence and ITS diversity of wood-associated Bursaphelenchus nematodes in Scots pine forests in Switzerland. Plant Pathol. 2015, 64, 1190–1197. [Google Scholar] [CrossRef]
- Đođ, N.; Cota, E.; Pernek, M. Wood nematode species spectrum in the Mediterranean pine forests of Croatia. Period. Biol. 2015, 117, 505–512. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Zhang, Y. Latitudinal variation in nematode diversity and ecological roles along the Chinese coast. Ecol. Evol. 2016, 6, 8018–8027. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J.R. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978. [Google Scholar] [CrossRef]
- Da Silva, J.V.L.; Hirschfeld, M.N.C.; Cares, J.E.; Esteves, A.M. Land use, soil properties and climate variables influence the nematode communities in the Caatinga dry forest. Appl. Soil Ecol. 2020, 150, 103474. [Google Scholar] [CrossRef]
- Fitoussi, N.; Pen-Mouratov, S.; Steinberger, Y. Soil free-living nematodes as bio-indicators for assaying the invasive effect of the alien plant Heterotheca subaxillaris in a coastal dune ecosystem. Appl. Soil Ecol. 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model–Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]


| Regional Unit | Locality | Tree Species | Bursaphelenchus spp. | Other Nematode spp. |
|---|---|---|---|---|
| Halkidiki | Kassandra | Pinus halepensis | B. leoni | Aphelenchus sp. |
| Pinus halepensis | B. mucronatus | Devibursaphelenchus sp. | ||
| Pinus halepensis | Diplenteron sp. | |||
| Pinus halepensis | Parasitorhabditis sp. | |||
| Pinus halepensis | Panagrolaimus sp. | |||
| Pinus halepensis | Pristionchus sp. | |||
| Pinus halepensis | Thonus sp. | |||
| Drama | Drama | Pinus sp. | Parasitorhabditis sp. | |
| Pinus sp. | Tylenchidae | |||
| Neurokopi | Pinus sp. | B. mucronatus | Bacterivore | |
| Pinus sylvestris | B. hellenicus | Aphelenchoides sp. | ||
| Pinus sylvestris | B. leoni | Anguinidae | ||
| Evros | Alexandroupoli | Pinus brutia | Ektaphelenchus sp. | |
| Pinus brutia | Panagrolaimus sp. | |||
| Pinus brutia | Parasitorhabditis sp. | |||
| Pinus brutia | Rhabditis sp. | |||
| Pinus brutia | Anguinidae | |||
| Pinus sp. | B. hellenicus | Clarkus sp. | ||
| Pinus sp. | Bursaphelenchus sp. | Eucehpalobus sp. | ||
| Pinus sp. | Laimaphelenchus sp. | |||
| Pinus sp. | Parasitorhabditis sp. | |||
| Pinus sp. | Plant parasitic | |||
| Florina | Florina | Pinus nigra | Laimaphelenchus sp. | |
| Karditsa | Mouzaki | Pinus brutia | Bursaphelenchus sp. | Aphelenchoides sp. |
| Pinus brutia | Rhabditis sp. | |||
| Pinus brutia | Anguinidae | |||
| Pinus brutia | Dolicodoridae | |||
| Lesvos | Lesvos | Pinus brutia | B. hellenicus | Eucehpalobus sp. |
| Pinus brutia | B. sexdentati | Plectus sp. | ||
| Pella | Aridaia | Abies borisii-regis | B. mucronatus | |
| Pinus sp. | Bursaphelenchus sp. | Aphelenchoides sp. | ||
| Pinus sp. | Laimaphelenchus sp. | |||
| Pella | Pinus sylvestris | Laimaphelenchus sp. | ||
| Pieria | Pieria | Pinus nigra | Panagrolaimus sp. | |
| Rodopi | Rodopi | Pinus maritima | B. hellenicus | Laimaphelenchus sp. |
| Pinus maritima | B. mucronatus | Bacterivore | ||
| Serres | Sidirokastro | Pinus brutia | Laimaphelenchus sp. | |
| Pinus brutia | Merlinius sp. | |||
| Pinus brutia | Anguinidae | |||
| Pinus brutia | Dolichodoridae | |||
| Pinus brutia | Rhabditidae | |||
| Thesprotia | Thesprotia | Pinus sp. | B. hellenicus | Clarkus sp. |
| Pinus sp. | Bursaphelenchus sp. | Tylencholaimellus sp. | ||
| Thessaloniki | Lagkadas | Pinus sp. | Bursaphelenchus sp. | Laimaphelenchus sp. |
| Pinus sp. | Parasitorhabditis sp. | |||
| Pinus sp. | Rhodolaimus sp. | |||
| Thessaloniki | Pinus maritima | B. sexdentati | Aphelenchus sp. | |
| Pinus maritima | Laimaphelenchus sp. | |||
| Pinus maritima | Panagrobelus sp. | |||
| Pinus maritima | Plectus sp. | |||
| Pinus sp. | B. hellenicus | Aphelenchoides sp. | ||
| Pinus sp. | B. leoni | Merlinius sp. | ||
| Pinus sp. | B. sexdentati | Parasitorhabditis sp. | ||
| Pinus sp. | Bursaphelenchus sp. | Anguinidae | ||
| Xanthi | Xanthi | Pinus sylvestris | Bursaphelenchus sp. | Laimaphelenchus sp. |
| Pinus sylvestris | Bacterivore |
| Bursaphelenhus spp. | Cyprus | Greece | Italy | Turkey |
|---|---|---|---|---|
| B. abietinus | ● | |||
| B. anamurius | ● | |||
| B. andrassyi | ● | |||
| B. eremus | ● | |||
| B. fraudulentus | ● | |||
| B. fungivorous | ● | |||
| B. hellenicus | ● | ● | ● | |
| B. idius | ● | |||
| B. leoni | ● | ● | ● | |
| B. minutus | ● | |||
| B. mucronatus | ● | ● | ||
| B. pinophilus | ● | |||
| B. sexdentati | ● | ● | ● | ● |
| B. tusciae | ● | |||
| B. vallesianus | ● | ● |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmezi, M.; Bataka, A.; Papachristos, D.; Avtzis, D.N. Nematodes in the Pine Forests of Northern and Central Greece. Insects 2022, 13, 194. https://doi.org/10.3390/insects13020194
Karmezi M, Bataka A, Papachristos D, Avtzis DN. Nematodes in the Pine Forests of Northern and Central Greece. Insects. 2022; 13(2):194. https://doi.org/10.3390/insects13020194
Chicago/Turabian StyleKarmezi, Maria, Alkmini Bataka, Dimitrios Papachristos, and Dimitrios N. Avtzis. 2022. "Nematodes in the Pine Forests of Northern and Central Greece" Insects 13, no. 2: 194. https://doi.org/10.3390/insects13020194
APA StyleKarmezi, M., Bataka, A., Papachristos, D., & Avtzis, D. N. (2022). Nematodes in the Pine Forests of Northern and Central Greece. Insects, 13(2), 194. https://doi.org/10.3390/insects13020194







