Nest Ecology and Prey Preference of the Mud Dauber Wasp Sceliphron formosum (Hymenoptera: Sphecidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mud Nest Collecting
2.2. Examination of Nest Contents
2.3. Statistical Analyses of Tenant Communities
3. Results
3.1. Survey and Observation of the Nests of Sceliphron formosum in ACT
3.2. Spider Prey Composition
3.3. Body Length to Leg Span Ratio (BLR)
3.4. Nest Composition
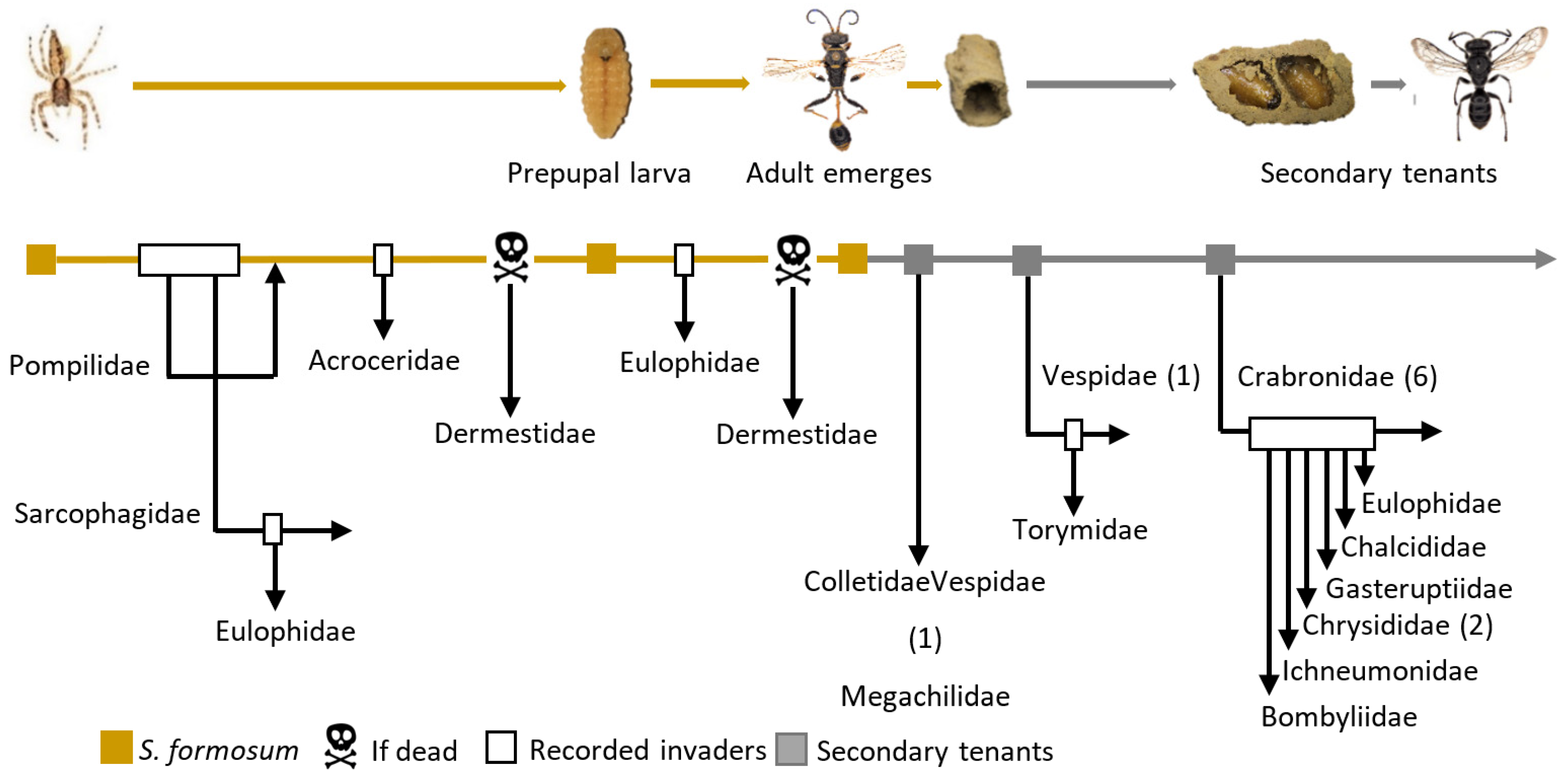
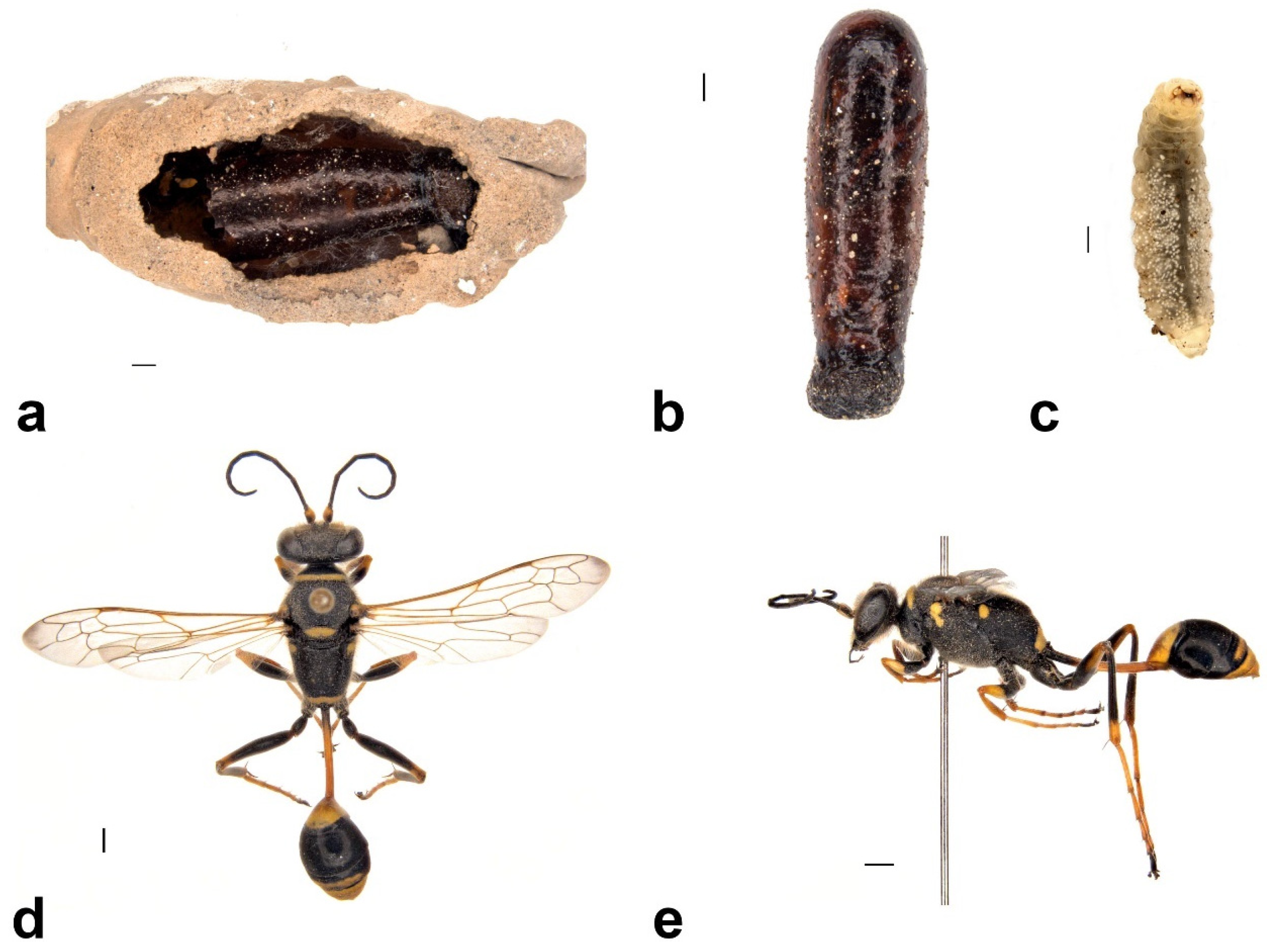
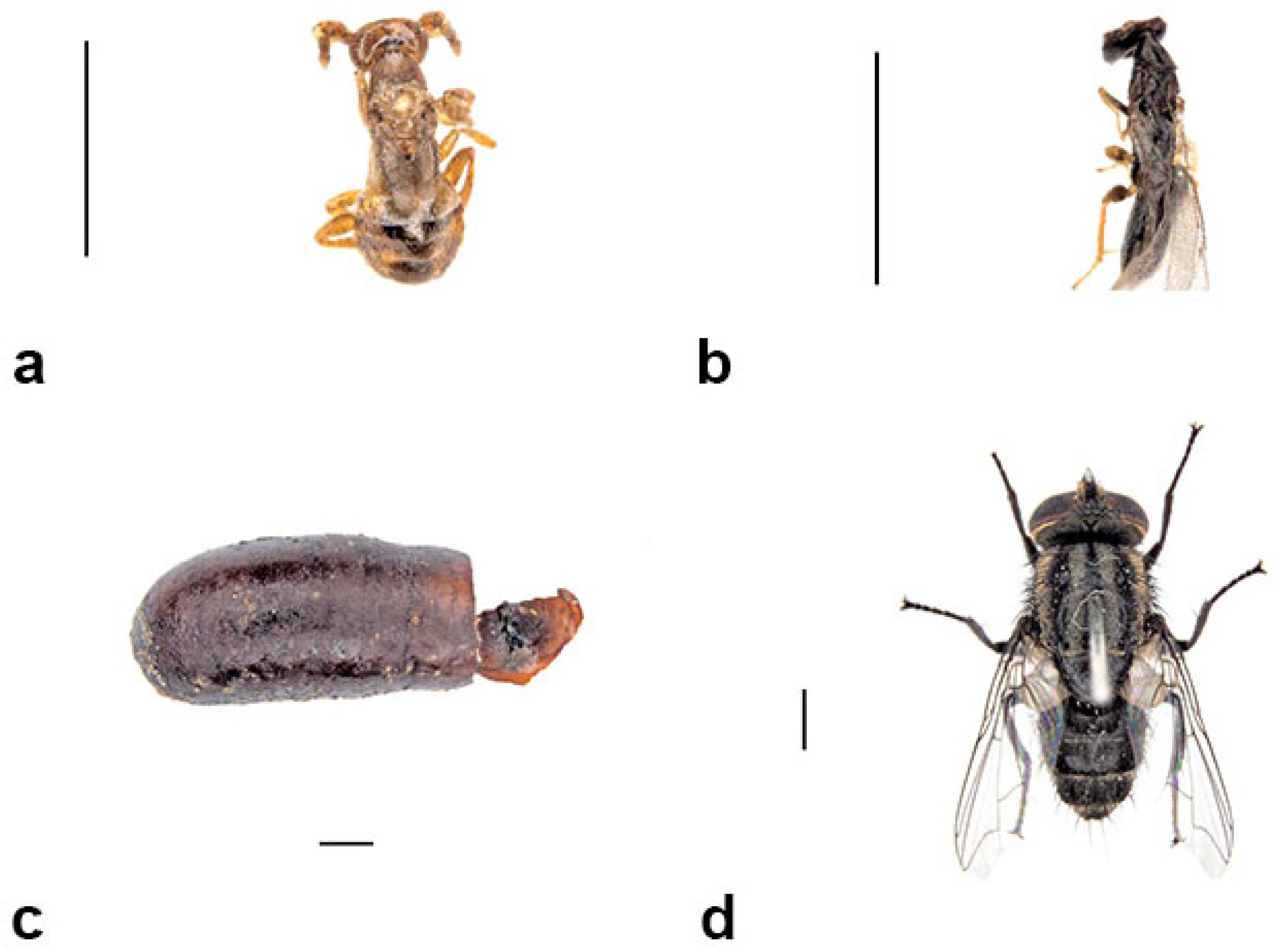
3.4.1. Group 1: Original Nest Builder
Sceliphron formosum (Smith, 1856) (Hymenoptera: Sphecidae)
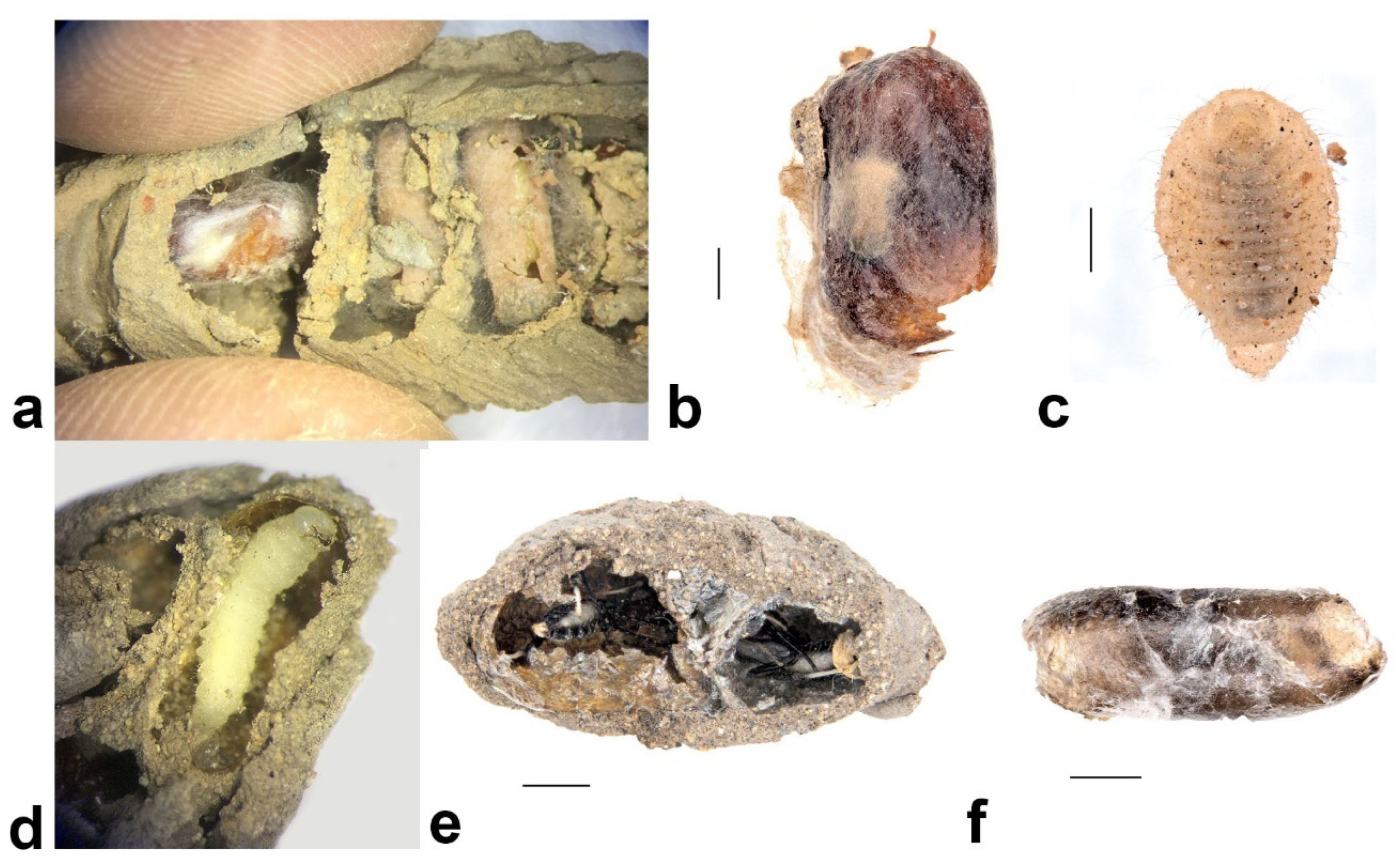
3.4.2. Group 2: Secondary Tenants That Exploit Abandoned Nests
Pison spp. (Hymenoptera: Crabronidae)
- (1)
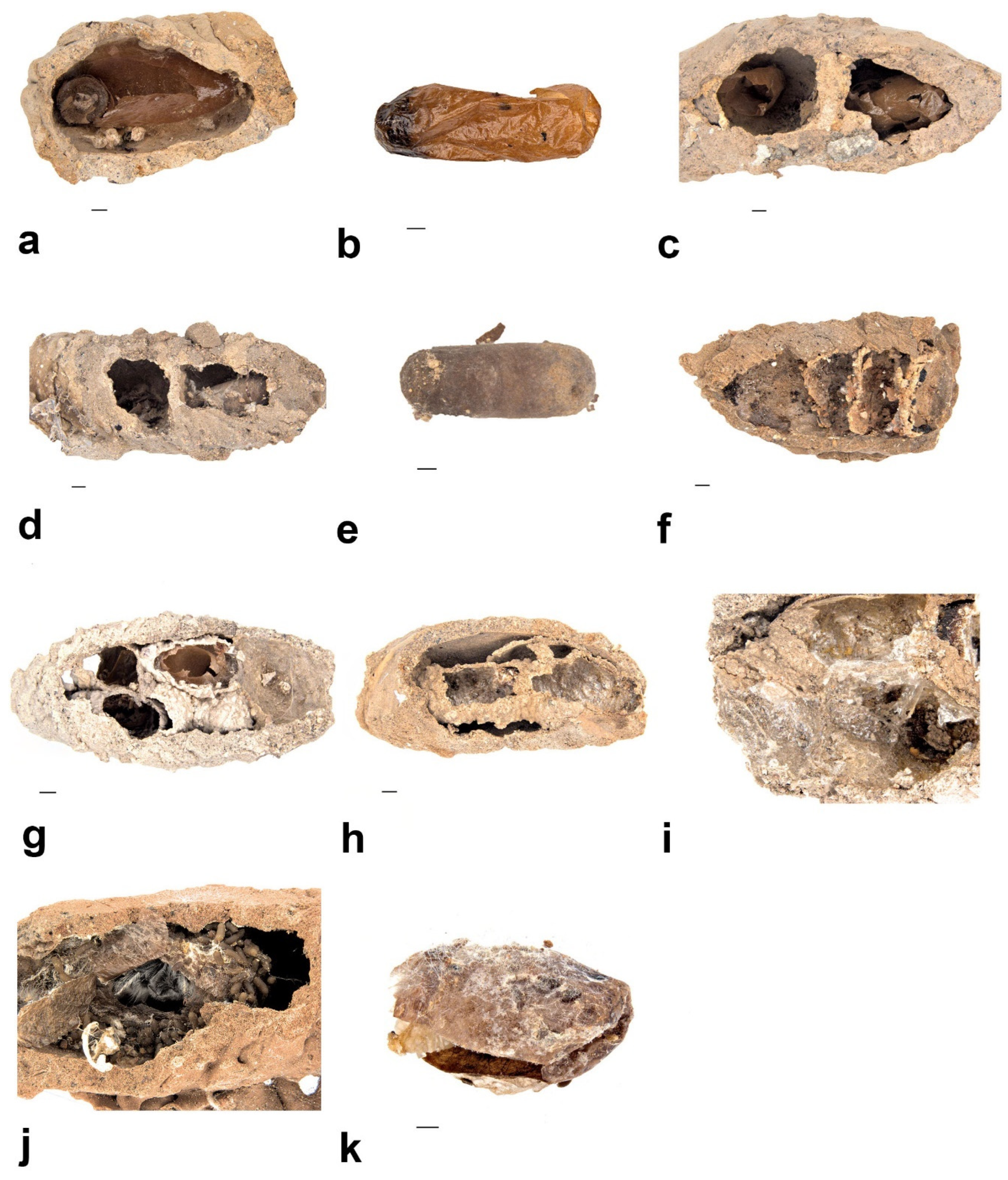
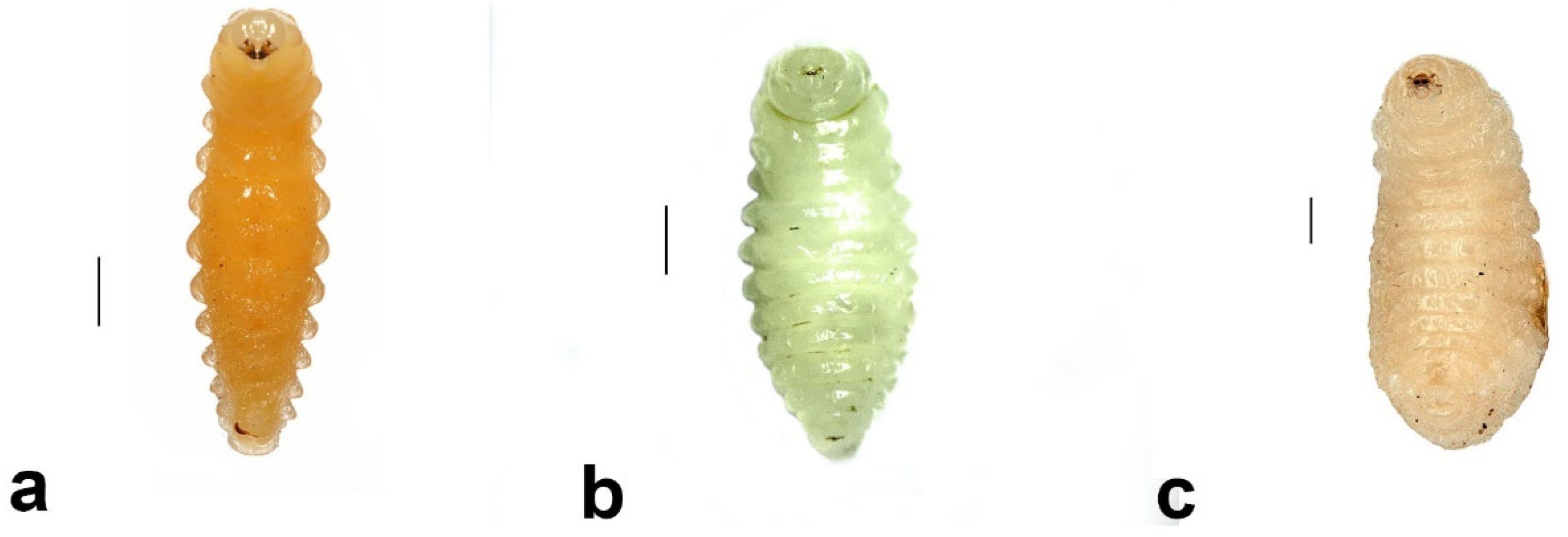
- Pison simillimum was the dominant tenant found in S. formosum nests. Females occupy the nest by building their own cells, which can make up one or two cells in a single nest (Figure 8a,c). The larvae are grub-like, the head capsule is clear, and the body light is yellow with protrusions on the side of segments (Figure 9a). The cocoon is oval, light-brown coloured, with a paper-like texture and a darkened cap that serves as a moulting exit (Figure 8b). The adults are entirely black, but have ferruginous tibia, tarsus and distinctive brown pubescence on the lower rim of each tergite (Figure 10a).
- (2)

- (3)
- (4)
- Pison priscum is a small-sized Pison. Adults make their own cells inside the nests of S. formosum. They usually build two to three cells in one nest, compartmentalized by mud walls (Figure 8f). Cocoon and larvae are similar to P. simillimum but smaller in size. Adult is entirely black in colour (Figure 9d).
- (5)
- Pison peletieri is another small-sized Pison. Adults were found building small ball-like mud nests inside empty mud nests (Figure 8g). The cocoon and larvae are similar to P. simillimum but smaller in size. The head, thorax and part of the femur of the adult are black, while the rest of the legs are ferruginous (Figure 9e).
- (6)
- Pison prostratum also build small ball-like nests inside empty nests and have a cocoon similar to that of P. simillimum. The main difference between Pison peletieri and Pison prostratum is body colouration. The adult is entirely black, except for the legs and ventral segments of the antennae (Figure 9f).
Eumeninae wasps (Hymenoptera: Vespidae)
Hylaeus nubilosus (Smith, 1853) (Hymenoptera: Colletidae)
Megachile aurifrons Smith, 1853 (Hymenoptera: Megachilidae)
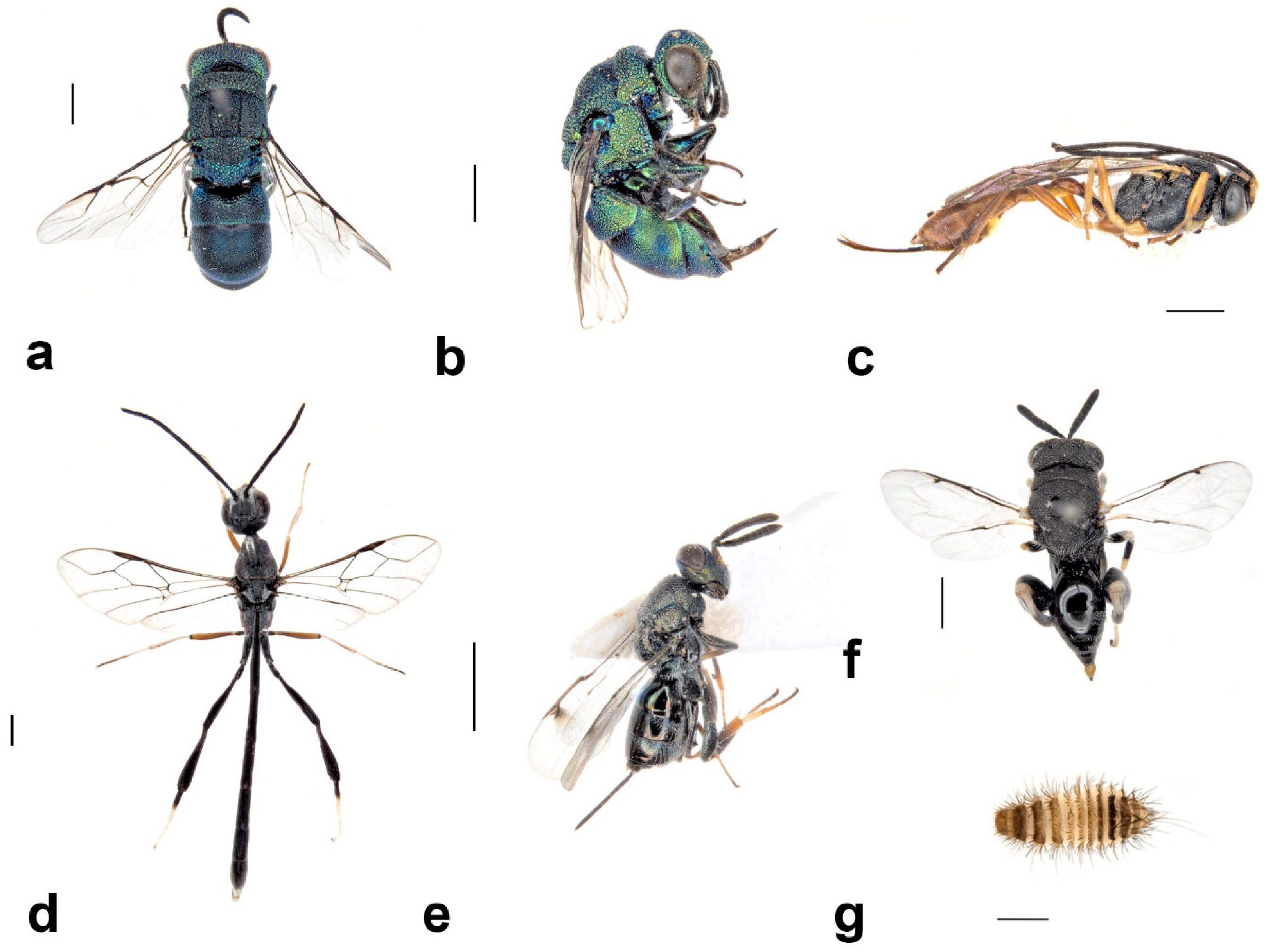
3.4.3. Group 3: Parasitoids of S. formosum
Melittobia australica Girault, 1912 (Hymenoptera: Eulophidae)
Amobia burnsi (Malloch, 1930) (Diptera: Sarcophagidae)
3.4.4. Group 4: Parasitoids of Secondary Tenants
Brachymeria sp. (Hymenoptera: Chalcididae)
Toryminae sp. (Hymenoptera: Torymidae)
Phrudus sp. (Hymenoptera: Ichneumonidae)
Gasteruption cinerescens Pasteels, 1957 (Hymenoptera: Gasteruptiidae)
Primeuchroeus reversus (Smith, 1874) (Hymenoptera: Chrysididae)
Primeuchroeus faustus (Smith, 1874) (Hymenoptera: Chrysididae)
Thraxan sp. (Diptera: Bombyliidae)
3.4.5. Group 5: Share Space with S. formosum
Epipompilus mirabundus Yuan & Rodriguez, 2020 (Hymenoptera: Pompilidae)
3.4.6. Group 6: The by-Catch Tenant
Ogcodes pygmaeus White, 1914 (Diptera: Acroceridae)
3.4.7. Group 7: Scavenger
Anthrenus sp. (Coleoptera: Dermestidae)
4. Discussion
4.1. Prey Preference
4.2. Nest Ecology
4.3. Host Associations
4.4. Notes on New Species
4.5. Australian Beneficial Pollinators
4.6. Australian Native Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, P.H. Taxonomic notes on the genus Sceliphron Klug (Hymenoptera: Sphecidae) from northern Vietnam, with description of a new species. Turk. J. Zool. 2016, 40, 686–690. [Google Scholar] [CrossRef]
- Naumann, I.D. The biology of mud nesting Hymenoptera (and their associates) and Isoptera in rock shelters of the Kakadu Region, Northern Territory. Aust. Nat. Parks Wildl. Serv. Spec. Pub. 1983, 10, 127–189. [Google Scholar]
- O’Neill, K.M. Solitary Wasps: Behavior and Natural History; Cornell University Press: New York, NY, USA, 2001; pp. 1–4. [Google Scholar]
- Evans, H.E.; Eberhard, M.J.W. The Wasps; University of Michigan Press: Ann Arbor, MI, USA, 1970. [Google Scholar]
- Elgar, M.A.; Jebb, M. Nest provision in the mud dauber wasp Sceliphron laetum (F. Smith): Body mass and taxa specific prey selection. Behaviour 1999, 136, 147–159. [Google Scholar]
- Hensen, R.V. Revision of the subgenus Prosceliphron Van der Vecht (Hymenoptera, Sphecidae). Tijdschr. Voor Entomol. 1987, 129, 217–261. [Google Scholar]
- Callan, E.M.C. Biological observations on the mud-dauber wasps Sceliphron formosum (F. Smith) (Hymenoptera: Sphecidae). Aust. Entomol. Mag. 1988, 14, 78–82. [Google Scholar]
- Blösch, M. Die Grabwespen Deutschlands. Lebensweise, Verhalten, Verbreitung; Goecke and Evers: Keltern, Germany, 2000. [Google Scholar]
- Coville, R.E. Spider-hunting sphecid-wasps. In Ecophysiology of Spiders; Nentwig, W., Ed.; Springer: New York, NY, USA, 1987; pp. 309–318. [Google Scholar]
- Blackledge, T.A.; Coddington, J.A.; Gillespie, R.G. Are three-dimensional spider webs defensive adaptations? Ecol. Lett. 2003, 6, 13–18. [Google Scholar] [CrossRef]
- Camillo, E. The natural history of the mud-dauber wasp Sceliphron fistularium (Hymenoptera: Sphecidae) in southeastern Brazil. Rev. Biol. Trop. 2002, 50, 127–134. [Google Scholar] [PubMed]
- Fateryga, A.V.; Kovblyuk, M.M. Nesting ecology of the wasp Sceliphron destillatorium (Illiger, 1807) (Hymenoptera, Sphecidae) in the Crimea. Entomol. Rev. 2014, 94, 330–336. [Google Scholar] [CrossRef]
- Polidori, C.; Trombino, L.; Fumagalli, C.; Andrietti, F. The nest of the mud-dauber wasp, Sceliphron spirifex (Hymenoptera: Sphecidae): An application of geological methods to structure and brood cells contents analysis. It. J. Zool. 2005, 72, 153–159. [Google Scholar] [CrossRef]
- Polidori, C.; Federici, M.; Pesarini, C.; Andrietti, F. Factors affecting spider prey selection by Sceliphron mud-dauber wasps (Hymenoptera: Sphecidae) in northern Italy. Anim. Biol. 2007, 57, 11–28. [Google Scholar]
- Uma, D.B.; Weiss, M.R. Chemical Mediation of Prey Recognition by Spider-Hunting Wasps. Ethology 2010, 116, 85–95. [Google Scholar] [CrossRef]
- White, E. Nest-building and provisioning in relation to sex in Sceliphron spirifex L. (Sphecidae). J. Anim. Ecol. 1962, 31, 317–332. [Google Scholar] [CrossRef]
- Blackledge, T.A.; Wenzel, J.W. Silk mediated defense by an orb web spider against predatory mud dauber wasps. Behaviour 2001, 138, 155–178. [Google Scholar] [CrossRef]
- Powell, E.C.; Taylor, L.A. Specialists and generalists coexist within a population of spider-hunting mud dauber wasps. Behav. Ecol. 2017, 28, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Campadelli, G.; Pagliano, G.; Scaramozzino, P.L.; Strumia, F. Parasitoids of Sceliphron caemenatrium (Drury) (Hymenoptera: Sphecidae) and other insects utilizing its nests in Romagna. Mus. Reg. Sci. Nat. Boll. (Turin) 1999, 16, 225–240. [Google Scholar]
- Van Achterberg, K. The Insect of Australia, 2nd ed.; Melbourne University Press: Melbourne, Australia, 1991. [Google Scholar]
- Dahms, E.C. Revision of the genus Melittobia (Chalcidoidea: Eulophidae) with the description of seven new species. Mem. Qld Mus. 1984, 21, 271–336. [Google Scholar]
- Evans, H.E. The Genus Epipompilus in Australia (Hymenoptera: Pompilidae). Pac. Insects 1962, 4, 773–782. [Google Scholar]
- Pulawski, W.J. A Revision of the Wasp Genus Pison Jurine, 1808 of Australia and New Zealand, New Guinea, and the Pacific Islands (Hymenoptera: Crabronidae). Proc. Calif. Acad. Sci. 2018, 4, 1–584. [Google Scholar]
- Richardson, B.J.; Whyte, R.; Żabka, M. A Key to the Genera of Australian Jumping Spiders (Aranaea: Salticidae). 2019. Available online: https://apps.lucidcentral.org/salticidae/ (accessed on 20 May 2021).
- Turner, R.E. Notes on the wasps of the genus Pison, and some allied genera. Proc. Zool. Soc. Lond. 1916, 86, 591–629. [Google Scholar] [CrossRef]
- Coleman, B.D. On random placement and species-area relations. Math. Biosci. 1981, 54, 191–215. [Google Scholar] [CrossRef]
- Coleman, B.D.; Mares, M.A.; Willig, M.R.; Hsieh, Y.H. Randomness, area, and species richness. Ecology 1982, 63, 1121–1133. [Google Scholar] [CrossRef]
- EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 9 and Earlier. User’s Guide and Application. Available online: http://purl.oclc.org/estimates (accessed on 28 May 2019).
- Li, X.K.; Yuan, D.; Rodriguez, J.; Yeates, D.K. Sand wasp (Hymenoptera: Crabronidae) parasites emerging from mud wasp nests (Hymenoptera: Sphecidae)—A reliable host record of Thraxan Yeates & Lambkin (Diptera: Bombyliidae: Anthracinae) with description of the pupal exuviae of three Thraxan species. Zootaxa 2019, 4609, 149–159. [Google Scholar]
- Yuan, D.; Rodriguez, J. Three new species of Epipompilus Kohl (Pompilidae, Pepsinae) from Australia. Zootaxa 2020, 4743, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, X.K.; Yeates, D.K.; Rodriguez, J. Unlucky spider flies (Acroceridae) trapped in a tomb of mud: An Australian predatory wasp (Sphecidae) provisions its nest with parasitised spiders (Salticidae). Pan Pac. Entomol. 2020, 95, 109–115. [Google Scholar] [CrossRef]
- Futuyma, D.J. Ecological specialization and generalization. In Evolutionary Ecology: Concepts and Case Studies; Fox, C.W., Roff, D.A., Fairbairn, D.J., Eds.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Araujo, M.; Gonzaga, M. Individual specialization in the hunting wasp Trypoxylon (Trypargilum) albonigrum (Hymenoptera, Crabronidae). Behav. Ecol. Sociobiol. 2007, 61, 1855–1863. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef]
- Marussich, W.A.; Faeth, S.H. Effects of urbanization on trophic dynamics of arthropod communities on a common desert host plant. Urban Ecosyst. 2009, 12, 265–286. [Google Scholar] [CrossRef]
- Fraser, J.B.; Frankie, G.W. An Ecological Comparison of Spiders from Urban and Natural Habitats in California. Hilgardia 1986, 54, 1–24. [Google Scholar] [CrossRef]
- Hanson, P.E.; Gauld, I.D. The Hymenoptera of Costa Rica; Oxford University Press: Oxford, UK, 1995; p. 920. [Google Scholar]
- Freeman, B.E.; Parnell, J.R. Mortality of Sceliphron assimile Dahlbom (Sphecidae) caused by the eulophid Melittobia chalybii Ashmead. J. Anim. Ecol. 1973, 42, 779–784. [Google Scholar] [CrossRef]
- Genaro, J.A. Inquilinos de Sceliphron assimile, con énfasis en Podium fulvipes (Hymenoptera: Vespidae, Sphecidae, Megachilidae). Caribb. J. Sci. 1994, 30, 268–270. [Google Scholar]
- Genaro, J.A. Nest parasites (Coleoptera, Diptera, Hymenoptera) of some wasps and bees (Vespidae, Sphecidae, Colletidae, Megachilidae, Anthophoridae) in Cuba. Caribb. J. Sci. 1996, 32, 239–240. [Google Scholar]
- González, J.M.; Genaroand, J.A.; Matthews, R.W. Species of Melittobia (Hymenoptera: Eulophidae) established in Bahamas, Costa Rica, Cuba, Hispaniola, Puerto Rico and Trinidad. Fla. Entomol. 2004, 87, 619–620. [Google Scholar] [CrossRef]
- Hunt, J.H. Survivorship, fecundity and recruitment in a mud dauber wasp, Sceliphron assimile (Hymenoptera: Sphecidae). Ann. Entomol. Soc. Am. 1993, 86, 51–59. [Google Scholar] [CrossRef]
- Tormos, J.; Polidori, C.; Asís, J.D.; Federici, M. Description of the Postdefecating Larva of Stilbum cyanura (Förster) and Observations on Adult Behaviour. J. Entomol. Sci. 2006, 41, 1–8. [Google Scholar] [CrossRef]
- Ashton, P.S. Species richness in plant communities. In Conservation Biology; Fiedler, P.I., Jain, K.S., Eds.; Chapman and Hall: London, UK, 1992; pp. 3–22. [Google Scholar]
- Dayton, P.K. The structure and regulation of same South American kelpcommunities. Ecol. Monogr. 1985, 55, 447–468. [Google Scholar] [CrossRef]
- Duran, I.R.; Castilla, J. Variation and persistance of the middle rocky intertidal community of central Chile, wirh aud wlthout human harvesting. Mar. Biol. 1989, 103, 555–562. [Google Scholar] [CrossRef]
- Gentry, A.H.; Dodson, C.H. Diversity and biogeography of neotropieal vascular epiphytes. Ann. Mo. Bot. Gard. 1987, 74, 205–233. [Google Scholar] [CrossRef]
- Gess, S.K.; Gess, F.W. Patterns of Usage of Snail Shells for Nesting by Wasps (Vespidae: Masarinae and Eumeninae) and Bees (Megachilidae: Megachilinae) in Southern Africa. J. Hym. Res. 2008, 17, 86–109. [Google Scholar]
- Paine, R.T.; Schanek, T.H. Convergence of ecologteal process between dependently evolved competitive dominants: A cunicate mussel comparison. Evolution 1983, 37, 821–831. [Google Scholar] [PubMed]
- Strong, D.R. Epiphyte loads, tree falls and perennial forest disruption: A mechanism for maintaining higher tree species richness in the tropics without animals. Biotropica 1977, 4, 215–218. [Google Scholar] [CrossRef]
- Gillung, J.P.; Borkent, C.J. Death comes on two wings: A review of dipteran natural enemies of arachnids. J. Aracchnology 2017, 45, 1–19. [Google Scholar] [CrossRef]
- Schlinger, E.I.; Gillung, J.P.; Borkent, C.J. New spider flies from the Neotropical Region (Diptera, Acroceridae) with a key to New World genera. Zookeys 2013, 270, 59–93. [Google Scholar]
- Schlinger, E.I. A revision of the genus Ogcodes Latreille, with particular reference to species of the Western hemisphere. Proc. United States Natl. Mus. 1960, 111, 227–336. [Google Scholar] [CrossRef]
- Roig-Alsina, A.; Barneche, J. The genus Epipompilus in Argentina (Hymenoptera: Pompilidae). Rev. Soc. Entomol. Argent. 2017, 76, 33–38. [Google Scholar] [CrossRef][Green Version]
- Villanueva-Bonilla, G.A.; Brescovit, A.D.; Santos, E.F.; Vasconcellos-Netro, J. First record of Epipompilus excelsus (Bradley, 1944) (Hymenoptera, Pompilidae) as a koinobiont ectoparasitoid of Ariadna mollis (Holmberg, 1876) (Araneae, Segestriidae). J. Hymenopt. Res. 2018, 66, 15–21. [Google Scholar] [CrossRef]
- Nilsson, L.A.; Jonsson, L.; Rason, L.; Randrianjohany, E. The pollination of Cymbidiella flabellata (Orchidaceae) in Madagascar: A system operated by sphecid wasps. Nord. J. Bot. 1986, 6, 411–422. [Google Scholar] [CrossRef]
- Hawthorn, L.R.; Bohart, G.E.; Toole, E.H.; Nye, W.P.; Levin, M.D. Carrot Seed Production as Affected by Insect Pollination. Utah Agr. Exp. Sta. Bull. 1960, 422, 18. [Google Scholar]
- Anderson, D.L.; Sedgley, M.; Short, J.R.T.; Allwood, A.J. Insect Pollination of Mango in Northern Australia. Aus. J. Agric. Res. 1982, 33, 541–548. [Google Scholar] [CrossRef]
- Bosch, J.; Kemp, W.P. Developing and establishing bee species as crop pollinators: The example of Osmia spp. (Hymenoptera: Megachilidae) and fruit trees. Bull. Entomol. Res. 2002, 92, 3–16. [Google Scholar]
- Diallo, B.O.; Ouedraogo, M.; Chevallier, M.; Joly, I.; Hossaert-McKey, M.; McKey, D. Potential pollinators of Tamarindus indica L. (Caesalpinioideae) in Sudanian region of Burkina Faso. Afr. J. Plant Sci. 2014, 8, 528–536. [Google Scholar]
- Pitts-Singer, T.L.; Cane, J.H. The Alfalfa Leafcutting Bee, Megachile rotundata: The world’s most intensively managed solitary bee. Annu. Rev. Entomol. 2011, 56, 221–237. [Google Scholar] [CrossRef]
- Gosek, J.; Ruszkowski, A.; Kaczmarska, K. Food plants and an economic importance of Hylaeus species of subgenera Spatulariella Popov, Abrupta Popov and Koptogaster Alfken (Hymenoptera, Colletidae). Pszczel. Zesz. Nauk. 1995, 39, 265–272. [Google Scholar]
- Colombari, F.; Battisti, A. Spread of the introduced biocontrol agent Torymus sinensis in north-eastern Italy: Dispersal through active flight or assisted by wind? BioControl 2016, 61, 127–139. [Google Scholar] [CrossRef]
- Matošević, D.; Mujezinović, O.; Dautbašić, M. First Record of Biocontrol Agent Torymus sinensis (Hymenoptera; Torymidae) in Bosnia and Herzegovina. South-East Eur. For. 2017, 8, 147–149. [Google Scholar] [CrossRef][Green Version]
- Mohamed, S.A.; Ramadan, M.M.; Ekesi, S. In and Out of Africa: Parasitoids Used for Biological Control of Fruit Flies. In Fruit Fly Research and Development in Africa—Towards a Sustainable Management Strategy to Improve Horticulture; Ekesi, S., Mohamed, S., De Meyer, M., Eds.; Springer: Cham, Switerland, 2016. [Google Scholar]
- Singh, M.P. Erionata thrax Linn, a serious pest of banana in Manipur and its potential biocontrol agent, Brachymeria euploeae (West). Insect Environ. 1997, 3, 51–55. [Google Scholar]
- Falcón-Brindis, A.; Rodriguez-Estrella, R.; Jimenez, M.L. A Fatal Nest Construction: Man-mixed Cement Used by Mud-dauber Wasps. Sociobiology 2018, 65, 524–526. [Google Scholar] [CrossRef]
- Nelson, D.M.; Starr, C.K. Comparative nesting success of the keyhole mud-dauber (Hymenoptera, Crabronidae, Trypoxylon nitidum) in different substrates. J. Hymenopt. Res. 2016, 52, 163–167. [Google Scholar]




| Spider Family | Numbers of Individuals in Nests |
|---|---|
| Salticidae | 514 (82.5%) |
| Araneidae | 42 (6.7%) |
| Hersiliidae | 29 (4.7%) |
| Sparassidae | 23 (3.7%) |
| Thomisidae | 8 (1.3%) |
| Amaurobiidae | 1 (0.2%) |
| unknown | 5 (0.8%) |
| Spider Prey | Number of Spiders |
|---|---|
| Opisthoncus parcidentatus | 94 (35.5%) |
| Opisthuncus sp. | 21 (7.9%) |
| Servaea narraweena | 59 (22.3%) |
| Servaea villosa | 16 (6%) |
| Servaea incana | 5 (1.9%) |
| Servaea sp. | 1 (0.4%) |
| Cytaea sp. | 38 (14.3%) |
| Helpis sp. | 8 (3%) |
| Simaethula sp. | 7 (2.6%) |
| Holoplatys sp. | 4 (1.5%) |
| Simaetha sp. | 3 (1.1%) |
| Zenodorus sp. | 1 (0.4%) |
| Bianor maculatus | 1 (0.4%) |
| Sandalodes sp. | 1 (0.4%) |
| Clynotis sp. | 1 (0.4%) |
| Other | 5 (1.9%) |
| Total | 265 |
| Type of Tenant | Family or Species | Number of Nests Occupied |
|---|---|---|
| 1. Nest builder | Sceliphron formosum (Sphecidae) | 162 |
| 2. Secondary tenants | Pison spp. (Crabronidae) | 266 |
| Eumeninae (Vespidae) | 101 | |
| Hylaeus nubilosus (Colletidae) | 15 | |
| Megachile aurifrons (Megachilidae) | 2 | |
| 3. Parasitoids of S. formosum | Amobia burnsi (Sarcophagidae) | 12 |
| Melittobia australica (Eulophidae) | 20 (type 3 + 4) | |
| 4. Parasitoids of secondary tenants | Melittobia australica (Eulophidae) | 20 (type 3 + 4) |
| Toryminae (Torymidae) | 1 | |
| Brachymeria sp. (Chalcididae) | 1 | |
| Phrudus sp. (Ichneumonidae) | 1 | |
| Gasteruption cinerescens (Gasteruptidae) | 7 | |
| Primeuchroeus faustus (Chrysididae) | 5 | |
| Primeuchroeus reversus (Chrysididae) | 2 | |
| Thraxan sp. (Bombyliidae) | 5 | |
| 5. Share space with S. formosum | Epipompilus sp. (Pompilidae) | 1 |
| 6. By-catch | Ogcodes pygmaeus (Acroceridae) | 14 |
| 7. Scavenger | Anthrenus sp. (Dermestidae) | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, D.; Beckman, J.; Florez Fernandez, J.; Rodriguez, J. Nest Ecology and Prey Preference of the Mud Dauber Wasp Sceliphron formosum (Hymenoptera: Sphecidae). Insects 2022, 13, 1136. https://doi.org/10.3390/insects13121136
Yuan D, Beckman J, Florez Fernandez J, Rodriguez J. Nest Ecology and Prey Preference of the Mud Dauber Wasp Sceliphron formosum (Hymenoptera: Sphecidae). Insects. 2022; 13(12):1136. https://doi.org/10.3390/insects13121136
Chicago/Turabian StyleYuan, David, Juliey Beckman, Jaime Florez Fernandez, and Juanita Rodriguez. 2022. "Nest Ecology and Prey Preference of the Mud Dauber Wasp Sceliphron formosum (Hymenoptera: Sphecidae)" Insects 13, no. 12: 1136. https://doi.org/10.3390/insects13121136
APA StyleYuan, D., Beckman, J., Florez Fernandez, J., & Rodriguez, J. (2022). Nest Ecology and Prey Preference of the Mud Dauber Wasp Sceliphron formosum (Hymenoptera: Sphecidae). Insects, 13(12), 1136. https://doi.org/10.3390/insects13121136






