Sex-Specific Effect of the Dietary Protein to Carbohydrate Ratio on Personality in the Dubia Cockroach
Abstract
:Simple Summary
Abstract
1. Introduction
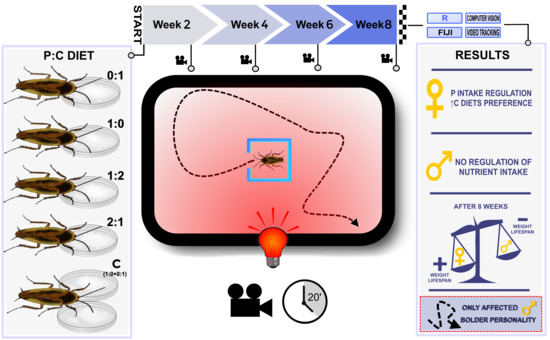
2. Material and Methods
2.1. Experimental Individuals
2.2. Diets
2.3. Behavioral Protocol
2.4. Trajectories Analyses
2.5. Statistical Analyses
3. Results
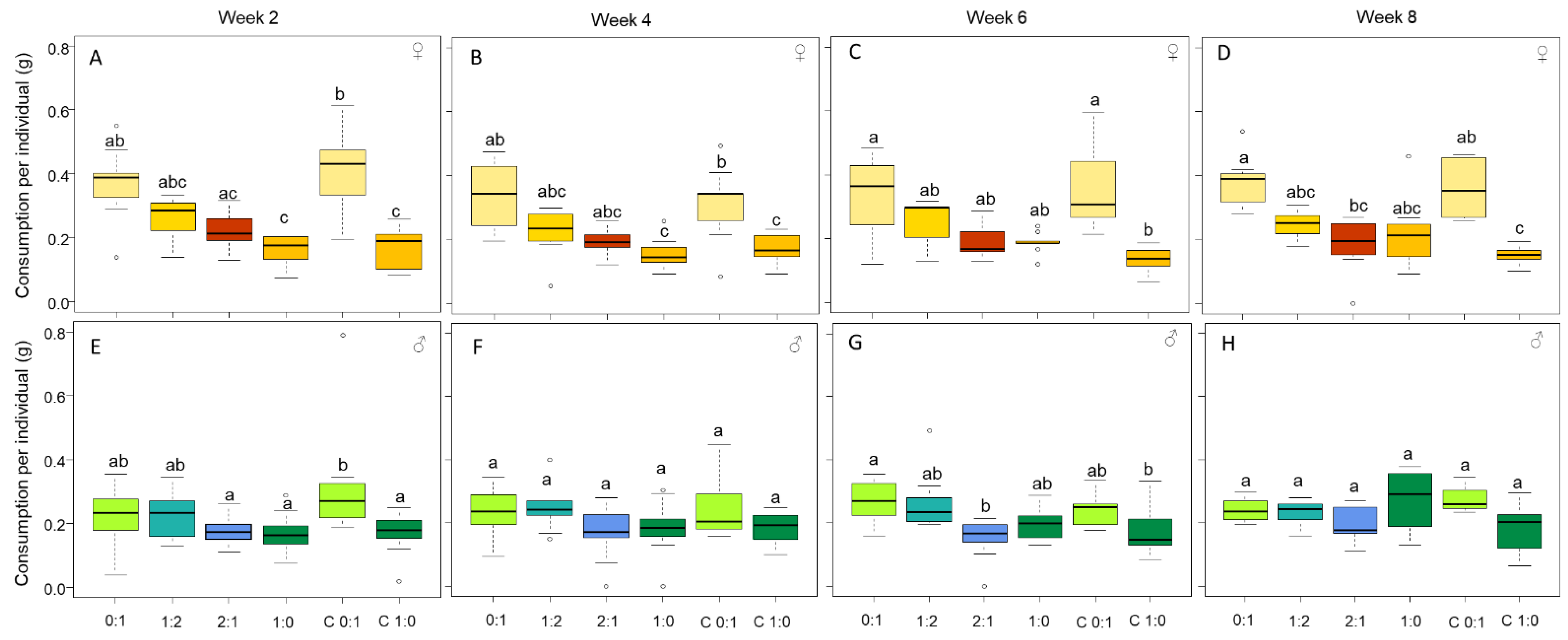
3.1. Females Regulate Their Nutrient Intake, Consume More Carbohydrates and Gain More Body Mass
3.2. Females Live Longer Than Males
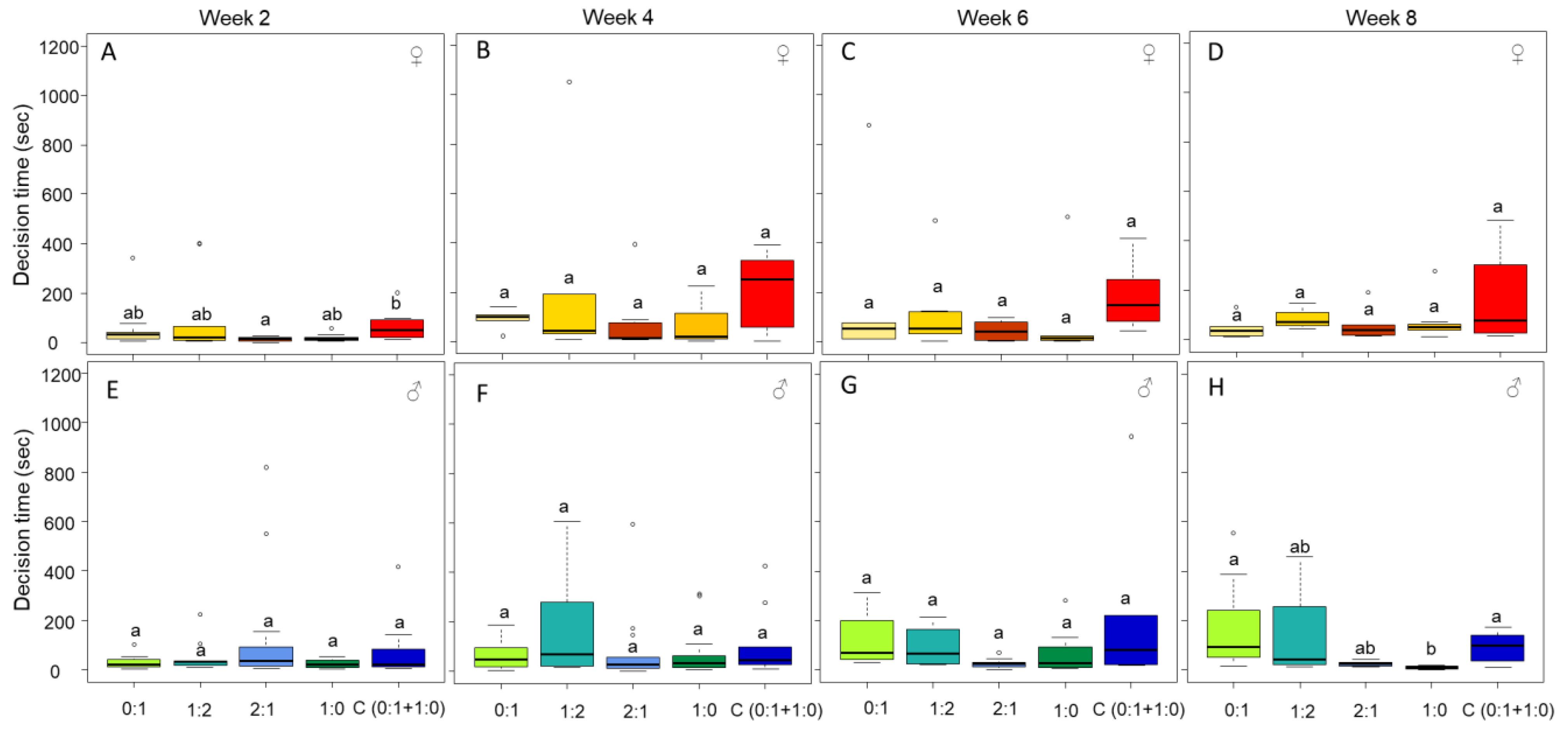
3.3. Only Male Personality Is Affected by the Diet over Time
3.4. Mobility and Exploration Is Affected by Diet (in Males) and Time
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dall, S.R.X.; Griffith, S.C. An empiricist guide to animal personality variation in ecology and evolution. Front. Ecol. Evol. 2014, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Stanley, C.R.; Mettke-Hofmann, C.; Preziosi, R.F. Personality in the cockroach Diploptera punctata: Evidence for stability across developmental stages despite age effects on boldness. PLoS ONE 2017, 12, e0176564. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.M.; Krause, J. Personality and metamorphosis: Is behavioral variation consistent across ontogenetic niche shifts? Behav. Ecol. 2012, 23, 1316–1323. [Google Scholar] [CrossRef] [Green Version]
- Amat, I.; Desouhant, E.; Gomes, E.; Moreau, J.; Monceau, K. Insect personality: What can we learn from metamorphosis? Curr. Opin. Insect Sci. 2018, 27, 46–51. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef] [Green Version]
- Dingemanse, N.J.; Réale, D. Natural selection and animal personality. Behaviour 2005, 142, 1159–1184. [Google Scholar] [CrossRef]
- Logue, D.M.; Mishra, S.; McCaffrey, D.; Ball, D.; Cade, W.H. A behavioral syndrome linking courtship behavior toward males and females predicts reproductive success from a single mating in the hissing cockroach, Gromphadorhina portentosa. Behav. Ecol. 2009, 20, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Stamps, J.A. Growth-mortality tradeoffs and personality traits in animals. Ecol. Lett. 2007, 10, 355–363. [Google Scholar] [CrossRef]
- Kendall, B.E.; Fox, G.A.; Stover, J.P. Boldness-aggression syndromes can reduce population density: Behavior and demographic heterogeneity. Behav. Ecol. 2017, 29, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Krams, I.; Kivleniece, I.; Kuusik, A.; Krama, T.; Mänd, R.; Rantala, M.J.; Znotiņa, S.; Freeberg, T.M.; Mänd, M. Predation promotes survival of beetles with lower resting metabolic rates. Èntomol. Exp. Appl. 2013, 148, 94–103. [Google Scholar] [CrossRef]
- Ward-Fear, G.; Brown, G.P.; Pearson, D.J.; West, A.; Rollins, L.A.; Shine, R. The ecological and life history correlates of boldness in free-ranging lizards. Ecosphere 2018, 9, 02125. [Google Scholar] [CrossRef]
- Belgrad, B.A.; Griffen, B.D. Predator–prey interactions mediated by prey personality and predator hunting mode. Proc. R. Soc. B Boil. Sci. 2016, 283, 20160408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgrad, B.A.; Griffen, B.D. Personality interacts with habitat quality to govern individual mortality and dispersal patterns. Ecol. Evol. 2018, 8, 7216–7227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davenport, J.M.; King, A.B.; Riley, A.W.; Hampson, M.E.; Constantinides, P. The non-consumptive effects of predators and personality on prey growth and mortality. Ethology 2019, 126, 363–371. [Google Scholar] [CrossRef]
- Niemelä, P.; Lattenkamp, E.; Dingemanse, N. Personality-related survival and sampling bias in wild cricket nymphs. Behav. Ecol. 2015, 26, 936–946. [Google Scholar] [CrossRef] [Green Version]
- Planas-Sitjà, I. Personality variation improves collective decision-making in cockroaches. Behav. Process. 2020, 177, 104147. [Google Scholar] [CrossRef]
- Planas-Sitjà, I.; Deneubourg, J.-L.; Gibon, C.; Sempo, G. Group personality during collective decision-making: A multi-level approach. Proc. R. Soc. B Boil. Sci. 2015, 282, 20142515. [Google Scholar] [CrossRef] [Green Version]
- Jandt, J.; Gordon, D. The behavioral ecology of variation in social insects. Curr. Opin. Insect Sci. 2016, 15, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Wray, M.K.; Mattila, H.R.; Seeley, T.D. Collective personalities in honeybee colonies are linked to colony fitness. Anim. Behav. 2011, 81, 559–568. [Google Scholar] [CrossRef]
- Scharf, I.; Modlmeier, A.; Fries, S.; Tirard, C.; Foitzik, S. Characterizing the Collective Personality of Ant Societies: Aggressive Colonies Do Not Abandon Their Home. PLoS ONE 2012, 7, e33314. [Google Scholar] [CrossRef]
- Kralj-Fišer, S.; Schuett, W. Studying personality variation in invertebrates: Why bother? Anim. Behav. 2014, 91, 41–52. [Google Scholar] [CrossRef]
- Biro, P.A.; Beckmann, C.; Stamps, J.A. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc. R. Soc. B Boil. Sci. 2010, 277, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, I.; Dingemanse, N. Parasitism and the evolutionary ecology of animal personality. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Lihoreau, M.; Brepson, L.; Rivault, C. The weight of the clan: Even in insects, social isolation can induce a behavioural syndrome. Behav. Process. 2009, 82, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Jäger, H.Y.; Han, C.S.; Dingemanse, N. Social experiences shape behavioral individuality and within-individual stability. Behav. Ecol. 2019, 30, 1012–1019. [Google Scholar] [CrossRef]
- Magura, T.; Mizser, S.; Horváth, R.; Nagy, D.; Tóth, M.; Csicsek, R.; Lövei, G. Are There Personality Differences between Rural vs. Urban-Living Individuals of a Specialist Ground Beetle, Carabus convexus? Insects 2021, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Dingemanse, N.J. Effect of diet on the structure of animal personality. Front. Zoo 2015, 12, S5. [Google Scholar] [CrossRef] [Green Version]
- Tremmel, M.; Müller, C. Insect personality depends on environmental conditions. Behav. Ecol. 2012, 24, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Logue, D.M.; Abiola, I.O.; Cade, W.H. Developmental environment affects risk-acceptance in the hissing cockroach, Gromphadorhina portentosa. J. Comp. Psychol. 2011, 125, 40–47. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity; Princeton University Press: Princeton, NJ, USA, 2012; ISBN 978-0-691-14565-5. [Google Scholar]
- Lihoreau, M.; Buhl, J.; Charleston, M.; Sword, G.A.; Raubenheimer, D.; Simpson, S.J. Nutritional ecology beyond the individual: A conceptual framework for integrating nutrition and social interactions. Ecol. Lett. 2015, 18, 273–286. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, J.; Lihoreau, M. Quantifying Nutritional Trade-Offs across Multidimensional Performance Landscapes. Am. Nat. 2019, 193, E168–E181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.S.; Dingemanse, N. You are what you eat: Diet shapes body composition, personality and behavioural stability. BMC Evol. Biol. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, J.; Bieman, D.N. Glucose aversion in the German cockroach, Blattella germanica. J. Insect Physiol. 1993, 39, 925–933. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Jones, S. Nutritional imbalance in an extreme generalist omnivore: Tolerance and recovery through complementary food selection. Anim. Behav. 2006, 71, 1253–1262. [Google Scholar] [CrossRef]
- Bunning, H.; Bassett, L.; Clowser, C.; Rapkin, J.; Jensen, K.; House, C.M.; Archer, C.R.; Hunt, J. Dietary choice for a balanced nutrient intake increases the mean and reduces the variance in the reproductive performance of male and female cockroaches. Ecol. Evol. 2016, 6, 4711–4730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchebti, S.; Durier, V.; Pasquaretta, C.; Rivault, C.; Lihoreau, M. Subsocial Cockroaches Nauphoeta cinerea Mate Indiscriminately with Kin Despite High Costs of Inbreeding. PLoS ONE 2016, 11, e0162548. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-Project.Org/ (accessed on 15 December 2021).
- Bunning, H.; Rapkin, J.; Belcher, L.; Archer, C.R.; Jensen, K.; Hunt, J. Protein and carbohydrate intake influence sperm number and fertility in male cockroaches, but not sperm viability. Proc. R. Soc. B Boil. Sci. 2015, 282, 20142144. [Google Scholar] [CrossRef]
- Jensen, K.; McClure, C.; Priest, N.; Hunt, J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell 2015, 14, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.; Schal, C.; Silverman, J. Adaptive contraction of diet breadth affects sexual maturation and specific nutrient consumption in an extreme generalist omnivore. J. Evol. Biol. 2015, 28, 906–916. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.; Silverman, J. Frequently mated males have higher protein preference in German cockroaches. Behav. Ecol. 2018, 29, 1453–1461. [Google Scholar] [CrossRef]
- South, S.H.; House, C.M.; Moore, A.J.; Simpson, S.J.; Hunt, J. Male cockroaches prefer a high carbohydrate diet that makes them more attractive to females: Implications for the study of condition dependence. Evolution 2011, 65, 1594–1606. [Google Scholar] [CrossRef]
- Cerreta, A.J.; Smith, D.C.; Heugten, K.A.; Minter, L.J. Comparative nutrient analysis of four species of cockroaches used as food for insectivores by life stage, species, and sex. Zoo Biol. 2021. [Google Scholar] [CrossRef]
- Taylor, R.L.; Freckleton, W.C. Sex differences in longevity of starved cockroaches (Leucophaea maderae). J. Invertebr. Pathol. 1969, 13, 68–73. [Google Scholar] [CrossRef]
- Arganda, S.; Bouchebti, S.; Bazazi, S.; Le Hesran, S.; Puga, C.; Latil, G.; Simpson, S.J.; Dussutour, A. Parsing the life-shortening effects of dietary protein: Effects of individual amino acids. Proc. R. Soc. B Boil. Sci. 2017, 284, 20162052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirk, C.W.W.; Boodhoo, C.; Human, H.; Nicolson, S. The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 2009, 41, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Paoli, P.P.; Donley, D.; Stabler, D.; Saseendranath, A.; Nicolson, S.; Simpson, S.J.; Wright, G.A. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 2014, 46, 1449–1458. [Google Scholar] [CrossRef] [Green Version]
- Horváth, G.; Rodríguez-Ruiz, G.; Martín, J.; López, P.; Herczeg, G. Maternal diet affects juvenile Carpetan rock lizard performance and personality. Ecol. Evol. 2019, 9, 14476–14488. [Google Scholar] [CrossRef]
- Schuett, W.; Dall, S. Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim. Behav. 2009, 77, 1041–1050. [Google Scholar] [CrossRef]
- Malloy, T.E.; Barcelos, S.; Arruda, E.; DeRosa, M.; Fonseca, C. Individual Differences and Cross-Situational Consistency of Dyadic Social Behavior. J. Pers. Soc. Psychol. 2005, 89, 643–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Oers, K.; Klunder, M.; Drent, P.J. Context dependence of personalities: Risk-taking behavior in a social and a nonsocial situation. Behav. Ecol. 2005, 16, 716–723. [Google Scholar] [CrossRef] [Green Version]
- McPherson, S.; Wada-Katsumata, A.; Hatano, E.; Silverman, J.; Schal, C. Comparison of Diet Preferences of Laboratory-Reared and Apartment-Collected German Cockroaches. J. Econ. Èntomol. 2021, 114, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Salazar, M.-O.; Bouchebti, S.; Lihoreau, M. Gregarious Cockroaches. In Encyclopedia of Social Insects; Starr, C.K., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 445–450. ISBN 978-3-030-28102-1. [Google Scholar]




| P:C Ratio | Amino Acids * | Sucrose | Cholesterol | Wesson’s Salt | Vitamin Mix |
|---|---|---|---|---|---|
| 0:1 | 0 | 96.45 | 0.55 | 2.50 | 0.50 |
| 1:2 | 32.15 | 64.3 | 0.55 | 2.50 | 0.50 |
| 2:1 | 64.3 | 32.15 | 0.55 | 2.50 | 0.50 |
| 1:0 | 96.45 | 0 | 0.55 | 2.50 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouchebti, S.; Cortés-Fossati, F.; Vales Estepa, Á.; Plaza Lozano, M.; S. Calovi, D.; Arganda, S. Sex-Specific Effect of the Dietary Protein to Carbohydrate Ratio on Personality in the Dubia Cockroach. Insects 2022, 13, 133. https://doi.org/10.3390/insects13020133
Bouchebti S, Cortés-Fossati F, Vales Estepa Á, Plaza Lozano M, S. Calovi D, Arganda S. Sex-Specific Effect of the Dietary Protein to Carbohydrate Ratio on Personality in the Dubia Cockroach. Insects. 2022; 13(2):133. https://doi.org/10.3390/insects13020133
Chicago/Turabian StyleBouchebti, Sofia, Fernando Cortés-Fossati, Ángela Vales Estepa, Maria Plaza Lozano, Daniel S. Calovi, and Sara Arganda. 2022. "Sex-Specific Effect of the Dietary Protein to Carbohydrate Ratio on Personality in the Dubia Cockroach" Insects 13, no. 2: 133. https://doi.org/10.3390/insects13020133
APA StyleBouchebti, S., Cortés-Fossati, F., Vales Estepa, Á., Plaza Lozano, M., S. Calovi, D., & Arganda, S. (2022). Sex-Specific Effect of the Dietary Protein to Carbohydrate Ratio on Personality in the Dubia Cockroach. Insects, 13(2), 133. https://doi.org/10.3390/insects13020133