Predicting the Potential Distribution of Pine Wilt Disease in China under Climate Change
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Incidence Rates for PWD
2.2. Selection and Comparison of Climate Variables
2.3. Optimization of Model Parameters and Model Building
2.4. Assessment of Multivariate Environmental Similarity Surface
3. Results
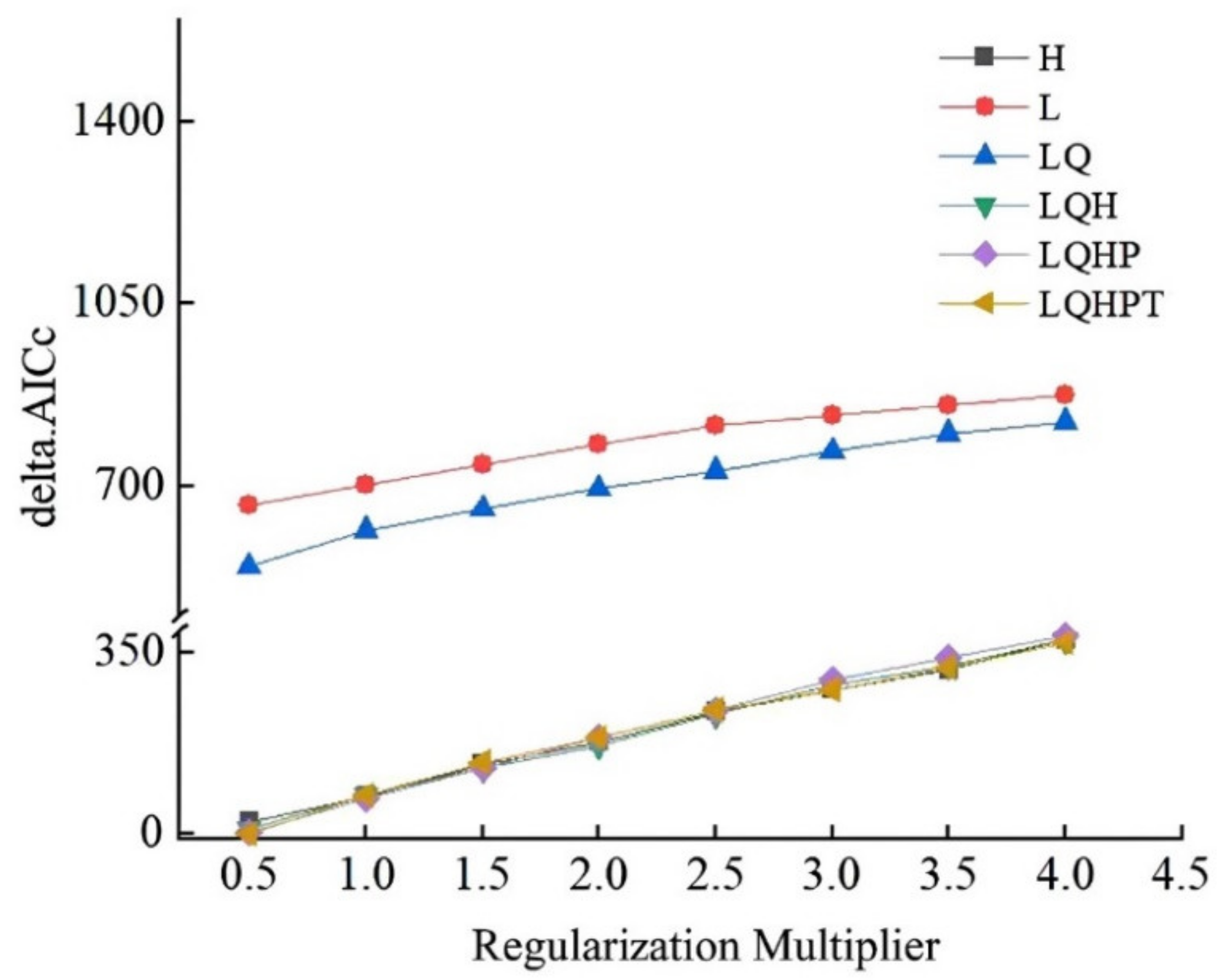
3.1. Model Optimization and Accuracy and Evaluation
3.2. Main Climate Variables Affecting Distribution of Pine Wilt Disease
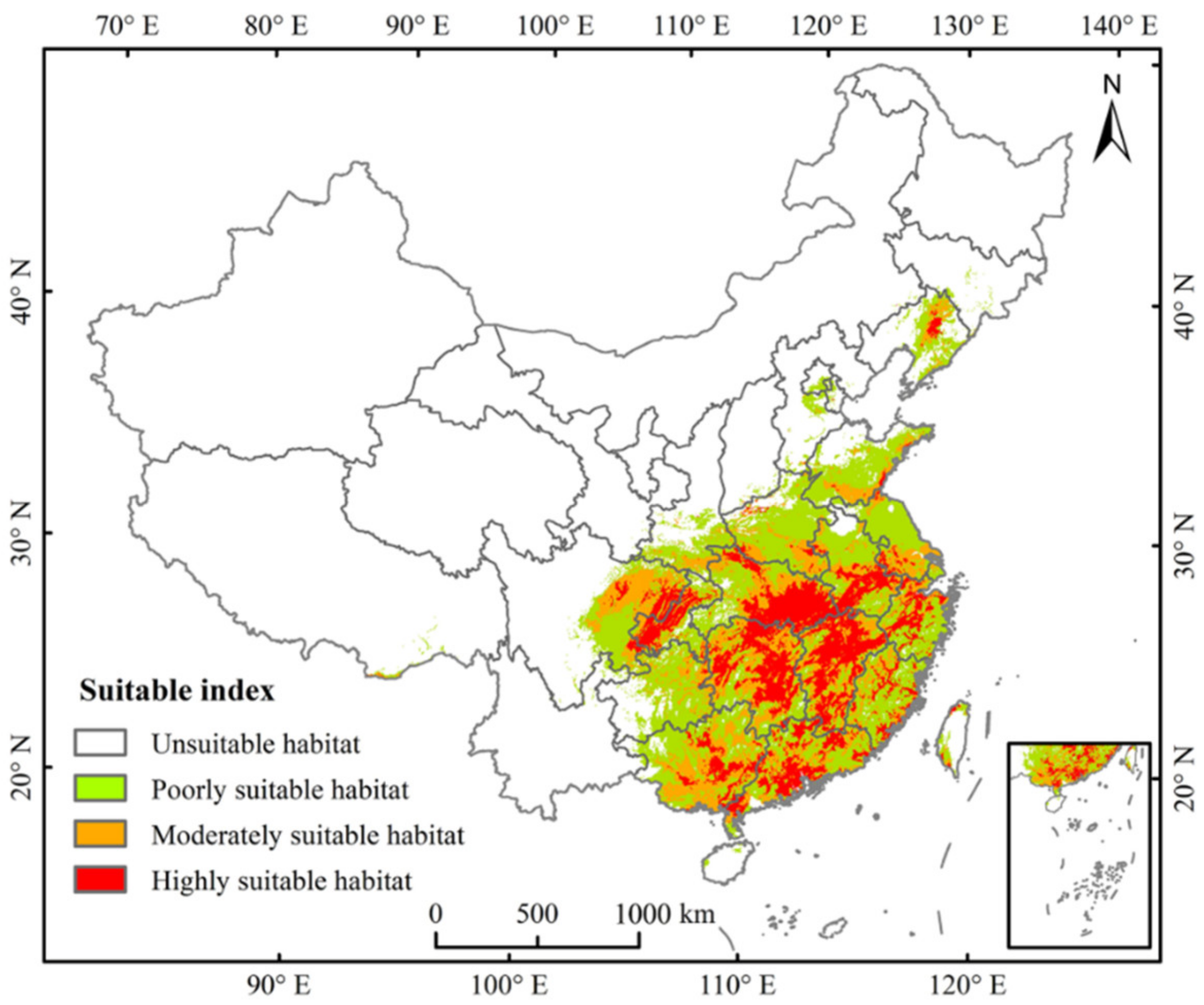
3.3. Current Potential Distribution
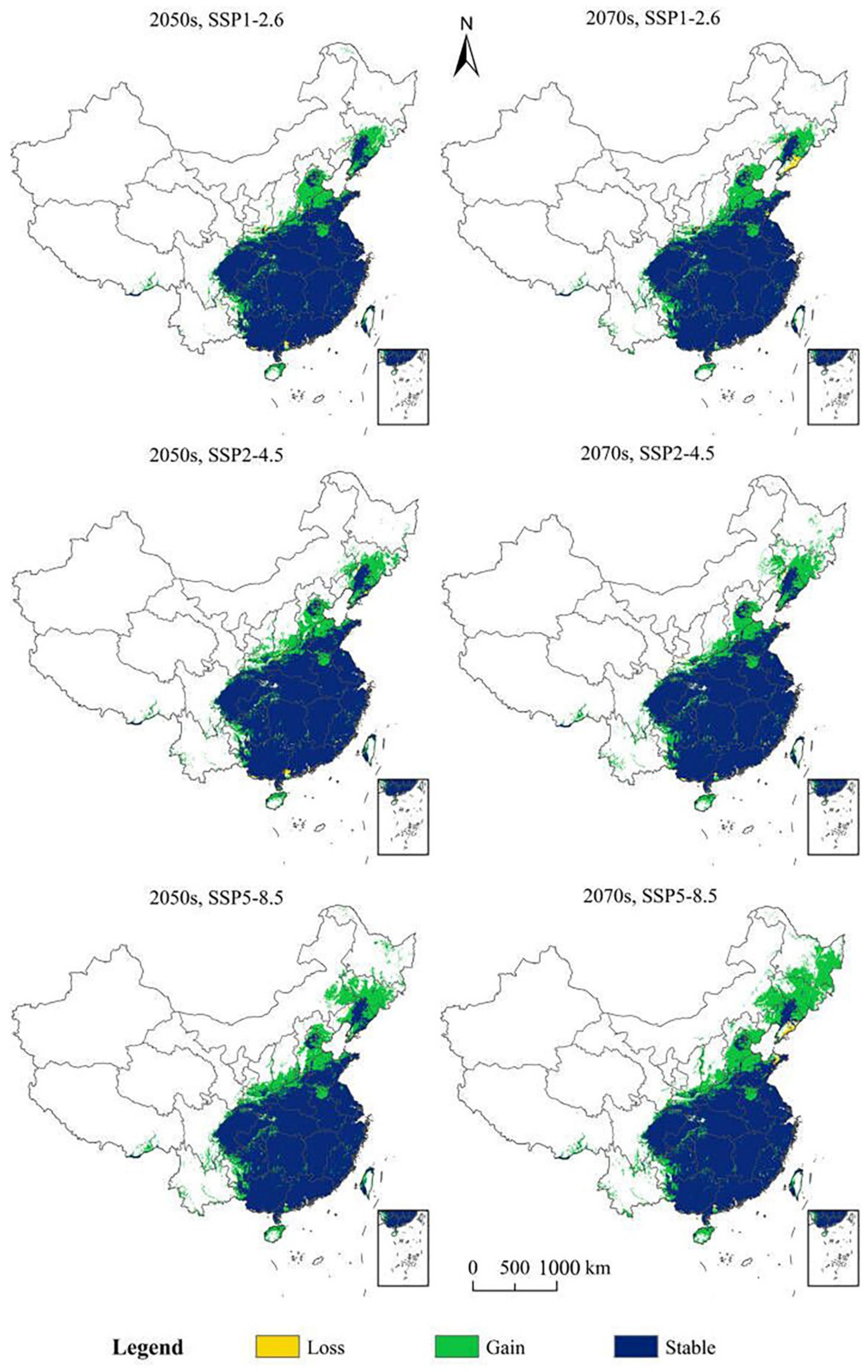
3.4. Potentially Suitable Climatic Distributions in the Future
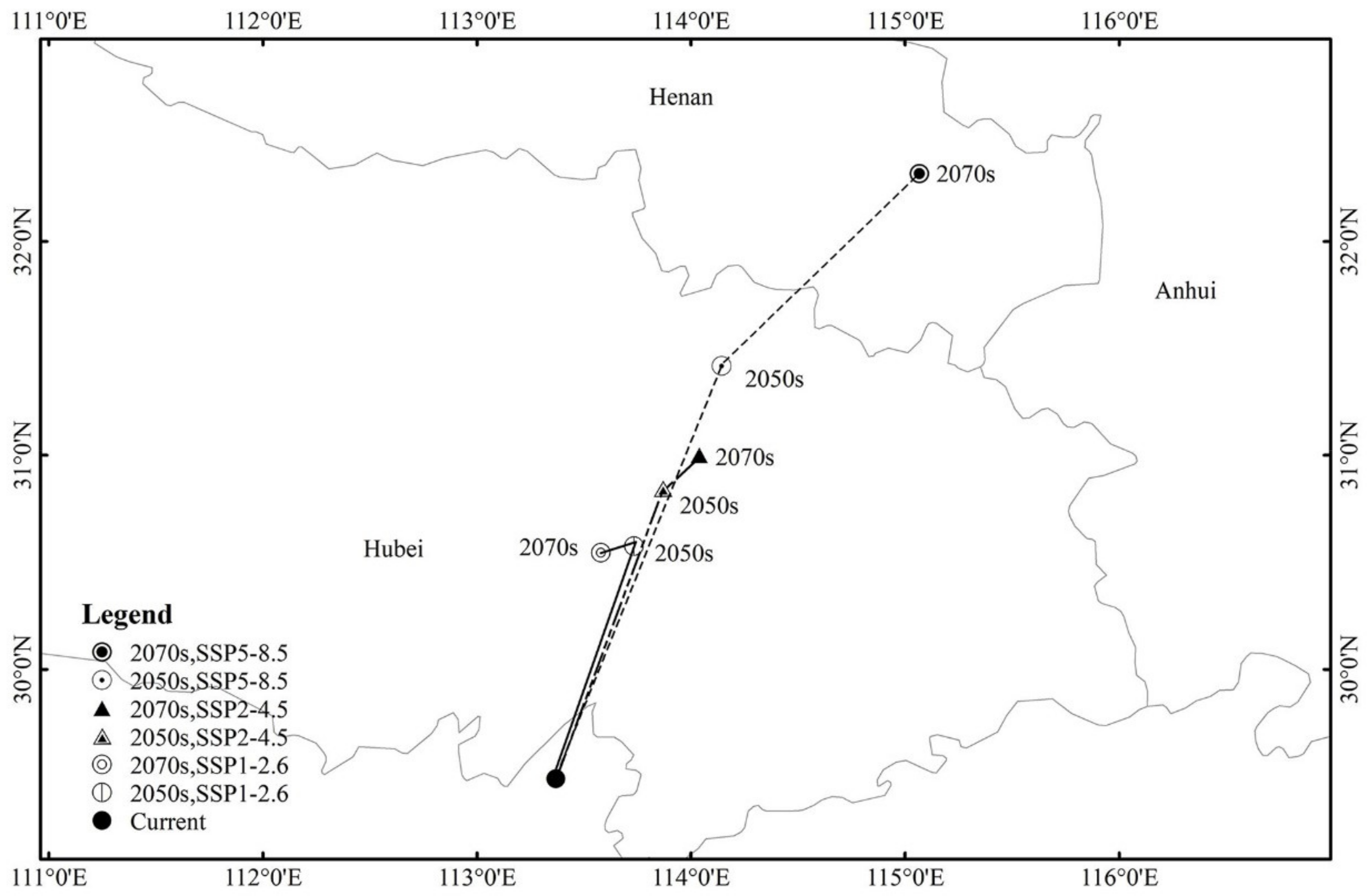
3.5. Highly Suitable Area Centroid Distributional Shifts under Climate Change for Pine Wilt Disease
3.6. Analysis of the Multivariate Environmental Similarity Surface (MESS) of Potential Area of Distribution for Pine Wilt Disease under Further Climate Change Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, F.; Tang, L.; He, H.; Yang, F.; Tao, J.; Wang, W. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent.Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef]
- Ouyang, X.; Pan, J.; Wu, Z.; Chen, A. Predicting potential distribution of Campsis grandiflora in China under climate change. Environ. Sci. Pollut. R. 2022, 29, 63629–63639. [Google Scholar] [CrossRef]
- Qi, Z.F.; Ye, X.Y.; Zhang, H.; Yu, Z.L. Land fragmentation and variation of ecosystem services in the context of rapid urbanization: The case of Taizhou city, China. Stoch. Env. Res. Risk A 2014, 28, 843–855. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Li, X.; Mao, F.; Du, H.; Zhou, G.; Xing, L.; Liu, T.; Han, N.; Liu, Y.; Zheng, J.; Dong, L. Spatiotemporal evolution and impacts of climate change on bamboo distribution in China. J. Environ. Manag. 2019, 248, 109265. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yu, Z.; Yang, C.; Ji, X.; Zhang, K. Trends in evapotranspiration and their responses to climate change and vegetation greening over the upper reaches of the Yellow River Basin. Agric. For. Meteorol. 2018, 263, 118–129. [Google Scholar] [CrossRef]
- Dieleman, C.M.; Branfireun, B.A.; McLaughlin, J.W.; Lindo, Z. Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability. Glob. Change Biol. 2015, 21, 388–395. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef]
- Ghareghan, F.; Ghanbarian, G.; Pourghasemi, H.R.; Safaeian, R. Prediction of habitat suitability of Morina persica L. species using artificial intelligence techniques. Ecol. Indic. 2020, 112, 106096. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, S.; Hao, M.; Fu, J.; Ding, F. Mapping the potential global codling moth (Cydia pomonella L.) distribution based on a machine learning method. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Ouyang, X.; Bai, S.; Brien Strachan, G.; Chen, A. Simulation of the potential suitable distribution of rare and endangered Satyrium species in China under climate change. Ecol. Evol. 2022, 12, e9054. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Zhao, H.; Li, M.; Han, W. Predicting the distribution of the invasive species Leptocybe invasa: Combining MaxEnt and geodetector models. Insects 2021, 12, 92. [Google Scholar] [CrossRef]
- Mehmud, S.; Kalita, N.; Roy, H.; Sahariah, D. Species distribution modelling of Calamus floribundus Griff.(Arecaceae) using Maxent in Assam. Acta Ecol. Sin. 2022, 42, 115–121. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Kroschel, J.; Sporleder, M.; Tonnang, H.E.; Juarez, H.; Carhuapoma, P.; Gonzales, J.; Simon, R. Predicting climate-change-caused changes in global temperature on potato tuber moth Phthorimaea operculella (Zeller) distribution and abundance using phenology modeling and GIS mapping. Agric. For. Meteorol. 2013, 170, 228–241. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.; DiTommaso, A.; Wang, R.; Wu, R. Predicting invasions of Wedelia trilobata (L.) Hitchc. with Maxent and GARP models. J. Plant Res. 2015, 128, 763–775. [Google Scholar] [CrossRef]
- Sanchez, A.C.; Osborne, P.E.; Haq, N. Identifying the global potential for baobab tree cultivation using ecological niche modelling. Agroforest. Syst. 2010, 80, 191–201. [Google Scholar] [CrossRef]
- Yan, X.; Wang, S.; Duan, Y.; Han, J.; Huang, D.; Zhou, J. Current and future distribution of the deciduous shrub Hydrangea macrophylla in China estimated by MaxEnt. Ecol. Evol. 2021, 11, 16099–16112. [Google Scholar] [CrossRef]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum entropy modeling to predict the impact of climate change on pine wilt disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant. Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Liu, J.; He, C.; Tang, Y.; Liu, W.; Xu, Y.; Li, Z.; Qin, X.; Jin, S. A review of Cremastra appendiculata (D. Don) Makino as a traditional herbal medicine and its main components. J. Ethnopharmacol. 2021, 279, 114357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, P.; Humble, L.M.; Sun, J. Within-tree distribution and attractant sampling of propagative pinewood nematode, Bursaphelenchus xylophilus: An early diagnosis approach. Forest. Ecol. Manag. 2009, 258, 1932–1937. [Google Scholar] [CrossRef]
- Kim, B.N.; Kim, J.H.; Ahn, J.Y.; Kim, S.; Cho, B.K.; Kim, Y.H.; Min, J. A short review of the pinewood nematode, Bursaphelenchus xylophilus. Toxicol. Environ. Health Sci. 2020, 12, 297–304. [Google Scholar]
- Tan, Q.Q.; Wu, H.Y.; Jiang, S.X.; Bing, H. Mortality and movement behaviour of Bursaphelenchus xylophilus under different dosages of copper sulphate. Plant. Protect. Sci. 2013, 49, 98–103. [Google Scholar] [CrossRef]
- Wang, W.; Peng, W.; Liu, X.; He, G.; Cai, Y. Spatiotemporal Dynamics and Factors Driving the Distributions of Pine Wilt Disease-Damaged Forests in China. Forests 2022, 13, 261. [Google Scholar] [CrossRef]
- Hao, Z.; Fang, G.; Huang, W.; Ye, H.; Zhang, B.; Li, X. Risk Prediction and Variable Analysis of Pine Wilt Disease by a Maximum Entropy Model. Forests 2022, 13, 342. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Riahi, K.; Moss, R.; Edmonds, J.; Thomson, A.; Nakicenovic, N.; Arnell, N. A proposal for a new scenario framework to support research and assessment in different climate research communities. Glob. Environ. Change 2012, 22, 21–35. [Google Scholar] [CrossRef]
- Zamora, P.; Rodríguez, V.; Renedo, F.; Sanz, A.V.; Domínguez, J.C.; Pérez-Escolar, G.; Martín, A.B. First report of Bursaphelenchus xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant. Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Zhang, M.G.; Slik, J.F.; Ma, K.P. Using species distribution modeling to delineate the botanical richness patterns and phytogeographical regions of China. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, K.; Xia, Q. A MaxEnt model for mineral prospectivity mapping. Nat. Resour. Res. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle; Springer: Berlin/Heidelberg, Germany, 1973; pp. 610–624. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Method Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, X.; Liu, S.; Zhang, J. The major factors influencing distribution of three species of Dendrobium: Analysis of potential ecologically suitable distributions. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100275. [Google Scholar] [CrossRef]
- Chen, R.; Huang, K.; Pan, S.; Xu, T.; Tan, J.; Hao, D. Jasmonate induced terpene-based defense in Pinus massoniana depresses Monochamus alternatus adult feeding. Pest Manag. Sci. 2021, 77, 731–740. [Google Scholar] [CrossRef]
- Gruffudd, H.R.; Jenkins, T.A.R.; Evans, H.F. Using an evapo-transpiration model (ETpN) to predict the risk and expression of symptoms of pine wilt disease (PWD) across Europe. Biol. Invasions. 2016, 18, 2823–2840. [Google Scholar] [CrossRef]
- Rutherford, T.A.; Webster, J.M. Distribution of pine wilt disease with respect to temperature in North America, Japan, and Europe. Can. J. For. Res. 1987, 17, 1050–1059. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef] [PubMed]
- Naoko, T.; Nobuhito, O.; Masanori, K. Separate estimation of N export into baseline N leakage without disturbance and N loss due to insect defoliation in a pine forest watershed in central Japan. Environ. Monit. Assess. 2013, 185, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Rebetez, M.; Dobbertin, M. Climate change may already threaten Scots pine stands in the Swiss Alps. Theor. Appl. Climatol. 2004, 79, 1–9. [Google Scholar] [CrossRef]
- Menéndez-Gutiérrez, M.; Alonso, M.; Toval, G.; Díaz, R. Variation in pinewood nematode susceptibility among Pinus pinaster Ait. provenances from the Iberian Peninsula and France. Ann. For. Sci. 2017, 74, 1–15. [Google Scholar] [CrossRef]
- Kanzaki, N.; Giblin-Davis, R.M. Diversity and plant pathogenicity of Bursaphelenchus and related nematodes in relation to their vector bionomics. Curr. For. Rep. 2018, 4, 85–100. [Google Scholar] [CrossRef]
- Volney, W.J.A.; Fleming, R.A. Climate change and impacts of boreal forest insects. Agr. Ecosyst. Environ. 2000, 82, 283–294. [Google Scholar]
- Cheng, G.; Lv, Q.; Feng, Y.; Li, Y.; Wang, Y.; Zhang, X. Temporal and spatial dynamic patteren of pine wilt disease distribution in China predicted under climate change scenario. Sci. Silvae Sin. 2015, 51, 119–126. [Google Scholar]
- Gao, R.; Wang, Z.; Shi, J.; Luo, Y. Effect of Bursaphelenchus xylophilus infection on leaf photosynthetic characteristics and resource-use efficiency of Pinus massoniana. Ecol. Evol. 2017, 7, 3455–3463. [Google Scholar] [CrossRef]
- Juan, S.; Youqing, L.; Haiwei, W.; Heliövaara, K.; Lizhuang, L. Impact of the invasion by Bursaphelenchus xylophilus on forest growth and related growth models of Pinus massoniana population. Acta Ecol. Sin. 2008, 28, 3193–3204. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model–Pine Wilt disease as a model case. Forest Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Jikumaru, S.; Togashi, K. Resistance of an indigenous biological system against expansion of the invasive nematode, Bursaphelenchus xylophilus, in cool areas of Japan. Nematology 2008, 10, 679–687. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Lu, F.; Guo, K.; Chen, A.; Chen, S.; Lin, H.; Zhou, X. Transcriptomic profiling of effects of emamectin benzoate on the pine wood nematode Bursaphelenchus xylophilus. Pest Manag. Sci. 2020, 76, 747–757. [Google Scholar] [CrossRef]
- Ouyang, X.; Fan, Q.; Chen, A.; Huang, J. Effects of trunk injection with emamectin benzoate on arthropod diversity. Pest Manag. Sci. 2022. [Google Scholar] [CrossRef]
- Liu, G.; Lin, X.; Xu, S.; Liu, G.; Liu, Z.; Liu, F.; Mu, W. Efficacy of fluopyram as a candidate trunk-injection agent against Bursaphelenchus xylophilus. Eur. J. Plant Pathol. 2020, 157, 403–411. [Google Scholar]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Insecticide dose and seasonal timing of trunk injection in apples influence efficacy and residues in nectar and plant parts. Pest Manag. Sci. 2019, 75, 1453–1463. [Google Scholar] [CrossRef]
- Byrne, F.J.; Krieger, R.I.; Doccola, J.; Morse, J.G. Seasonal timing of neonicotinoid and organophosphate trunk injections to optimize the management of avocado thrips in California avocado groves. Crop Prot. 2014, 57, 20–26. [Google Scholar] [CrossRef]
- Aćimović, S.G.; VanWoerkom, A.H.; Reeb, P.D.; Vandervoort, C.; Garavaglia, T.; Cregg, B.M.; Wise, J.C. Spatial and temporal distribution of trunk-injected imidacloprid in apple tree canopies. Pest Manag. Sci. 2014, 70, 1751–1760. [Google Scholar] [CrossRef]
- VanWoerkom, A.; Aćimović, S.; Sundin, G.; Cregg, B.; Mota-Sanchez, D.; Vandervoort, C.; Wise, J. Trunk injection: An alternative technique for pesticide delivery in apples. Crop Prot. 2014, 65, 173–185. [Google Scholar]
- Zhang, X.W.; Han, Q.; Wang, T.; Wu, L.; Chen, A.L. Preparation of 10% emamectin benzoate soluble granule for trunkinjection and its control efficacy on pine wilt disease. Chin. J. Pestic. Sci. 2019, 21, 538–544. [Google Scholar]
- Takai, K.; Soejima, T.; Suzuki, T.; Kawazu, K. Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Manag. Sci. 2000, 56, 937–941. [Google Scholar]



| Climatic Factors | Contribution (%) |
|---|---|
| Rainfall of wettest quarter (bio16) | 45.7 |
| The maximum temperature of warmest month (bio5) | 27.5 |
| Rainfall in driest month (bio14) | 16.1 |
| Isothermality (bio3) | 3.7 |
| Rainfall seasonality (coefficient of variation) (bio15) | 3.2 |
| Mean temperature of driest quarter (bio9) | 1.9 |
| Temperature seasonality (standard deviation × 100) (bio4) | 1.8 |
| Province Municipality Autonomous Regions | Poorly Suitable Habitat (104 km2) 10–30% | Moderately Habitat (104 km2) 30–50% | Highly Habitat (104 km2) 50–100% | Total Suitable Habitat (104 km2) |
|---|---|---|---|---|
| Beijing | 0.41 | 0.00 | 0.00 | 0.41 |
| Tianjin | 0.20 | 0.00 | 0.01 | 0.21 |
| Hebei | 1.27 | 0.07 | 0.01 | 1.35 |
| Shanxi | 0.18 | 0.01 | 0.00 | 0.19 |
| Inner Mongolia | 0.04 | 0.00 | 0.00 | 0.04 |
| Liaoning | 3.96 | 1.27 | 0.55 | 5.78 |
| Jilin | 0.39 | 0.02 | 0.01 | 0.42 |
| Heilongjiang | 0.00 | 0.00 | 0.00 | 0.00 |
| Shanghai | 0.42 | 0.10 | 0.00 | 0.52 |
| Jiangsu | 5.93 | 2.61 | 0.57 | 9.11 |
| Zhejiang | 4.61 | 2.05 | 2.27 | 8.93 |
| Anhui | 6.16 | 2.79 | 3.07 | 12.02 |
| Fujian | 6.15 | 2.52 | 1.81 | 10.48 |
| Jiangxi | 2.93 | 4.41 | 7.84 | 15.18 |
| Shandong | 7.65 | 2.25 | 0.22 | 10.12 |
| Henan | 9.45 | 3.21 | 0.50 | 13.16 |
| Hubei | 5.59 | 4.06 | 7.04 | 16.69 |
| Hunan | 4.71 | 6.74 | 7.60 | 19.05 |
| Guangdong | 5.55 | 4.18 | 5.14 | 14.87 |
| Guangxi | 8.77 | 7.66 | 2.61 | 19.04 |
| Hainan | 0.18 | 0.00 | 0.00 | 0.18 |
| Chongqing | 2.72 | 1.67 | 2.62 | 7.01 |
| Sichuan | 6.66 | 5.95 | 1.89 | 14.5 |
| Guizhou | 7.98 | 2.22 | 0.14 | 10.25 |
| Yunnan | 0.65 | 0.01 | 0.00 | 0.66 |
| Tibet | 0.30 | 0.14 | 0.01 | 0.45 |
| Shaanxi | 4.30 | 1.25 | 0.02 | 5.57 |
| Gansu | 0.04 | 0.00 | 0.00 | 0.04 |
| Qinghai | 0.00 | 0.00 | 0.00 | 0.00 |
| Ningxia | 0.00 | 0.00 | 0.00 | 0.00 |
| Xinjiang | 0.00 | 0.00 | 0.00 | 0.00 |
| Macao | 0.00 | 0.00 | 0.00 | 0.00 |
| Taiwan | 0.64 | 0.15 | 0.18 | 0.97 |
| Xianggang | 0.04 | 0.02 | 0.00 | 0.06 |
| Total (China) | 97.88 | 55.36 | 44.11 | 197.35 |
| Decades Scenarios | Predicted Area/104 km2 | |||
|---|---|---|---|---|
| Poorly Suitable Habitat | Moderately Suitable Habitat | Highly Suitable Habitat | Total Suitable Habitat | |
| Current | 97.88 | 55.36 | 44.11 | 197.35 |
| 2050s, SSP1-2.6 | 54.2 | 35.23 | 150.93 | 240.36 |
| 2050s, SSP2-4.5 | 61.89 | 33.75 | 151.35 | 246.99 |
| 2050s, SSP5-8.5 | 61.45 | 33.14 | 172.64 | 267.23 |
| 2070s, SSP1-2.6 | 56.73 | 30.95 | 156.01 | 243.69 |
| 2070s, SSP2-4.5 | 60.68 | 33.91 | 157.38 | 251.97 |
| 2070s, SSP5-8.5 | 73.92 | 39.24 | 173.13 | 286.29 |
| Decades, Scenarios | Predicted Area/104 km2 | ||
|---|---|---|---|
| Loss | Gain | Stable | |
| 2050s, SSP1-2.6 | 1.52 | 44.01 | 196.35 |
| 2050s, SSP2-4.5 | 1.56 | 50.72 | 196.33 |
| 2050s, SSP5-8.5 | 0.42 | 69.92 | 197.39 |
| 2070s, SSP1-2.6 | 2.34 | 48.17 | 195.52 |
| 2070s, SSP2-4.5 | 1.11 | 55.24 | 196.79 |
| 2070s, SSP5-8.5 | 1.82 | 90.48 | 196.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, X.; Chen, A.; Li, Y.; Han, X.; Lin, H. Predicting the Potential Distribution of Pine Wilt Disease in China under Climate Change. Insects 2022, 13, 1147. https://doi.org/10.3390/insects13121147
Ouyang X, Chen A, Li Y, Han X, Lin H. Predicting the Potential Distribution of Pine Wilt Disease in China under Climate Change. Insects. 2022; 13(12):1147. https://doi.org/10.3390/insects13121147
Chicago/Turabian StyleOuyang, Xianheng, Anliang Chen, Yan Li, Xiaoxiao Han, and Haiping Lin. 2022. "Predicting the Potential Distribution of Pine Wilt Disease in China under Climate Change" Insects 13, no. 12: 1147. https://doi.org/10.3390/insects13121147
APA StyleOuyang, X., Chen, A., Li, Y., Han, X., & Lin, H. (2022). Predicting the Potential Distribution of Pine Wilt Disease in China under Climate Change. Insects, 13(12), 1147. https://doi.org/10.3390/insects13121147






