When the Beetles Hit the Fan: The Fan-Trap, an Inexpensive, Light and Scalable Insect Trap under a Creative Commons License, for Monitoring and Experimental Use
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Present State of the Art: Available Commercial Traps
1.2. A Cheap and Versatile Alternative: Bottle-Traps
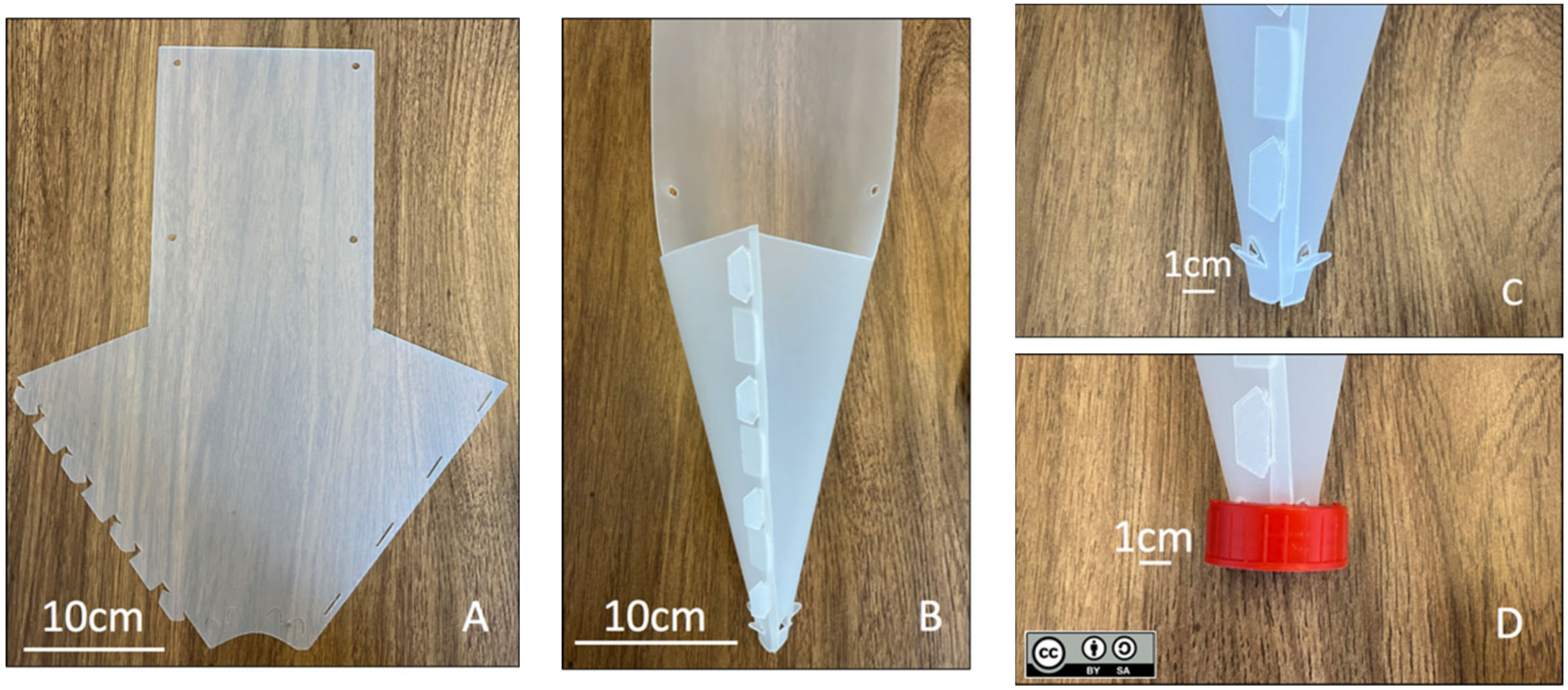
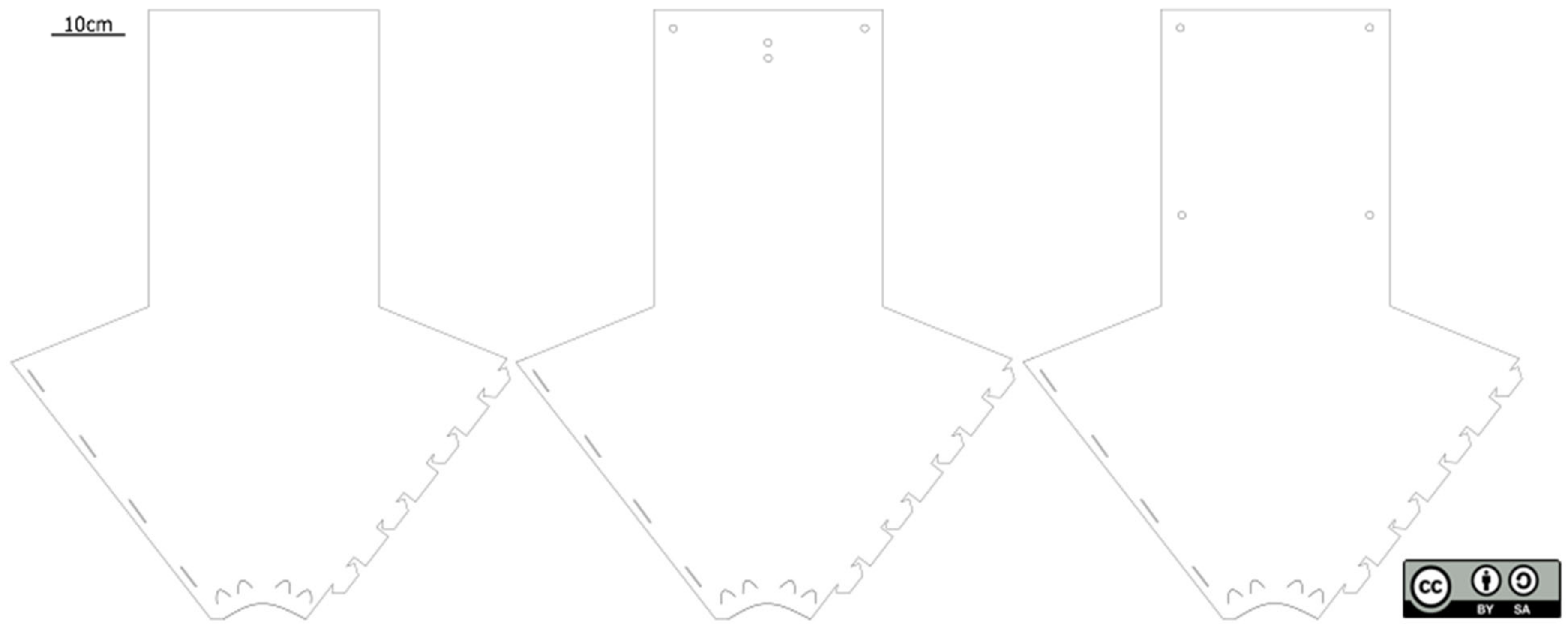
2. The Fan-Trap—A Description
3. Experimental Support
3.1. Material and Methods
3.1.1. Comparisons with Bottle-Traps
3.1.2. Comparisons with Bottle Traps and Commercial Trap Models
3.2. Results
Comparisons with Bottle-Traps
4. Discussion
- For monitoring potentially incoming non-native species, one needs to reach a high probability (sometimes prescribed by the national regulatory agencies, e.g., [26,27,28,29]) to detect an unwanted organism above a certain level of prevalence. As discussed above, an optimal trap size should be adopted in this respect. Another limiting factor here is the attraction radius of the lure in the trap, i.e., the radius around the trap within which randomly flying beetles can detect components of the chosen lure. For many beetles, these attraction radii are comparatively short. Byers [30] calculated a radius of 1.4 to 16 m for I. typographus, but Franklin and Grégoire [16] provide a slightly larger estimate (at least 50 m). From a network of Multifunnel traps baited with frontalin and turpentine, Turchin and Odendaal [31] calculated that a trap attraction range for Dendroctonus frontalis is about 0.1 ha. Jactel et al. [32] calculated a 92–123 m radius for Monochamus galloprovincialis, whilst Torres-Vila et al. [33] reported a 50 m radius for the same species. These rather short distances mean that, for maximum monitoring accuracy, traps should be deployed at densities high enough to avoid gaps between their respective areas of attractiveness. For example, D. frontalis should be monitored with traps positioned 20 m from each other. This would be in favor of deploying many cheaper but perhaps less efficient traps.
- For measuring population trends in well-established species, the trapping results are compared to each other, e.g., against time to measure phenology or density changes, or against space to measure population expansion. The trapping data in this case are relative (one trap against the others) and even small catches could be compared to each other.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nahrung, H.F.; Liebhold, A.M.; Brockerhoff, E.G.; Rassati, D. Forest Insect Biosecurity: Processes, Patterns, Predictions, Pitfalls. Annu. Rev. Entomol. 2022, 68, 1. [Google Scholar] [CrossRef]
- Rassati, D.; Petrucco Toffolo, E.; Roques, A.; Battisti, A.; Faccoli, M. Trapping wood boring beetles in Italian ports: A pilot study. J. Pest Sci. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Rassati, D.; Faccoli, M.; Marini, L.; Haack, R.A.; Battisti, A.; Petrucco Toffolo, E. Exploring the role of wood waste landfills in early detection of non-native wood-boring beetles. J. Pest Sci. 2015, 88, 563–572. [Google Scholar] [CrossRef]
- Fan, J.T.; Denux, O.; Courtin, C.; Bernard, A.; Javal, M.; Millar, J.G.; Hanks, L.M.; Roques, A. Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J. Pest Sci. 2019, 92, 281–297. [Google Scholar] [CrossRef]
- Rabaglia, R.J.; Cognato, A.I.; Hoebeke, E.R.; Johnson, C.W.; LaBonte, J.R.; Carter, M.E.; Vlach, J.J. Early Detection and Rapid Response: A 10-Year Summary of the USDA Forest Service Program of Surveillance for Non-Native Bark and Ambrosia Beetles. Am. Entomol. 2019, 65, 29–42. [Google Scholar] [CrossRef]
- Hoch, G.; Connell, J.; Roques, A. Testing multi-lure traps for surveillance of native and alien longhorn beetles (Coleoptera, Cerambycidae) at ports of entry and in forests in Austria. Manag. Biol. Invasions 2020, 11, 677–688. [Google Scholar] [CrossRef]
- Pawson, S.M.; Kerr, J.L.; Somchit, C.; Wardhaugh, C.W. Flight activity of wood-and bark-boring insects at New Zealand ports. N. Z. J. For. Sci. 2020, 50, 14. [Google Scholar] [CrossRef]
- Thurston, G.S.; Slater, A.; Nei, I.; Roberts, J.; Hamilton, K.M.; Sweeney, J.D.; Kimoto, T. New Canadian and Provincial Records of Coleoptera Resulting from Annual Canadian Food Inspection Agency Surveillance for Detection of Non-Native, Potentially Invasive Forest Insects. Insects 2022, 13, 708. [Google Scholar] [CrossRef]
- Galko, J.; Nikolov, C.; Kunca, A.; Vakula, J.; Gubka, A.; Zúbrik, M.; Rell, S.; Konôpka, B. Effectiveness of pheromone traps for the European spruce bark beetle: A comparative study of four commercial products and two new models. For. J. 2016, 62, 207–215. [Google Scholar] [CrossRef]
- Šramel, N.; Kavčič, A.; Kolšek, M.; de Groot, M. A cost-benefit analysis of different traps for monitoring European spruce bark beetle (Ips typographus). Austrian J. For. Sci. 2022, 139, 137–168. [Google Scholar]
- Rieske, L.K.; Raffa, K.F. Use of ethanol-and-turpentine-baited flight traps to monitor Pissodes weevils (Coleoptera: Curculionidae) in Christmas tree plantations. Gt. Lakes Entomol. 1993, 26, 155–160. Available online: https://scholar.valpo.edu/tgle/vol26/iss2/8 (accessed on 12 August 2022).
- Steininger, M.S.; Hulcr, J.; Šigut, M.; Lucky, A. Simple and efficient trap for bark and ambrosia beetles (Coleoptera: Curculionidae) to facilitate invasive species monitoring et citizen involvement. J. Econ. Entomol. 2015, 108, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Reding, M.E.; Schultz, P.B.; Ranger, C.M.; Oliver, J.B. Optimizing ethanol-baited traps for monitoring damaging ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in ornamental nurseries. J. Econ. Entomol. 2011, 104, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Olenici, N.; Duduman, M.L.; Popa, I.; Isaia, G.; Paraschiv, M. Geographical Distribution of Three Forest Invasive Beetle Species in Romania. Insects 2022, 13, 621. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.; Debruyne, C.; Grégoire, J.-C. Recapture of Ips typographus (Col., Scolytidae) with attractants of low release rates: Localized dispersion and environmental influences. Agric. For. Entomol. 2000, 2, 259–270. [Google Scholar] [CrossRef]
- Franklin, A.; Grégoire, J.-C. Dose-dependent response and preliminary attraction range of Ips typographus (Col., Scolytidae) to pheromones of low release rates. J. Chem. Ecol. 2001, 27, 2425–2435. [Google Scholar] [CrossRef]
- Grégoire, J.-C.; Piel, F.; De Proft, M.; Gilbert, M. Spatial distribution of ambrosia-beetle catches: A possibly useful knowledge to improve mass-trapping. Integr. Pest Manag. Rev. 2001, 6, 237–242. [Google Scholar] [CrossRef]
- Meurisse, N.; Couillien, D.; Grégoire, J.-C. Kairomones traps: A tool for monitoring the invasive spruce bark beetle, Dendroctonus micans (Coleoptera: Scolytinae) and its specific predator, Rhizophagus grandis (Coleoptera: Monotomidae). J. Appl. Ecol. 2008, 45, 537–548. [Google Scholar] [CrossRef]
- Piel, F.; Gilbert, M.; Franklin, A.; Grégoire, J.-C. Occurrence of Ips typographus (Col., Scolytidae) along an urbanization gradient in Brussels, Belgium. Agric. For. Entomol. 2005, 7, 161–167. [Google Scholar] [CrossRef]
- Piel, F.; Grégoire, J.-C.; Knížek, M. New occurrence of Ips duplicatus Sahlberg in Herstal Liege, Belgium. Bull. OEPP 2006, 36, 529–530. [Google Scholar] [CrossRef]
- Warzée, N.; Gilbert, M.; Grégoire, J.-C. Predator/prey ratios: A measure of bark-beetle population status influenced by stand composition in different French stands after the 1999 storms. Ann. For. Sci. 2006, 63, 301–308. [Google Scholar] [CrossRef][Green Version]
- OWSF. Observatoire Wallon de la Santé des Forêts. Available online: http://owsf.environnement.wallonie.be/fr/ips-typographe.html?IDC=5773 (accessed on 12 August 2022).
- Leather, S. Entomological Classics—The Window (Pane) Flight Intercept Trap. Don’t Forget the Roundabouts. 2015. Available online: https://simonleather.wordpress.com/2015/11/04/entomological-classics-the-window-pane-flight-intercept-trap/ (accessed on 17 January 2022).
- Gahleitner, M.; Paulik, C. Polypropylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Hoboken, NJ, USA, 2014; pp. 1–44. [Google Scholar] [CrossRef]
- Byers, J.A.; Anderbrant, O.; Löqvist, J. Effective attraction radius. J. Chem. Ecol. 1989, 15, 749–765. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). ISPM (International Standards for Phytosanitary Measures) 31. Methodologies for Sampling of Consignments. FAO, 2016a. Available online: https://www.ippc.int/en/publications/588/ (accessed on 12 August 2022).
- FAO (Food and Agriculture Organization of the United Nations). Plant Pest Surveillance: A Guide to Understand the Principal Requirements of Surveillance Programmes for National Plant Protection Organizations, Version 1.1; FAO: Rome, Italy, 2016. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). ISPM (International Standards for Phytosanitary Measures) 8. Determination of Pest Status in an Area. FAO. 2017. Available online: https://www.ippc.int/en/publications/612/ (accessed on 12 August 2022).
- FAO (Food and Agriculture Organization of the United Nations). ISPM (International Standards for Phytosanitary Measures) 6. Surveillance. FAO. 2018. Available online: https://www.ippc.int/en/publications/615 (accessed on 12 August 2022).
- Byers, J.A. Effects of attraction radius and flight paths on catch of scolytid beetles dispersing outward through rings of pheromone traps. J. Chem. Ecol. 1999, 25, 985–1005. [Google Scholar] [CrossRef]
- Turchin, P.; Odendaal, F.J. Measuring the effective sampling area of a pheromone trap for monitoring population density of southern pine beetle (Col., Scolytidae). Environ. Entomol. 1996, 25, 582–588. [Google Scholar] [CrossRef]
- Jactel, H.; Bonifacio, L.; Van Halder, I.; Vétillard, F.; Robinet, C.; David, G. A novel, easy method for estimating pheromone trap attraction range: Application to the pine sawyer beetle Monochamus galloprovincialis. Agric. For. Entomol. 2019, 21, 8–14. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Zugasti, C.; De-Juan, J.M.; Oliva, M.J.; Montero, C.; Mendiola, F.J.; Conejo, Y.; Sanchez, A.; Fernandez, F.; Poncer, F.; et al. Mark-recapture of Monochamus galloprovincialis with semiochemical-baited traps: Population density, attraction distance, flight behaviour and mass trapping efficiency. For. Int. J. For. Res. 2015, 88, 224–236. [Google Scholar] [CrossRef]
- Cavaletto, G.; Faccoli, M.; Marini, L.; Spaethe, J.; Magnani, G.; Rassati, D. Effect of trap color on captures of bark-and wood-boring beetles (Coleoptera; Buprestidae and Scolytinae) and associated predators. Insects 2020, 11, 749. [Google Scholar] [CrossRef]
- Cavaletto, G.; Faccoli, M.; Marini, L.; Spaethe, J.; Giannone, F.; Moino, S.; Rassati, D. Exploiting trap color to improve surveys of longhorn beetles. J. Pest Sci. 2021, 94, 871–883. [Google Scholar] [CrossRef]
| Species | Trap | N | Mean | SEM |
|---|---|---|---|---|
| Ips typographus | Intercept | 10 | 2106.70 | 327.35 |
| Theysohn | 10 | 1965.70 | 276.46 | |
| Bottle-traps | 10 | 252.30 | 34.08 | |
| Fan-traps | 10 | 229.80 | 40.77 | |
| Trypodendron domesticum | Intercept | 10 | 30.60 | 5.33 |
| Theysohn | 10 | 22.70 | 4.38 | |
| Bottle-traps | 10 | 7.10 | 1.21 | |
| Fan-traps | 10 | 6.40 | 1.07 | |
| T. signatum | Intercept | 10 | 292.90 | 72.88 |
| Theysohn | 10 | 166.90 | 18.51 | |
| Fan-traps | 10 | 115.20 | 10.83 | |
| Bottle-traps | 10 | 100.50 | 8.75 | |
| T. lineatum | Intercept | 10 | 111.70 | 28.09 |
| Theysohn | 10 | 50.10 | 4.39 | |
| Fan-traps | 10 | 35.20 | 3.97 | |
| Bottle-traps | 10 | 32.30 | 4.55 | |
| Anisandrus dispar | Theysohn | 10 | 77.20 | 19.64 |
| Fan-traps | 10 | 59.80 | 16.60 | |
| Intercept | 10 | 45.00 | 7.54 | |
| Bottle-traps | 10 | 39.50 | 12.10 |
| Species | Trap | N | Mean | SEM |
|---|---|---|---|---|
| Ips typographus/cm2 | Bottle-traps | 10 | 0.781 | 0.106 |
| Fan-traps | 10 | 0.766 | 0.136 | |
| Theysohn | 10 | 0.328 | 0.046 | |
| Intercept | 10 | 0.251 | 0.039 | |
| Trypodendron domesticum/cm2 | Bottle-traps | 10 | 0.022 | 0.004 |
| Fan-traps | 10 | 0.021 | 0.004 | |
| Theysohn | 10 | 0.004 | 0.001 | |
| Intercept | 10 | 0.004 | 0.001 | |
| T. signatum/cm2 | Fan-traps | 10 | 0.384 | 0.036 |
| Bottle-traps | 10 | 0.311 | 0.027 | |
| Intercept | 10 | 0.035 | 0.009 | |
| Theysohn | 10 | 0.028 | 0.003 | |
| T. lineatum/cm2 | Fan-traps | 10 | 0.117 | 0.013 |
| Bottle-traps | 10 | 0.100 | 0.014 | |
| Intercept | 10 | 0.013 | 0.003 | |
| Theysohn | 10 | 0.008 | 0.001 | |
| Anisandrus dispar/cm2 | Bottle-traps | 10 | 0.122 | 0.037 |
| Fan-traps | 10 | 0.199 | 0.055 | |
| Theysohn | 10 | 0.013 | 0.003 | |
| Intercept | 10 | 0.005 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grégoire, J.-C.; Caiti, E.; Hasbroucq, S.; Molenberg, J.-M.; Willenz, S. When the Beetles Hit the Fan: The Fan-Trap, an Inexpensive, Light and Scalable Insect Trap under a Creative Commons License, for Monitoring and Experimental Use. Insects 2022, 13, 1122. https://doi.org/10.3390/insects13121122
Grégoire J-C, Caiti E, Hasbroucq S, Molenberg J-M, Willenz S. When the Beetles Hit the Fan: The Fan-Trap, an Inexpensive, Light and Scalable Insect Trap under a Creative Commons License, for Monitoring and Experimental Use. Insects. 2022; 13(12):1122. https://doi.org/10.3390/insects13121122
Chicago/Turabian StyleGrégoire, Jean-Claude, Emilio Caiti, Séverine Hasbroucq, Jean-Marc Molenberg, and Sylvain Willenz. 2022. "When the Beetles Hit the Fan: The Fan-Trap, an Inexpensive, Light and Scalable Insect Trap under a Creative Commons License, for Monitoring and Experimental Use" Insects 13, no. 12: 1122. https://doi.org/10.3390/insects13121122
APA StyleGrégoire, J.-C., Caiti, E., Hasbroucq, S., Molenberg, J.-M., & Willenz, S. (2022). When the Beetles Hit the Fan: The Fan-Trap, an Inexpensive, Light and Scalable Insect Trap under a Creative Commons License, for Monitoring and Experimental Use. Insects, 13(12), 1122. https://doi.org/10.3390/insects13121122






