Nutritional Compositions of Aquatic Insects Living in Rice Fields, with a Particular Focus on Odonate Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
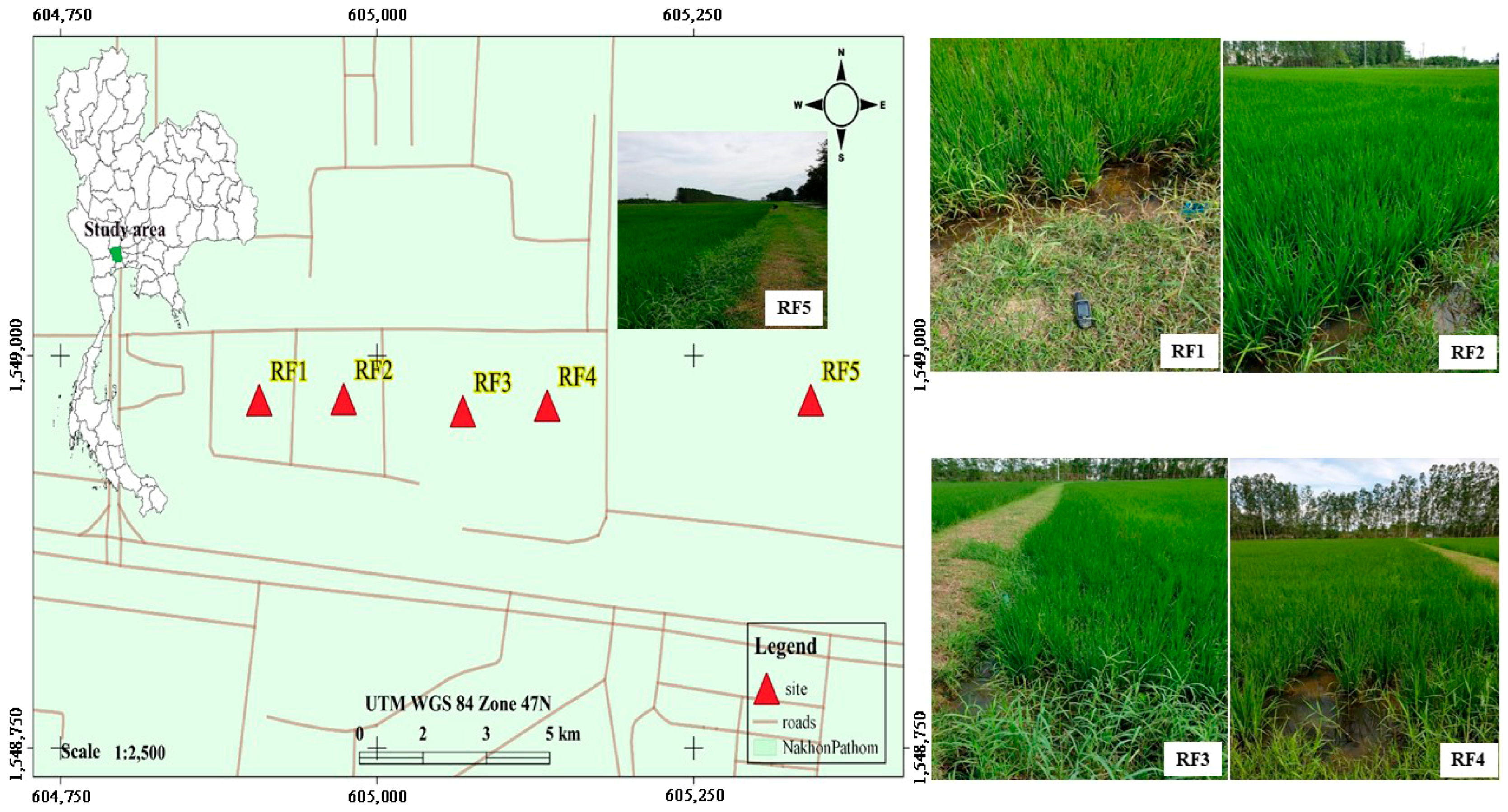
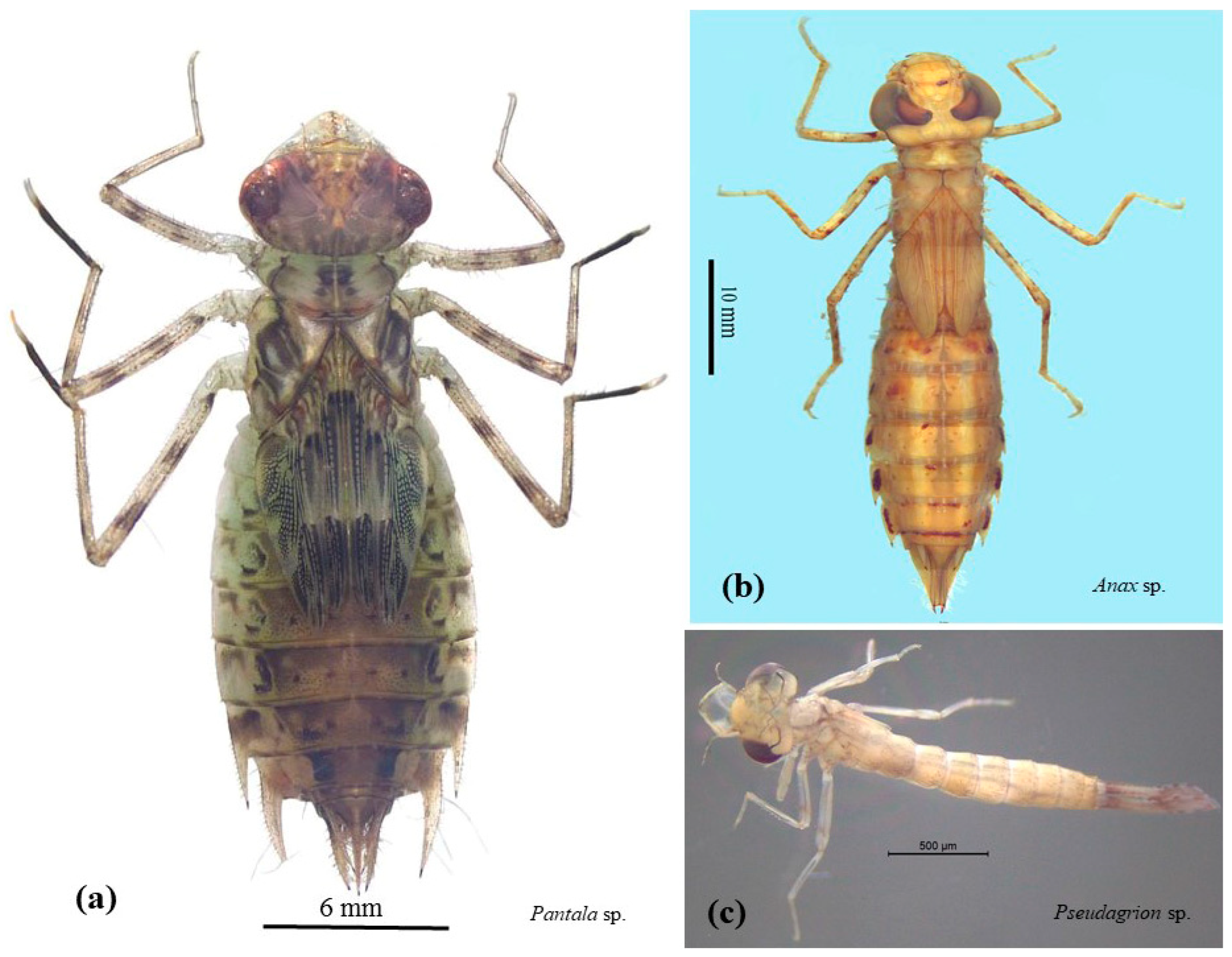
2.1. A Sampling of Aquatic Insects
2.2. Sample Preparation for Nutrition Analysis
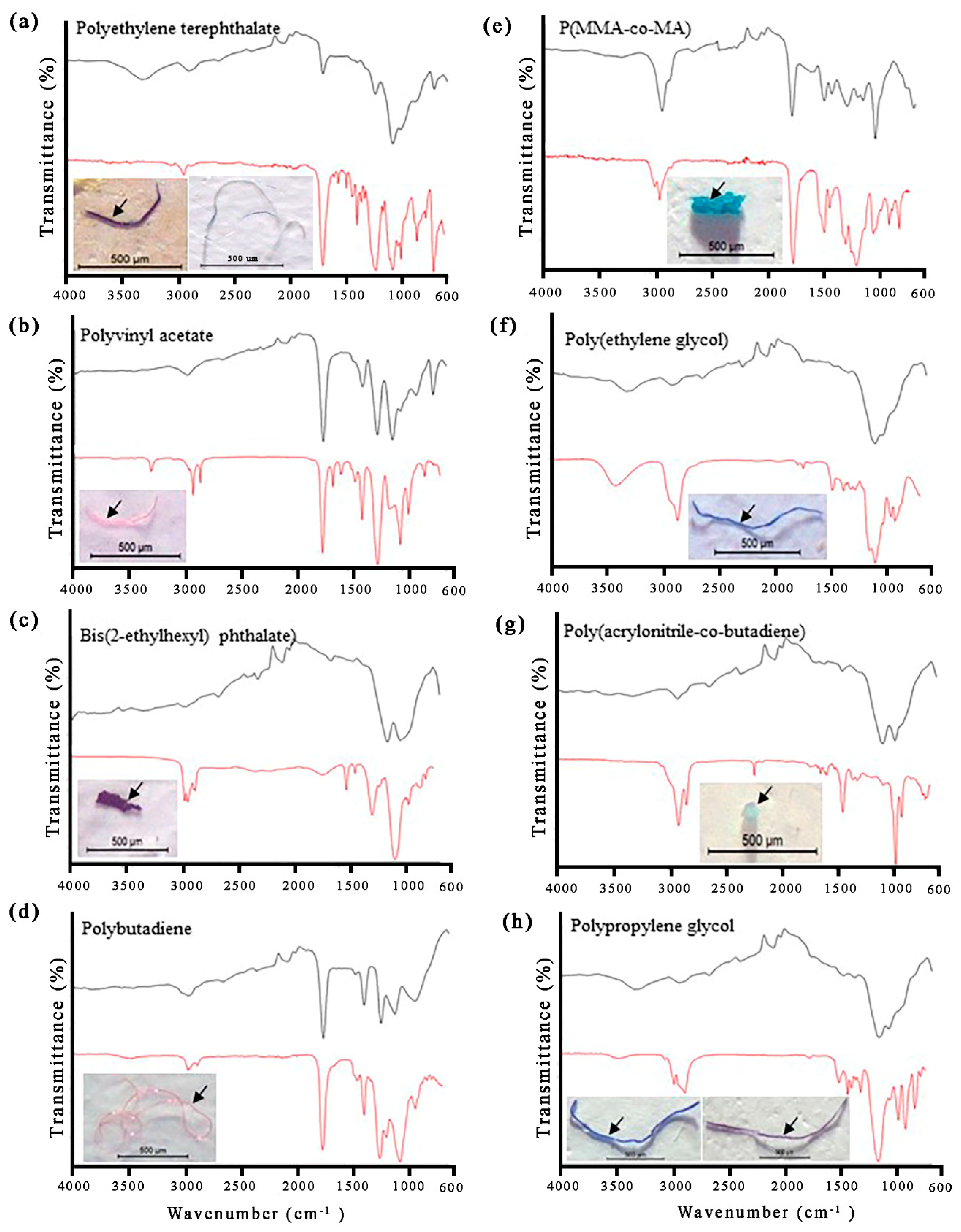
2.3. Microplastics Extraction and Identification
2.4. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Fatty Acid Composition
3.3. Amino Acids Composition
3.4. Mineral Contents and Heavy Metals of the Odonate Larvae (Pantala sp.)
3.5. Microplastic Accumulation in Odonate Larvae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN (United Nations). World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019; p. 46. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Looking at Edible Insects from a Food Safety Perspective. Challenges and Opportunities for the Sector; FAO: Rome, Italy, 2021. [Google Scholar]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef]
- Das, J.K.; Hazarika, A.K. Study on the diversity of aquatic edible insects in Baksa district of Assam with reference to Hemipteran species (Bellostomatidae and Nepidae). Clar.-Int. Multidiscip. J. 2019, 8, 15–21. [Google Scholar] [CrossRef]
- Narzari, S.; Sarmah, J. Nutritional aspects of an aquatic edible insect Sympetrum sp. (Odonata: Libellulidae) of Assam, northeast India. Int. J. Food Sci. Nutr. 2017, 2, 38–42. [Google Scholar]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.D.; Williams, S.S. Aquatic Insects and their Potential to Contribute to the Diet of the Globally Expanding Human Population. Insects 2017, 8, 72. [Google Scholar] [CrossRef]
- Hotaling, S.; Kelley, J.L.; Frandsen, P.B. Aquatic Insects Are Dramatically Underrepresented in Genomic Research. Insects 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.D.; Williams, S.S.; van Huis, A. Can we farm aquatic insects for human food or livestock feed? J. Insects Food Feed. 2021, 7, 121–127. [Google Scholar] [CrossRef]
- Macadam, C.R.; Stockan, J.A. The diversity of aquatic insects used as human food. J. Insects Food Feed. 2017, 3, 203–209. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, M.; Ding, W.F.; Chen, X.M. Overview of edible insect resources and common species utilisation in China. J. Insects Food Feed. 2020, 6, 13–25. [Google Scholar] [CrossRef]
- Tanaka, R.; Oda, M. Cyclic dipeptide of D-ornithine obtained from the dobsonfly, Protohermes grandis Thunberg. Biosci. Biotechnol. Biochem. 2009, 73, 1669–1670. [Google Scholar] [CrossRef]
- Cao, C.Q. Rearing hellgrammites for food and medicine in China. J. Insects Food Feed. 2016, 2, 263–267. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Wang, S.Y.; Ye, S.D.; Chen, Y. Three edible odonata species and their nutritive value. Lin Ye Ke Xue Yan Jiu 2001, 14, 421–424. [Google Scholar]
- Chakravorty, J.; Ghosh, S.; Meyer-Rochow, V.B. Comparative Survey of Entomophagy and Entomotherapeutic Practices in Six Tribes of Eastern Arunachal Pradesh (India). J. Ethnobiol. Ethnomed. 2013, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- DeFoliart, G. Insects as human food: Gene DeFoliart discusses some nutritional and economic aspects. Crop. Prot. 1992, 11, 395–399. [Google Scholar] [CrossRef]
- Barennes, H.; Phimmasane, M.; Rajaonarivo, C. Insect Consumption to Address Undernutrition, a National Survey on the Prevalence of Insect Consumption among Adults and Vendors in Laos. PLoS ONE 2015, 10, e0136458. [Google Scholar] [CrossRef] [PubMed]
- Hanboonsong, Y. Edible insects and associated food habits in Thailand. In Forest Insects as food: Humans Bite Back. Proceedings of the Workshop on Asia-Pacific Resources and Their Potential for Development, Chiang Mai, Thailand, 19–21 February 2008; Durst, P.B., Johnson, D.V., Leslie, R.N., Shono, K., Eds.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010; pp. 173–182. [Google Scholar]
- Xiaoming, C.; Ying, F.; Hong, Z.; Zhiyong, C. Review of the nutritive value of edible insects. In Forest Insects as Food: Humans Bite Back. Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2010; pp. 85–92. [Google Scholar]
- Wakhid, W.; Rauf, A.; Krisanti, M.; Sumertajaya, I.M.; Maryana, N. Species richness and diversity of aquatic insects inhabiting rice fields in Bogor, West Java, Indonesia. Biodiversitas J. Biol. Divers. 2019, 21, 34–42. [Google Scholar] [CrossRef]
- Kolakowski, B.M.; Johaniuk, K.; Zhang, H.; Yamamoto, E. Analysis of Microbiological and Chemical Hazards in Edible Insects Available to Canadian Consumers. J. Food Prot. 2021, 84, 1575–1581. [Google Scholar] [CrossRef]
- Aydogan, Z.; Sisman, T.; Incekara, U.; Gurol, A. Heavy metal accumulation in some aquatic insects (Coleoptera: Hydrophilidae) and tissues of Chondrostoma regium (Heckel, 1843) relevant to their concentration in water and sediments from Karasu River, Erzurum, Turkey. Environ. Sci. Pollut. Res. Int. 2017, 24, 9566–9574. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, C.Y.; Sun, L.; He, Z.; Yang, P.L.; Liao, H.J.; Feng, Y. Edible Aquatic Insects: Diversities, Nutrition, and Safety. Foods 2021, 10, 3033. [Google Scholar] [CrossRef]
- Yahya, A.N.; Mohamed, S.K.; Mohamed, A.G. Environmental Pollution by Heavy Metals in the Aquatic Ecosystems of Egypt. Open Access J. Toxicol. 2018, 3, 555603. [Google Scholar] [CrossRef]
- Zhao, G.; Ye, S.; Yuan, H.; Ding, X.; Wang, J. Surface sediment properties and heavy metal pollution assessment in the Pearl River Estuary, China. Environ. Sci. Pollut. Res. Int. 2017, 24, 2966–2979. [Google Scholar] [CrossRef] [PubMed]
- WHO. 10 Chemicals of Public Health Concern. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 5 June 2022).
- Kallenbach, E.M.F.; Rødland, E.S.; Buenaventura, N.T.; Hurley, R. Microplastics in Terrestrial and Freshwater Environments. In Microplastic in the Environment: Pattern and Process; Bennett, E.R., Ed.; Springer Nature: Berlin, Germany, 2022; pp. 87–130. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; King, A.E.; Yu, L.H. Analytical Chemistry of Plastic Debris: Sampling, Methods, and Instrumentation. In Microplastic in the Environment: Pattern and Process; Bennett, E.R., Ed.; Springer Nature: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Wang, Z.; Taylor, S.E.; Sharma, P.; Flury, M. Poor extraction efficiencies of polystyrene nano- and microplastics from biosolids and soil. PLoS ONE 2018, 13, e0208009. [Google Scholar] [CrossRef] [PubMed]
- Chaukura, N.; Kefeni, K.K.; Chikurunhe, I.; Nyambiya, I.; Gwenzi, W.; Moyo, W.; Nkambule, T.T.; Mamba, B.B.; Abulude, F.O. Microplastics in the aquatic environment—The occurrence, sources, ecological impacts, fate, and remediation challenges. Pollutants 2021, 1, 95–118. [Google Scholar] [CrossRef]
- Dudgeon, D. Tropical Asian Streams: Zoobenthos, Ecology and Conservation; Hong Kong University Press: Hong Kong, China, 1999. [Google Scholar]
- Yule, C.M.; Sen, Y.H. Freshwater Invertebrates of the Malaysian Region; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2004. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; George, W., Latimer, J., Eds.; AOAC: Rockville, MD, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; Greorge, W., Latimer, J., Eds.; AOAC: Rockville, MD, USA, 2019. [Google Scholar]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef]
- Choudhury, K.; Sarma, D.; Sapruna, P.J.; Soren, A.D. Proximate and mineral compositions of Samia cynthia ricini and Dytiscus marginalis, commonly consumed by the Bodo tribe in Assam, India. Bull. Natl. Res. Cent. 2020, 44, 152. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Plachý, V.; Božik, M.; Adámková, A.; Vrabec, V. Effect of sex on the nutritional value of house cricket, Acheta domestica L. Food Chem. 2019, 272, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Bbosa, T.; Tamale Ndagire, C.; Muzira Mukisa, I.; Fiaboe, K.K.M.; Nakimbugwe, D. Nutritional Characteristics of Selected Insects in Uganda for Use as Alternative Protein Sources in Food and Feed. J. Insect Sci. 2019, 19, 23. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Hall, W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr. Res. Rev. 2009, 22, 18–38. [Google Scholar] [CrossRef]
- Sprecher, H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochem. Biophys. Acta 2000, 1486, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Raksakantong, P.; Meeso, N.; Kubola, J.; Siriamornpun, S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Res. Int. 2010, 43, 350–355. [Google Scholar] [CrossRef]
- Longvah, T.; Mangthya, K.; Ramulu, P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403. [Google Scholar] [CrossRef]
- Amadi, E.; Kiin-Kabari, D. Nutritional composition and microbiology of some edible insects commonly eaten in Africa, hurdles and future prospects: A critical review. J. Food Microbiol. Saf. Hyg. 2016, 1, 1000107. [Google Scholar]
- Christensen, D.L.; Orech, F.O.; Mungai, M.N.; Larsen, T.; Friis, H.; Aagaard-Hansen, J. Entomophagy among the Luo of Kenya: A potential mineral source? Int. J. Food Sci. Nutr. 2006, 57, 198–203. [Google Scholar] [CrossRef]
- Das, J.K.; Hazarika, A.K. Macronutrient and mineral content of edible Coleopteran with reference to the Baksa district, India. Clar.-Int. Multidiscip. J. 2019, 8, 15–20. [Google Scholar] [CrossRef]
- Palanog, A.D.; Calayugan, M.I.C.; Descalsota-Empleo, G.I.; Amparado, A.; Inabangan-Asilo, M.A.; Arocena, E.C.; Sta Cruz, P.C.; Borromeo, T.H.; Lalusin, A.; Hernandez, J.E.; et al. Zinc and Iron Nutrition Status in the Philippines Population and Local Soils. Front. Nutr. 2019, 6, 81. [Google Scholar] [CrossRef]
- Nasirian, H.; Irvine, K.N. Odonata larvae as a bioindicator of metal contamination in aquatic environments: Application to ecologically important wetlands in Iran. Environ. Monit. Assess. 2017, 189, 436. [Google Scholar] [CrossRef]
- Simon, E.; Kis, O.; Jakab, T.; Kolozsvari, I.; Malnas, K.; Harangi, S.; Baranyai, E.; Miskolczi, M.; Tothmeresz, B.; Devai, G. Assessment of contamination based on trace element concentrations in Gomphus flavipes (Odonata: Insect) larvae of the Upper Tisza Region. Ecotoxicol. Environ. Saf. 2017, 136, 55–61. [Google Scholar] [CrossRef]
- Addo-Bediako, A.; Malakane, K. Preliminary Assessment of Chemical Elements in Sediments and Larvae of Gomphidae (Odonata) from the Blyde River of the Olifants River System, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 8135. [Google Scholar] [CrossRef]
- Cid, N.; Ibanez, C.; Palanques, A.; Prat, N. Patterns of metal bioaccumulation in two filter-feeding macroinvertebrates: Exposure distribution, inter-species differences and variability across developmental stages. Sci. Total Environ. 2010, 408, 2795–2806. [Google Scholar] [CrossRef] [PubMed]
- FAO-WHO. General Standard for Contaminants and Toxins in Food and Feed (GSCTFF). Codex Alimentarius Commission. 2015. Available online: www.fao.org/input/download/standards/17/CXS_193e_2015.pdf (accessed on 18 November 2022).
- Ministry of Public Health. Standard of Contaminated Food. Standard of Contaminated Food. Ministry of Public Health in Thailand. 2020. Available online: https://fsvps.gov.ru/sites/default/files/files/ehksport-import/tailand/umz_414.pdf (accessed on 18 November 2022).
- Choprathumma, C.; Thongkam, T.; Jaiyen, C.; Tusai, T.; Apilux, A.; Kladsomboon, S. Determination of toxic heavy metal contaminated in food crops in Nakhon Pathom province, Thailand. Khon Kaen Agric. J. 2019, 47, 83–94. [Google Scholar] [CrossRef]
- Akindele, E.O.; Ehlers, S.M.; Koop, J.H.E. Freshwater insects of different feeding guilds ingest microplastics in two Gulf of Guinea tributaries in Nigeria. Environ. Sci. Pollut. Res. Int. 2020, 27, 33373–33379. [Google Scholar] [CrossRef] [PubMed]
- Maneechan, W.; Prommi, T.O. Occurrence of microplastics in edible aquatic insect Pantala sp. (Odonata: Libellulidae) from rice fields. PeerJ 2022, 10, e12902. [Google Scholar] [CrossRef]
- Bertoli, M.; Pastorino, P.; Lesa, D.; Renzi, M.; Anselmi, S.; Prearo, M.; Pizzul, E. Microplastics accumulation in functional feeding guilds and functional habit groups of freshwater macrobenthic invertebrates: Novel insights in a riverine ecosystem. Sci. Total Environ. 2022, 804, 150207. [Google Scholar] [CrossRef]
| Parameter | Value (Means ± SD, n = 3) |
|---|---|
| Moisture | 30.86 ± 0.04 |
| Ash | 5.61 ± 0.08 |
| Fat | 5.29 ± 0.09 |
| Protein | 49.45 ± 0.32 |
| Total carbohydrate | 8.80 ± 0.29 |
| Total energy, (Kcal/100 g) | 280.55 ± 0.28 |
| Energy from fat, (Kcal/100 g) | 47.58 ± 0.82 |
| Saturated Fatty Acid (g/100 g) | |
|---|---|
| Lauric acid (C12:0) | 0.02 ± 0.00 |
| Myristic acid (C14:0) | 0.10 ± 0.01 |
| Pentadecanoic acid (C15:0) | 0.14 ± 0.00 |
| Palmitic acid (C16:0) | 1.19 ± 0.02 |
| Heptadecanoic acid (C17:0) | 0.24 ± 0.01 |
| Stearic acid (C18:0) | 0.79 ± 0.02 |
| Arachidic acid (C20:0) | 0.10 ± 0.00 |
| Behenic acid (C22:0) | 0.07 ± 0.01 |
| Total SFA | 2.65 |
| Unsaturated fatty acid (g/100 g) | |
| Palmitoleic acid (C16:1) | 0.27 ± 0.01 |
| cis-10-Heptadecenoic acid (C17:1n10) | 0.14 ± 0.01 |
| cis-9-Oleic acid (C18:1n9c) | 0.63 ± 0.02 |
| Total MUFA | 1.04 |
| Linoleic acid (C18:2n6c) | 0.55 ± 0.01 |
| gamma-Linolenic acid (C18:3n6) | 0.04 ± 0.00 |
| alpha-Linolenic acid (C18:3n3) | 0.33 ± 0.01 |
| cis-11,14-Eicosadienoic acid (C20:2) | 0.02 ± 0.00 |
| cis-8,11,14-Eicosatrienoic acid (C20:3n6) | 0.02 ± 0.01 |
| Arachidonic acid (C20:4n6) | 0.30 ± 0.01 |
| Eicosapentaenoic acid (C20:5n3) | 0.16 ± 0.01 |
| Total PUFA | 1.42 |
| Non-Essential AA (g/100 g) | |
|---|---|
| Aspartic acid | 2.92 ± 0.03 |
| Glutamic acid | 4.92 ± 0.07 |
| Serine | 1.68 ± 0.01 |
| Glycine | 1.89 ± 0.02 |
| Alanine | 3.21 ± 0.03 |
| Proline | 5.11 ± 0.50 |
| Arginine | 5.46 ± 0.05 |
| Tyrosine | 2.30 ± 0.01 |
| Cystine | 1.25 ± 0.01 |
| Essential AA (g/100 g) | |
| Threonine | 1.76 ± 0.01 |
| Histidine | 1.23 ± 0.01 |
| Valine | 2.73 ± 0.03 |
| Methionine | 0.85 ± 0.02 |
| Isoleucine | 1.63 ± 0.02 |
| Phenylalanine | 1.33 ± 0.02 |
| Tryptophan | 0.21 ± 0.00 |
| Leucine | 2.49 ± 0.03 |
| Lysine | 2.24 ± 0.03 |
| Total amino acids | 43.21 |
| Total essential amino acids | 14.47 |
| Mineral Content | Concentration |
|---|---|
| Sodium | 654.30 ± 5.82 |
| Calcium | 284.64 ± 11.92 |
| Iron | 86.74 ± 4.29 |
| Potassium | 803.74 ± 6.12 |
| Magnesium | 87.59 ± 1.29 |
| Phosphorus | 615.76 ± 13.90 |
| Zinc | 6.18 ± 0.04 |
| Copper | 1.48 ± 0.01 |
| Lead (mg/kg) | 0.18 ± 0.01 |
| Cadmium (mg/kg) | 0.17 ± 0.00 |
| Arsenic (mg/kg) | 3.51 ± 0.12 |
| Sites | Odonate Larvae | No. * | Total MP Items | Shape | Size (µm) | MP Items/Individual | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiber | Fragment | Rod | <100 | 200–250 | 250–500 | >500 | |||||
| RF1 | Coenagrionidae | 95 | 15 | 14 | 1 | 3 | 3 | 1 | 8 | 0.16 | |
| Libellulidae | 49 | 30 | 24 | 6 | 21 | 3 | 4 | 2 | 0.61 | ||
| RF2 | Coenagrionidae | 113 | 23 | 23 | 15 | 4 | 3 | 1 | 0.20 | ||
| Libellulidae | 48 | 44 | 36 | 8 | 21 | 11 | 6 | 6 | 0.92 | ||
| RF3 | Coenagrionidae | 53 | 12 | 11 | 1 | 2 | 4 | 2 | 4 | 0.23 | |
| Libellulidae | 44 | 22 | 7 | 13 | 2 | 15 | 3 | 2 | 2 | 0.50 | |
| RF4 | Coenagrionidae | 7 | 33 | 24 | 9 | 19 | 6 | 4 | 4 | 4.71 | |
| Libellulidae | 1 | 17 | 16 | 1 | 6 | 5 | 4 | 2 | 17.00 | ||
| RF5 | Libellulidae | 8 | 11 | 10 | 1 | 5 | 4 | 2 | 1.38 | ||
| Aeshnidae | 1 | 12 | 12 | 4 | 3 | 3 | 2 | 12.00 | |||
| Total (%) | 219 (100) | 177 (80.82) | 40 (18.26) | 2 (0.91) | 111 (50.68) | 46 (21.00) | 31 (14.16) | 31 (14.16) | |||
| Sites | Odonate Larvae | Color | Total MPs | |||||
|---|---|---|---|---|---|---|---|---|
| Blue | Violet | Pink | Transparent | Yellow | Red | |||
| RF1 | Coenagrionidae | 3 | 3 | 8 | 1 | 15 | ||
| Libellulidae | 1 | 2 | 6 | 21 | 30 | |||
| RF2 | Coenagrionidae | 3 | 20 | 23 | ||||
| Libellulidae | 1 | 13 | 3 | 27 | 44 | |||
| RF3 | Coenagrionidae | 1 | 6 | 1 | 4 | 12 | ||
| Libellulidae | 2 | 14 | 1 | 5 | 22 | |||
| RF4 | Coenagrionidae | 8 | 3 | 22 | 33 | |||
| Libellulidae | 3 | 3 | 1 | 10 | 17 | |||
| RF5 | Libellulidae | 1 | 9 | 1 | 11 | |||
| Aeshnidae | 2 | 1 | 9 | 12 | ||||
| Total (%) | 14 (6.39) | 50 (22.83) | 18 (8.22) | 135 (61.64) | 1 (0.46) | 1 (0.46) | 219 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maneechan, W.; Vitheepradit, A.; Prommi, T.O. Nutritional Compositions of Aquatic Insects Living in Rice Fields, with a Particular Focus on Odonate Larvae. Insects 2022, 13, 1131. https://doi.org/10.3390/insects13121131
Maneechan W, Vitheepradit A, Prommi TO. Nutritional Compositions of Aquatic Insects Living in Rice Fields, with a Particular Focus on Odonate Larvae. Insects. 2022; 13(12):1131. https://doi.org/10.3390/insects13121131
Chicago/Turabian StyleManeechan, Witwisitpong, Akekawat Vitheepradit, and Taeng On Prommi. 2022. "Nutritional Compositions of Aquatic Insects Living in Rice Fields, with a Particular Focus on Odonate Larvae" Insects 13, no. 12: 1131. https://doi.org/10.3390/insects13121131
APA StyleManeechan, W., Vitheepradit, A., & Prommi, T. O. (2022). Nutritional Compositions of Aquatic Insects Living in Rice Fields, with a Particular Focus on Odonate Larvae. Insects, 13(12), 1131. https://doi.org/10.3390/insects13121131







