Simple Summary
The biology of many species of cuckoo wasps (Chrysididae) is largely unknown, except for, if at all, the host species of these brood parasites. Cuticular hydrocarbons (CHCs), which form a waxy layer on the body surface of insects, have been shown to be mimicked by host-specific cuckoo wasp species. We studied the CHC profiles of a rather generalist brood parasitic cuckoo wasp species, Trichrysis cyanea, in detail. We found sex-related differences in CHCs and three different chemotypes among females. Genetic data reject the possibility that these different chemotypes represent different (cryptic) species. The CHC polymorphism could be an adaptation for females to sneak into nesting holes of hosts with the different female chemotypes representing adaptations to a broader host range. However, since information on the CHC profiles of the hosts is missing, it remains unclear whether these different CHC profiles are used for chemical mimicry or are simply age-related, reflecting the females’ reproductive state.
Abstract
Cuckoo wasps (Chrysididae, Hymenoptera) are known for their parasitoid or cleptoparasitic life histories. Indeed, the biology of only a few species has been studied in detail and often only little more is known than the host species. By mimicking their hosts’ cuticular hydrocarbon (CHC) profiles, species that parasitize single (or a few closely related) host species manage to deceive their hosts. However, the variability of the CHC profile in generalist cuckoo-wasp species is still unknown. Here, we used gas chromatography—mass spectrometry (GC-MS) and DNA barcoding to study intraspecific variation in cuticular hydrocarbons of one less host-specific species of cuckoo wasps, Trichrysis cyanea. Cuticular hydrocarbon (CHC) patterns were found to differ between males and females. Additionally, we found chemical polymorphism among females, which formed three distinct chemical subgroups characterized by different alkene patterns. A lack of divergence in the DNA barcoding region suggests that these different chemotypes do not represent cryptic species. Whether this intrasexual CHC-profile variation is an adaptation (mimicry) to different host species, or simply signaling the reproductive status, remains unclear.
1. Introduction
The cuticle of insects is covered by a thin layer of wax. These epicuticular lipids mainly consist of hydrocarbons with a typical length between 19 and 50 carbon atoms [1,2]. The three major chemical groups of cuticular hydrocarbons (CHCs) are n-alkanes, n-alkenes, as well as methyl branched alkanes. Different species have evolved with different CHC profiles. Notably, not only the number and identity of the CHC molecules on the cuticle varies among species, but also their relative abundance. These profiles are often species-specific in arthropods, such that CHCs are regarded as potentially useful markers in chemical taxonomy and systematics (e.g., [3]). Indeed, CHCs have been successfully used for resolving morphologically cryptic species complexes (e.g., [4,5]). CHCs have several functions in insects (and other arthropods). They prevent desiccation by building a hydrophobic barrier against water loss through the cuticula. They are also used for chemical communication, e.g., as pheromones, for mate recognition and mate choice, and they can impart information about gender, age, fertility, health and colony and caste affiliation [2,6,7]. In addition, CHCs serve as a barrier against microorganisms and play an important role in predator/parasite-prey interactions [7,8].
Parasitism is among the most successful evolutionary strategies, with around 40% of all known species having evolved parasitic lifestyles and a vast amount of species being parasitized [9,10]. Parasites optimize host utilization and hosts develop strategies to escape from parasites as much as possible, leading to corresponding adaptations in both hosts and parasites (e.g., [11,12,13]). Among parasitic insects, it has been suggested that 6.5–20% of all species may be parasitoid wasps [14], or more than 75% of all wasp species are parasitoids [15]. One family of wasps that includes species with parasitoid lifestyles is the family Chrysididae, also known as “cuckoo wasps”, with about 3000 species hitherto described. Their hosts are sawflies, aculeate wasps and bees, silk moths and walking sticks [16], but, in general, little is known about the biology of many species and usually, if at all, only the name of the host species is available [17,18,19]. Previous studies on the reproductive biology of chrysidid species implicate an important role of CHCs. Females of some species sneak into the nesting holes of their hosts to lay their eggs without being detected by their hosts. To be able to do so, many cuckoo wasps appear to mimic the CHC profiles of their hosts, but the exact strategies might differ among species, ranging from chemical insignificance, i.e., a much-reduced amount of CHCs as compared to the host [20], to weak mimicry, i.e., CHC profiles intermediate to those of different host species, in species parasitizing a few closely related host species [21], and strong mimicry of single host species [22]. Other species do not need any form of mimicry as the females open already closed nesting holes of their hosts [23] or lay their eggs into their hosts’ prey (e.g., [24]).
Trichrysis cyanea (Linnaeus, 1758) is a common cuckoo wasp species in the Palearctic region. It is a cleptoparasite that lays its eggs into the nests of different hosts and produces two to three generations per year. Known host species belong to the genera Trypoxylon (Crabronidae), Auplopus (Pompilidae) and Deuteragenia (Pompilidae), but other species (mainly from the families Crabronidae and Pompilidae) have also been reported as potential hosts [17,25]. Thus, this species has a rather broad host spectrum, but it is still unclear to what extent T. cyanea has adapted its life strategies to its various hosts. In the present study, using gas chromatography coupled to mass spectrometry (GC-MS) and DNA barcoding, we investigated CHC-profiles in T. cyanea. Specifically, we inferred (i) whether male and female T. cyanea have different CHC profiles, (ii) how variable CHC profiles are within sexes, and (iii) in case there are different CHC profiles, whether this indicates cryptic species.
2. Materials and Methods
2.1. Sampling and Species Determination
In total, 24 females and 28 males of T. cyanea were collected at 14 locations in Eastern Austria in Styria, Lower Austria, Vienna and Burgenland from 2017 to 2018 (a map is provided in Figure S1). In most cases, at least two individuals were collected per sampling site, in a distance less than 250 m from each other. In addition, twelve individuals from six other species (Chrysis fulgida Linnaeus, 1761; Chrysis inaequalis Dahlbom, 1845; Chrysis iris Christ, 1791; Chrysura laevigata (Abeille de Perrin, 1879); Chrysis gracillima (Förster, 1853) and Hedychridium roseum (Rossi, 1790)) were collected and used as outgroup (Table S1). Specimens were identified following Linsenmaier [26].
2.2. Gas Chromatography—Mass Spectrometry
CHCs were extracted by whole body extraction of single individuals for 30 min in 70–150 µL of hexane, depending on the size of the specimens. Aliquots of the extracts (1.5 µL) were analyzed by GC-MS using a Trace gas chromatograph (Thermo, Vienna, Austria) equipped with a ZB-5 fused silica capillary column (Phenomenex, Aschaffenburg, Germany) coupled with a DCQ I mass spectrometer (Thermo, Vienna, Austria). Detailed information and a description of the settings can be found in [27]. CHCs were identified by diagnostic ions, indicating saturated or unsaturated hydrocarbon fragments (CnH2n + 1-ions; CnH2n − 1-ions), molecular weight (M+) and possible methyl branches. In case of unsaturated compounds, the position of double bonds was determined by dimethyl disulfide (DMDS) derivatization, following [28] with some modifications. In detail, we added 100 µL DMDS (dimethyl disulfide; Sigma Aldrich, Vienna, Austria) and 50 µL iodine solution to 20 µL of the extracts diluted with 80 µL of hexane. The reaction mixtures were kept at 50 °C overnight, then diluted with 500 µL of hexane and 500 µL of 5% sodium thiosulfate. After repeated extraction with hexane (500 µL, twice), the organic phase was dried over anhydrous magnesium sulfate and reduced to dryness with N2, re-dissolved in 30 µL hexane and analyzed by GC-MS. DMDS-derivatized alkenes were identified on the basis of the characteristic cleavage at the carbon-atom at the site of derivatization, leading to two intense ions that corresponded to the parts of the molecule at both sides of the double bond. In case of minute quantities, derivatives did not show all mass spectrometric characteristics, but could be assigned due to their retention time in other samples. Gas chromatographic retention indices (RI) were calculated using an alkane standard mixture and a calculated (median) retention time of extract compounds [29]. We were able to unambiguously identify all components with a relative abundance of more than 1% that occurred in at least 10% of the individual extracts and quantified these for statistical analysis. Isomers with lower amounts were considered if they could be identified unambiguously. The peak areas were calculated using Xcalibur 2.0.7™ (Thermo Fisher Scientific, Vienna, Austria) and the relative amounts (%) were calculated as peak area relative to the total area of all listed compounds. Statistical analyses were conducted with PAST 3.17 [30]. Non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarity [31] was performed to identify chemical clusters. One-way PERMANOVA [32] was used to test whether the differences between clusters were statistically significant.
2.3. DNA Extraction, DNA Barcoding and Genetic Analyses
After extracting the CHCs, one leg was cut off and stored in 250 μL of >99% ethanol at −20 °C. Genomic DNA was extracted following a rapid Chelex protocol [33]. The first part of the mitochondrial cytochrome c oxidase subunit 1 (COI) gene was amplified, purified and sequenced following [34]. The primers used for PCR and cycle sequencing were LCO1490 and HCO2198 [35], with an annealing temperature of 50 °C. Failed PCRs were repeated using the Phusion Flash High-Fidelity PCR Master Mix (Thermo Fisher Scientific) following the manufacturer’s instructions.
Nucleotide sequences were edited in MEGA version X [36] and checked by eye. The nucleotide sequences were aligned using the ClustalW algorithm implemented in MEGA and checked for mitochondrial pseudogenes (numts) by translating them into amino acid sequences and looking for internal stop codons. For phylogenetic tree inference, a maximum likelihood (ML) tree was inferred in PhyML 3.0 [37], applying the best-fitting substitution model (GTR + I + G) selected by the Smart Model Selection tool [38] based on the Bayesian Information Criterion (BIC), with 1000 bootstrap replicates to assess statistical branch support. Visualization of the ML tree was done in MEGA version 5 [39]. The nucleotide sequences were deposited on BOLD (BACHR001-21-BACHR0064-21) and GenBank (OM415512-OM415571).
3. Results
3.1. Cuticular Hydrocarbon Patterns (CHCs)
In total, 23 compounds were identified and used for further analyses. These compounds were found to be alkanes and alkenes, ranging from 21 to 29 carbon atoms (see Table 1). We were not able to separate 13-nonacosene and 14-nonacosene, which occurred only in males, as well as 9- and 10-heneicosene from one single male sample. From characteristic fragments of the DMDS-derivatives, however, the identity of the two contributing compounds for each peak could be concluded. For example, derivatized 13- and 14-nonacosene eluted within one peak, exhibiting a mixed spectrum with a molecular ion at m/z 500, and additional fragments from cleavage at position of double bonds at m/z 229 + m/z 271 (13-nonacosene), and m/z 243 + m/z 257 (14-nonacosene). As we were not able to calculate the relative amount of the single isomeric forms of these two mixtures separately, we treated them as a combined character in further analyses.

Table 1.
List of all components found and analyzed at the cuticula of male and female specimens of T. cyanea, with their retention index and abbreviation.
Furthermore, a few additional but inconsistently occurring compounds were found in the samples. These were hentriacontane (C31), hentriacontene (C31:1), and some methyl-branched hydrocarbons that occurred in traces in some of the extracts. The low amounts of these compounds allowed a tentative identification only. These compounds were not considered for statistics.
A comparison of individual CHC-patterns by NMDS clearly indicated chemical sexual dimorphism (Figure 1; p < 0.0001). While the male specimens cluster together, the females appear to be chemically more heterogeneous and form three distinct subclusters (Figure 1 and Figure S2).
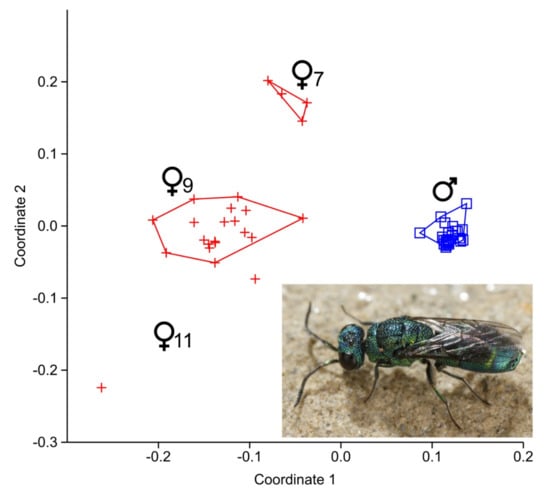
Figure 1.
Non-metric multidimensional scaling (NMDS) based on Bray-Curtis distances of all individual cuticular hydrocarbon (CHC) profiles of Trichrysis cyanea. Males are indicated by blue squares, females by red crosses. Note the distinct female clusters (also see dendrogram in Figure S2). The different female clusters are named after the position of double-bonds of the main CHCs. The photo shows a living individual of T. cyanea. Photo: Gernot Kunz.
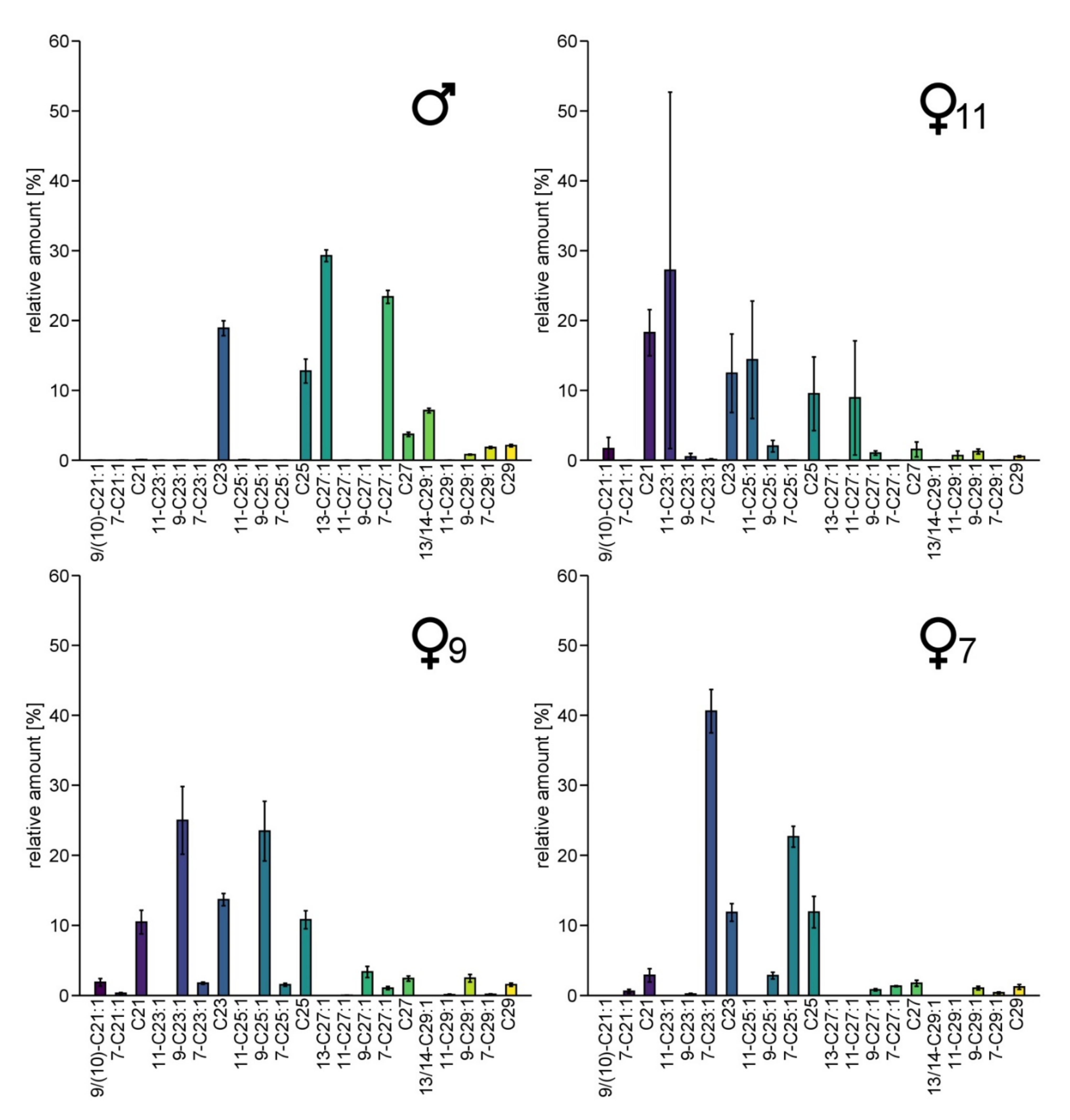
CHC-profiles of males showed large amounts of 13-heptacosene, 7-heptacosene, 13- and 14-nonacosene and 7-nonacosene (Figure 2). Females exhibited larger amounts of tricosene and pentacosene than males, with varying double bond positions, either in position 7, 9 or 11, respectively. According to the position of the double bond of the main alkenes, we identified three different female chemotypes, named ♀7, ♀9 and ♀11 (this terminology is also used in the figures). Only two ♀11 specimens are included in our dataset. Unlike the specimens of the other two clusters, their CHC-profiles show rather high variation in the relative abundance of particular CHCs, but as they were the only two female samples containing 11-C23:1 and 11-C25:1, we classified them as belonging to the same distinct subgroup. We were also able to detect 11-C27:1 not only in the two ♀11-females, but in traces (relative amount of 0.17–0.23%) in three other female samples. Thus, the different female subgroups are characterized not only by different components but also by different amounts of commonly shared substances. No correlation between the CHC-patterns and geography was observed, and all chemotypes were found at the sampling site with the largest sampling size (Figure S2).
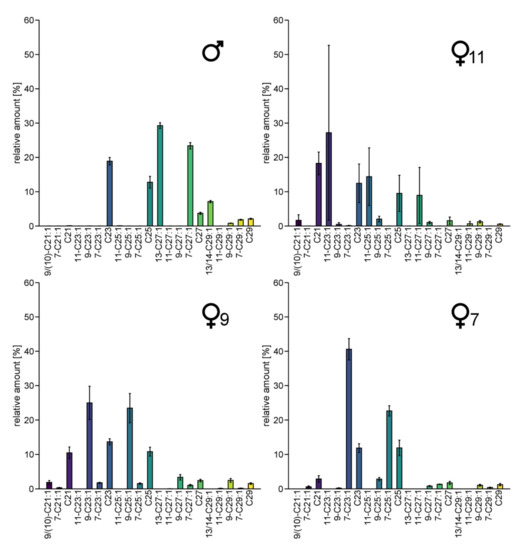
Figure 2.
Average amount of CHCs in males and the three groups of females of Trichrysis cyanea that differ in their CHC profiles from each other. The female specimens were grouped regarding the most frequent double bond position. The relative amounts [%] are calculated as peak area relative to the total area of all listed compounds. Whiskers represent the standard error. Number of individuals per group: ♂: n = 25 ♀7: n = 4, ♀9: n = 18, ♀11: n = 2.
3.2. DNA Barcoding
A total of 60 nucleotide sequences ranging from 541 to 657 base pairs (bp) in length were obtained, translating to a sequencing success rate of 94%. A deletion of three bp (position 345 to 347) was present in T. cyanea, C. gracillima and C. laevigata. All species represented by multiple samples were inferred as monophyletic entities in the phylogenetic analysis (Figure S3). We found little intraspecific variation in the COI nucleotide sequences of T. cyanea (pairwise distances of 0–0.7%; all 48 sequences are included in a single BIN (BOLD:AAH7935). Most T. cyanea individuals shared a single haplotype; four samples differed from this main haplotype by one substitution. These four specimens were all collected in Styria at three different sampling sites.
4. Discussion
In this study, we investigated CHC profiles of male and female T. cyanea and found a clear sexual dimorphism. In addition, we found three distinct CHC-profiles in females while males expressed a single profile with little variation.
Sexually dimorphic CHC-profiles have already been reported for other chrysidids, namely Chrysis pseudobrevitarsis and Chrysis parabrevitarsis [5], and it is very likely that this is a general pattern in the family. Usually, both sexes of cuckoo wasp species inhabit the same habitat and, thus, abiotic factors are not expected to affect their CHC-profiles differently. Males and females may utilize different CHC compounds for different communication purposes, with CHC compounds of males probably mainly serving for mate recognition. In females, the CHC-inventory might be further shaped by the need to deceive their hosts. For example, females of Hedychrum rutilans mimic the CHCs of their host Philanthus triangulum, but they produce only low amounts of host-CHCs [22]. Furthermore, the amount of hydrocarbons on the cuticle of cleptoparasitic H. rutilans is five times less compared to the host, a phenomenon termed chemical insignificance [20]. Female Chrysis mediata Linsenmeier, 1951 and Pseudochrysis neglecta (Shuckard, 1837) are able to mimic different chemotypes of their host species Odynerus spinipes (Linnaeus, 1758) [23,40], whereas the chrysidid Parnopes grandior (Pallas, 1771) evolved a weak mimicry to multiple host species of the genus Bembix, mimicking only one single intermediate CHC-profile that includes major characteristics of all its hosts [21]. Comparably, the different chemotypes found in T. cyanea females may indicate an adaptation to different hosts. Common hosts of T. cyanea are species of the genera Trypoxylon (Crabronidae), Auplopus (Pompilidae) and Deuteragenia (Pompilidae) [25], but additional hosts (other crabronid and pompilid species) have also been reported [17]. Unfortunately, no data are available on host-CHCs to explicitly test which strategy of host deceit T. cyanea is following.
Theoretically, the different CHC-profiles could have indicated the presence of cryptic species within the morphospecies T. cyanea, as has been shown for other chrysidids [5]. Our DNA barcoding data, however, strongly argue against cryptic diversity, unless divergence was very recent. Indeed, the genetic diversity in our T. cyanea samples was very low, which was unexpected given the generally high substitution rate of mitochondrial genes in Hymenoptera, and especially in Apocrita [41,42,43,44].
Distinctly different female chemotypes in T. cyanea may also reflect age-related differences, with CHC-modifications possibly occurring in different phases of maturation. Thus, different CHC-chemotypes of T. cyanea may characterize the cuticle chemistry of pre-reproductive, reproductive and post-reproductive (already mated) individuals. Pre-reproductive phases in insects can last between one day and several weeks (summarized in [45]). It has been shown that CHCs clearly correlate with reproductive status in some species [46,47], e.g., they are expressed in larger amounts at sexual maturity [45,48,49], and that they might be used as short-range contact pheromones [6,50]. Trichrysis cyanea produces multiple generations over a year, and well-founded data on CHC-modification during maturation would require an in-depth investigation of CHCs over a whole flight period.
5. Conclusions
In this study, we have shown that in the cuckoo wasp Trichrysis cyanea, CHC-profiles not only differ between the sexes but also among females. While sexual dimorphism can be explained by different communication purposes in males and females, the reasons underlying the evolution of distinct female chemotypes remain elusive. DNA barcoding results suggest that these female chemotypes do not represent distinct species, but whether these chemotypes are adaptations (mimicry) to different host species, or are simply signaling the reproductive status, remains unclear. To fully understand the complex function of the different CHC-profiles in T. cyanea, future studies will have to extend analyses of CHC-profiles also to the hosts and female T. cyanea of different and well identified maturation status. As T. cyanea is parasitizing a large number of host species from different families and produces multiple generations a year, this, however, will be a challenging endeavor. Nonetheless, such studies will provide important insights into how a generalist parasite has adapted to a broad host range and to what extent CHCs indeed play a role in this process.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects13020159/s1, Table S1: Specimen details, Figure S1: map of sampling sites, Figure S2: Dendrogram of CHC-cluster analysis; Figure S3: Maximum likelihood tree of partial COI sequences.
Author Contributions
Conceptualization, D.F., G.R. and S.K.; methodology, D.F. and L.Z.; software, D.F. and G.R.; validation, D.F., L.Z., G.R. and S.K.; formal analysis, D.F. and L.Z.; investigation, D.F., L.Z., G.R. and S.K.; resources, G.R. and S.K.; data curation, D.F. and L.Z.; writing—original draft preparation, D.F., L.Z., G.R. and S.K.; writing—review and editing, D.F., L.Z., G.R. and S.K.; visualization, D.F.; supervision, G.R. and S.K.; project administration, G.R. and S.K.; funding acquisition, G.R. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Austrian Federal Ministry of Science, Research and Economy in the frame of an ABOL-associated project within the framework of “Hochschulraum-Strukturmittel” Funds. The Open Access costs were covered by the University of Graz.
Data Availability Statement
The CHC data is available from the first author upon request. The DNA sequence data generated in this study are available on GenBank under the accession numbers listed in the “Material and methods” section and are also available on BOLD via the Process IDs BACHR001-21-BACHR0064-21.
Acknowledgments
We are grateful to Gernot Kunz, Gregor Degasperi and Elisabeth Papenberg for support in collecting specimens and to Gernot Kunz for also providing a photo. We give thanks to Andrea Lienhard and Julia Blesl for her assistance in the lab and Michaela Bodner for her advice in data interpretation and helpful comments on this manuscript. The authors acknowledge the financial support by the University of Graz.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Drijfhout, F.P.; Kather, R.; Martin, S.J. The role of cuticular hydrocarbons in insects. In Behavioral and Chemical Ecology; Zhang, W., Liu, H., Eds.; Nova Science Publishers: New York, NY, USA, 2010; ISBN 1607410990. [Google Scholar]
- Blomquist, G.J.; Bagnères, A.-G. (Eds.) Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-89814-0. [Google Scholar]
- Kather, R.; Martin, S.J. Cuticular hydrocarbon profiles as a taxonomic tool: Advantages, limitations and technical aspects. Physiol. Entomol. 2012, 37, 25–32. [Google Scholar] [CrossRef]
- Hartke, J.; Sprenger, P.P.; Sahm, J.; Winterberg, H.; Orivel, J.; Baur, H.; Beuerle, T.; Schmitt, T.; Feldmeyer, B.; Menzel, F. Cuticular hydrocarbons as potential mediators of cryptic species divergence in a mutualistic ant association. Ecol. Evol. 2019, 9, 9160–9176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soon, V.; Castillo-Cajas, R.F.; Johansson, N.; Paukkunen, J.; Rosa, P.; Ødegaard, F.; Schmitt, T.; Niehuis, O. Cuticular Hydrocarbon Profile Analyses Help Clarify the Species Identity of Dry-Mounted Cuckoo Wasps (Hymenoptera: Chrysididae), Including Type Material, and Reveal Evidence for a Cryptic Species. Insect Syst. Divers. 2021, 5, 3. [Google Scholar] [CrossRef]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 2015, 37, 822–830. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical Ecology, Biochemistry, and Molecular Biology of Insect Hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Howard, R.W.; Blomquist, G.J. Ecological, Behavioral, and Biochemical Aspects of Insect Hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- De Meeûs, T.; Renaud, F. Parasites within the new phylogeny of eukaryotes. Trends Parasitol. 2002, 18, 247–251. [Google Scholar] [CrossRef]
- Dobson, A.; Lafferty, K.D.; Kuris, A.M.; Hechinger, R.F.; Jetz, W. Colloquium paper: Homage to Linnaeus: How many parasites? How many hosts? Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. S1), 11482–11489. [Google Scholar] [CrossRef] [Green Version]
- Kilner, R.M.; Langmore, N.E. Cuckoos versus hosts in insects and birds: Adaptations, counter-adaptations and outcomes. Biol. Rev. Camb. Philos. Soc. 2011, 86, 836–852. [Google Scholar] [CrossRef]
- Feeney, W.E.; Welbergen, J.A.; Langmore, N.E. Advances in the Study of Coevolution Between Avian Brood Parasites and Their Hosts. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 227–246. [Google Scholar] [CrossRef]
- Haverkamp, A.; Smid, H.M. A neuronal arms race: The role of learning in parasitoid-host interactions. Curr. Opin. Insect Sci. 2020, 42, 47–54. [Google Scholar] [CrossRef]
- Lasalle, J.; Gauld, I.D. Parasitic Hymenoptera and the biodiversity crisis. REDIA 1991, 74, 315–334. [Google Scholar]
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic hymenoptera. Annu. Rev. Entomol. 2006, 51, 233–258. [Google Scholar] [CrossRef]
- Kimsey, L.S.; Bohart, R.M. The Chrysidid Wasps of the World; Oxford University Press: Oxford, UK, 1990; ISBN 0198540108. [Google Scholar]
- Wiesbauer, H.; Rosa, P.; Zettel, H. Die Goldwespen Mitteleuropas: Biologie, Lebensräume, Artenporträts; Ulmer: Stuttgart, Germany, 2020; ISBN 978-3-8186-1149-1. [Google Scholar]
- Wiśniowski, B. Cuckoo-Wasps (Hymenoptera: Chrysididae) of Poland: Diversity, Identification, Distribution; Ojców National Park: Ojców, Poland, 2015; ISBN 978-83-919746-1-2. [Google Scholar]
- Rosa, P. I Crisidi Della Valle d’Aosta: (Hymenoptera, Chrysididae); Museo Regionale di Scienze Naturali: Saint-Pierre, Italy, 2006. [Google Scholar]
- Kroiss, J.; Schmitt, T.; Strohm, E. Low level of cuticular hydrocarbons in a parasitoid of a solitary digger wasp and its potential for concealment. Entomol. Sci. 2009, 12, 9–16. [Google Scholar] [CrossRef]
- Polidori, C.; Ballesteros, Y.; Wurdack, M.; Asís, J.D.; Tormos, J.; Baños-Picón, L.; Schmitt, T. Low Host Specialization in the Cuckoo Wasp, Parnopes grandior, Weakens Chemical Mimicry but Does Not Lead to Local Adaption. Insects 2020, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Strohm, E.; Kroiss, J.; Herzner, G.; Laurien-Kehnen, C.; Boland, W.; Schreier, P.; Schmitt, T. A cuckoo in wolves’ clothing? Chemical mimicry in a specialized cuckoo wasp of the European beewolf (Hymenoptera, Chrysididae and Crabronidae). Front. Zool. 2008, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Wurdack, M.; Herbertz, S.; Dowling, D.; Kroiss, J.; Strohm, E.; Baur, H.; Niehuis, O.; Schmitt, T. Striking cuticular hydrocarbon dimorphism in the mason wasp Odynerus spinipes and its possible evolutionary cause (Hymenoptera: Chrysididae, Vespidae). Proc. Biol. Sci. 2015, 282, 1–9. [Google Scholar] [CrossRef]
- Winterhagen, P. Strategy for sneaking into a host’s home: The cuckoo wasp Omalus biaccinctus (Hymenoptera: Chrysididae) inserts its eggs into living aphids that are the prey of its host. Eur. J. Entomol. 2015, 112, 557–559. [Google Scholar] [CrossRef] [Green Version]
- Pauli, T.; Castillo-Cajas, R.F.; Rosa, P.; Kukowka, S.; Berg, A.; van den Berghe, E.; Fornoff, F.; Hopfenmüller, S.; Niehuis, M.; Peters, R.S.; et al. Phylogenetic analysis of cuckoo wasps (Hymenoptera: Chrysididae) reveals a partially artificial classification at the genus level and a species-rich clade of bee parasitoids. Syst. Entomol. 2019, 44, 322–335. [Google Scholar] [CrossRef]
- Linsenmaier, W. Revision der Familie Chrysididae (Hymenoptera) mit besonderer Berücksichtigung der europäischen Spezies. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 1959, 32, 1–232. [Google Scholar] [CrossRef]
- Bodner, M.; Raspotnig, G. Millipedes that smell like bugs: (E)-alkenals in the defensive secretion of the julid diplopod Allajulus dicentrus. J. Chem. Ecol. 2012, 38, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Roan, C.S.; Yost, R.A.; Hector, J. Dimethyl disulfide derivatives of long chain alkenes, alkadienes, and alkatrienes for gas chromatography/mass spectrometry. Anal. Chem. 1989, 61, 1564–1571. [Google Scholar] [CrossRef]
- Van den Dool, H.; Dec Kratz, P. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Richlen, M.L.; Barber, P.H. A technique for the rapid extraction of microalgal DNA from single live and preserved cells. Mol. Ecol. Notes 2005, 5, 688–691. [Google Scholar] [CrossRef]
- Zangl, L.; Kunz, G.; Berg, C.; Koblmüller, S. First records of the parthenogenetic Surinam cockroach Pycnoscelus surinamensis (Insecta: Blattodea: Blaberidae) for Central Europe. J. Appl. Entomol. 2019, 143, 308–313. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Lefort, V.; Longueville, J.-E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Aalysis Using Maximum Likelihood, Evolutionary Eistance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Moris, V.C.; Christmann, K.; Wirtgen, A.; Belokobylskij, S.A.; Berg, A.; Liebig, W.-H.; Soon, V.; Baur, H.; Schmitt, T.; Niehuis, O. Cuticular hydrocarbons on old museum specimens of the spiny mason wasp, Odynerus spinipes (Hymenoptera: Vespidae: Eumeninae), shed light on the distribution and on regional frequencies of distinct chemotypes. Chemoecology 2021, 31, 311–322. [Google Scholar] [CrossRef]
- Crozier, R.H.; Crozier, Y.C.; Mackinlay, A.G. The CO-I and CO-II Region of Honeybee Mitochondrial DNA: Evidence for Variation in Insect Mitochondrial Evolutionary Rates. Mol. Biol. Evol. 1989, 6, 399–411. [Google Scholar] [CrossRef]
- Kaltenpoth, M.; Showers Corneli, P.; Dunn, D.M.; Weiss, R.B.; Strohm, E.; Seger, J. Accelerated Evolution of Mitochondrial but Not Nuclear Genomes of Hymenoptera: New Evidence from Crabronid Wasps. PLoS ONE 2012, 7, e32826. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.C.S.G.; Raychoudhury, R.; Lavrov, D.V.; Werren, J.H. Rapidly Evolving Mitochondrial Genome and Directional Selection in Mitochondrial Genes in the Parasitic Wasp Nasonia (Hymenoptera: Pteromalidae). Mol. Biol. Evol. 2008, 25, 2167–2180. [Google Scholar] [CrossRef] [Green Version]
- Turčinavičienė, J.; Radzevičiūtė, R.; Budrienė, A.; Budrys, E. Species identification and genetic differentiation of European cavity-nesting wasps (Hymenoptera: Vespidae, Pompilidae, Crabronidae) inferred from DNA barcoding data. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2016, 27, 476–482. [Google Scholar] [CrossRef]
- Butterworth, N.J.; Drijfhout, F.P.; Byrne, P.G.; Keller, P.A.; Wallman, J.F. Major Transitions in Cuticular Hydrocarbon Expression Coincide with Sexual Maturity in a Blowfly (Diptera: Calliphoridae). J. Chem. Ecol. 2020, 46, 610–618. [Google Scholar] [CrossRef]
- Amsalem, E.; Hefetz, A. The appeasement effect of sterility signaling in dominance contests among Bombus terrestris workers. Behav. Ecol. Sociobiol. 2010, 64, 1685–1694. [Google Scholar] [CrossRef]
- Peeters, C.; Monnin, T.; Malosse, C. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 1999, 266, 1323–1327. [Google Scholar] [CrossRef] [Green Version]
- Everaerts, C.; Farine, J.-P.; Cobb, M.; Ferveur, J.-F. Drosophila cuticular hydrocarbons revisited: Mating status alters cuticular profiles. PLoS ONE 2010, 5, e9607. [Google Scholar] [CrossRef]
- Arienti, M.; Antony, C.; Wicker-Thomas, C.; Delbecque, J.-P.; Jallon, J.-M. Ontogeny of Drosophila melanogaster female sex-appeal and cuticular hydrocarbons. Integr. Zool. 2010, 5, 272–282. [Google Scholar] [CrossRef]
- Kather, R.; Martin, S.J. Evolution of Cuticular Hydrocarbons in the Hymenoptera: A Meta-Analysis. J. Chem. Ecol. 2015, 41, 871–883. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).