Prevalence and Distribution of Three Bumblebee Pathogens from the Czech Republic
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
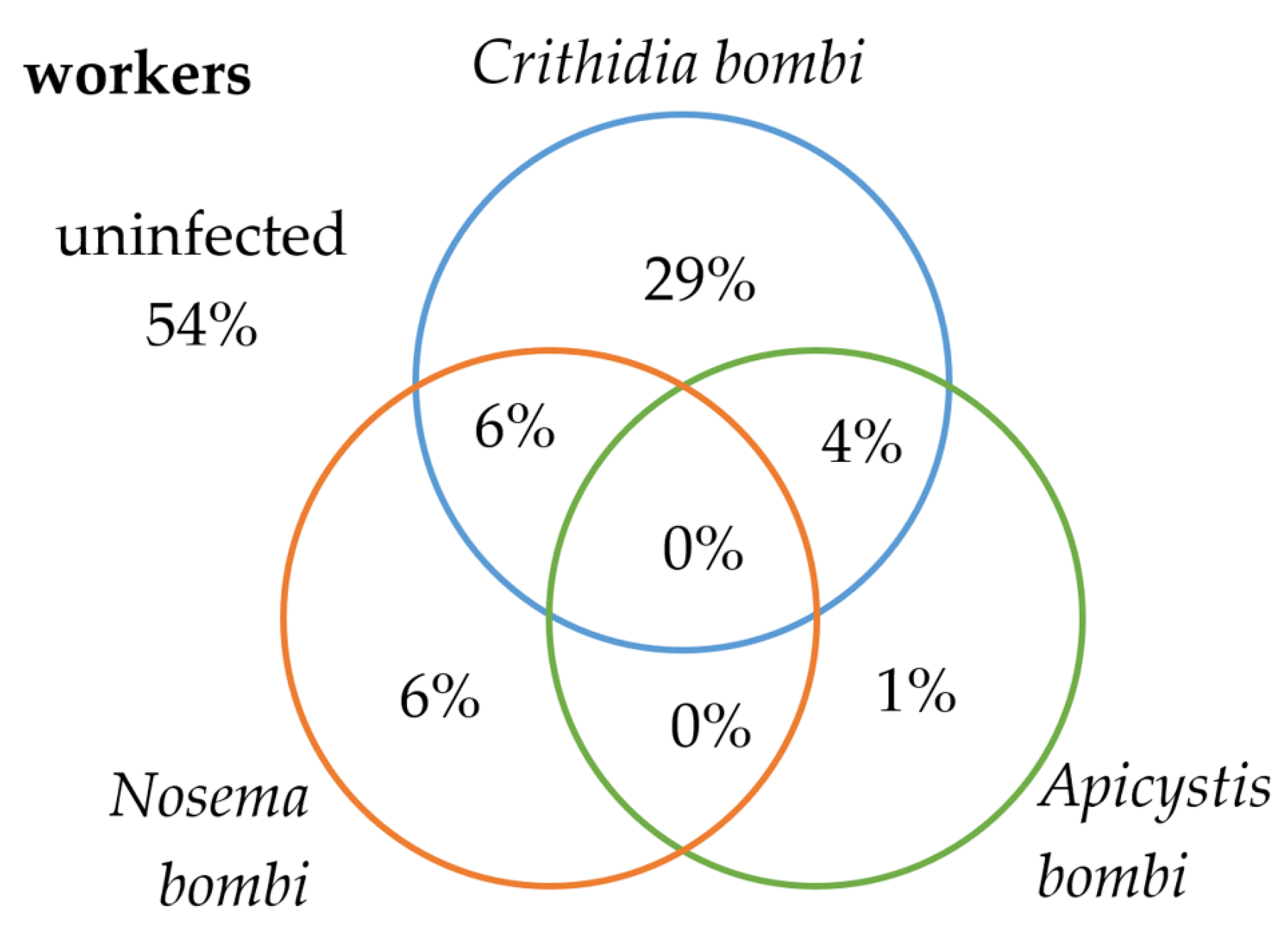
3. Results
3.1. Bombus terrestris/lucorum
3.2. Bombus lapidarius
3.3. Comparison of B. terrestris/lucorum and B. lapidarius
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Steele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Velthuis, H.H.W.; van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; Criado, M.G.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014; pp. 1–84. [Google Scholar]
- Williams, P.H.; Osborne, J.L. Bumblebee vulnerability and conservation world-wide. Apidologie 2009, 40, 367–387. [Google Scholar] [CrossRef]
- Goulson, D.; Whitehorn, P.; Fowley, M. Influence of urbanisation on the prevalence of protozoan parasites of bumblebees. Ecol. Entomol. 2012, 37, 83–89. [Google Scholar] [CrossRef]
- Cameron, S.A.; Sadd, B.M. Global trends in bumble bee health. Annu. Rev. Entomol. 2020, 65, 209–232. [Google Scholar] [CrossRef]
- Schmid-Hempel, P.; Schmid-Hempel, R. Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav. Ecol. Sociobiol. 1993, 33, 319–327. [Google Scholar] [CrossRef]
- Otterstatter, M.C.; Thomson, J.D. Contact networks and transmission of an intestinal pathogen in bumble bee (Bombus impatiens) colonies. Oecologia 2007, 154, 411–421. [Google Scholar] [CrossRef]
- Durrer, S.; Schmid-Hempel, P. Shared use of flowers leads to horizontal pathogen transmission. Proc. Biol. Sci. 1994, 258, 299–302. [Google Scholar]
- Gegear, R.J.; Otterstatter, M.C.; Thomson, J.D. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. R. Soc. B. 2006, 273, 1073–1078. [Google Scholar] [CrossRef]
- Fauser, A.; Sandrock, C.; Neumann, P.; Sadd, B. Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator. Ecol. Entomol. 2017, 42, 306–314. [Google Scholar] [CrossRef]
- Brown, M.J.F.; Schmid-Hempel, R.; Schmid-Hempel, P. Strong context-dependent virulence in a host-parasite system: Reconciling genetic evidence with theory. J. Anim. Ecol. 2003, 72, 994–1002. [Google Scholar] [CrossRef]
- Rutrecht, S.T.; Brown, M.J.F. Within colony dynamics of Nosema bombi infections: Disease establishment, epidemiology and potential vertical transmission. Apidologie 2008, 39, 504–514. [Google Scholar] [CrossRef]
- Otti, O.; Schmid-Hempel, P. Nosema bombi: A pollinator parasite with detrimental fitness effects. J. Invertebr. Pathol. 2007, 96, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Otti, O.; Schmid-Hempel, P. A field experiment on the effect of Nosema bombi in colonies of the bumblebee Bombus terrestris. Ecol. Entomol. 2008, 33, 577–582. [Google Scholar] [CrossRef]
- Van Der Steen, J.J. Infection and transmission of Nosema bombi in Bombus terrestris colonies and its effect on hibernation, mating and colony founding. Apidologie 2008, 39, 273–282. [Google Scholar] [CrossRef]
- Manlik, O.; Schmid-Hempel, R.; Schmid-Hempel, P. Parasite infection of specific host genotypes relates to changes in prevalence in two natural populations of bumblebees. Infect. Genet. Evol. 2017, 56, 125–132. [Google Scholar] [CrossRef]
- McNeil, D.J.; McCormick, E.; Heimann, A.C.; Kammerer, M.; Douglas, M.R.; Goslee, S.C.; Grozinger, C.M.; Hines, H.M. Bumble bees in landscapes with abundant floral resources have lower pathogen loads. Sci. Rep. 2020, 10, 22306. [Google Scholar] [CrossRef]
- Manlik, O.; Mundra, S.; Schmid-Hempel, R.; Schmid-Hempel, P. Impact of climate change on parasite infection of an important pollinator depends on host genotypes. Glob. Chang. Biol. 2022, in press. [Google Scholar] [CrossRef]
- Brown, M.J.F. Microsporidia: An emerging threat to bumblebees? Trends Parasitol. 2017, 33, 754–762. [Google Scholar] [CrossRef]
- Colla, S.R.; Otterstatter, M.C.; Gegear, R.J.; Thomson, J.D. Plight of the bumble bee: Pathogen spillover from commercial to wild populations. Biol. Conserv. 2006, 129, 461–467. [Google Scholar] [CrossRef]
- Jones, C.M.; Brown, M.J.F.; Ings, T. Parasites and genetic diversity in an invasive bumblebee. J. Anim. Ecol. 2014, 83, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Graystock, P.; Meeus, I.; Smagghe, G.; Goulson, D.; Hughes, W.O.H. The effects of single and mixed infections of Apicystis bombi and deformed wing virus in Bombus terrestris. Parasitology 2015, 143, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Arese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Aizen, M.A.; Smith-Ramírez, C.; Morales, C.L.; Vieli, L.; Sáez, A.; Barahona-Segovia, R.M.; Arbetman, M.P.; Montalva, J.; Garibaldi, L.A.; Inouye, D.W.; et al. Coordinated species importation policies are needed to reduce serious invasions globally: The case of alien bumblebees in South America. J. Appl. Ecol. 2019, 56, 100–106. [Google Scholar] [CrossRef]
- Shykoff, J.A.; Schmid-Hempel, P. Incidence and effects of four parasites in natural populations of bumble bees in Switzerland. Apidologie 1991, 22, 117–125. [Google Scholar] [CrossRef]
- Popp, M.; Erler, S.; Lattorff, H.M. Seasonal variability of prevalence and occurrence of multiple infections shape the population structure of Crithidia bombi, an intestinal parasite of bumblebees (Bombus spp.). MicrobiologyOpen 2012, 4, 362–372. [Google Scholar] [CrossRef]
- Graystock, P.; Goulson, D.; Hughes, W.O. The relationship between managed bees and the prevalence of parasites in bumblebees. PeerJ 2014, 2, e522. [Google Scholar] [CrossRef]
- Gallot-Lavallée, M.; Schmid-Hempel, R.; Vandame, R.; Vergara, C.H.; Schmid-Hempel, P. Large scale patterns of abundance and distribution of parasites in Mexican bumblebees. J. Invertebr. Pathol. 2016, 133, 73–82. [Google Scholar] [CrossRef]
- Jabal-Uriel, C.; Martín-Hernández, R.; Ornosa, C.; Higes, M.; Berriatua, E.; de la Rua, P. Short communication: First data on the prevalence and distribution of pathogens in bumblebees (Bombus terrestris and Bombus pascuorum) from Spain. Span. J. Agric. Res. 2017, 15, e05SC01. [Google Scholar] [CrossRef]
- Parsche, S.; Lattorff, M.H.G. The relative contributions of host density and genetic diversity on prevalence of a multi-host parasite in bumblebees. Biol. J. Linn. Soc. 2018, 125, 900–910. [Google Scholar] [CrossRef]
- Ocepek, M.P.; Toplak, I.; Zajc, U.; Bevk, D. The pathogens spillover and incidence correlation in bumblebees and honeybees in Slovenia. Pathogens 2021, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, R.; Tognazzo, M. Molecular divergence defines two distinct lineages of Crithidia bombi (Trypanosomatidae), parasites of bumblebees. J. Eukaryot. Microbiol. 2010, 57, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Meeus, I.; de Graaf, D.C.; Jans, K.; Smagghe, G. Multiplex PCR detection of slowly-evolving trypanosomatids and neogregarines in bumblebees using broad-range primers. J. Appl. Microbiol. 2010, 109, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Klee, J.; Tay, W.T.; Paxton, R.J. Specific and sensitive detection of Nosema bombi (Microsporidia: Nosematidae) in bumble bees (Bombus spp.; Hymenoptera: Apidae) by PCR of partial rRNA gene sequences. J. Invertebr. Pathol. 2006, 91, 98–104. [Google Scholar] [CrossRef]
- ArcGIS Pro. Available online: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview (accessed on 8 July 2022).
- Copernicus. Available online: https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 (accessed on 24 August 2022).
- Gillespie, S. Factors affecting parasite prevalence among wild bumblebees. Ecol. Entomol. 2010, 35, 737–747. [Google Scholar] [CrossRef]
- Huth-Schwarz, A.; Settele, J.; Moritz, R.F.; Kraus, F.B. Factors influencing Nosema bombi infections in natural populations of Bombus terrestris (Hymenoptera: Apidae). J. Invertebr. Pathol. 2012, 110, 48–53. [Google Scholar] [CrossRef]
- Alizon, S.; de Roode, J.C.; Michalakis, Y. Multiple infections and the evolution of virulence. Ecol. Lett. 2013, 16, 556–567. [Google Scholar] [CrossRef]
- Mráz, P.; Hýbl, M.; Kopecký, M.; Bohatá, A.; Hoštičková, I.; Šipoš, J.; Vočadlová, K.; Čurn, V. Screening of honey bee pathogens in the Czech Republic and their prevalence in various habitats. Insects 2021, 12, 1051. [Google Scholar] [CrossRef]
- Theodorou, P.; Radzevičiūtė, R.; Settele, J.; Schweiger, O.; Murray, T.; Paxton, R. Pollination services enhanced with urbanization despite increasing pollinator parasitism. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160561. [Google Scholar] [CrossRef]
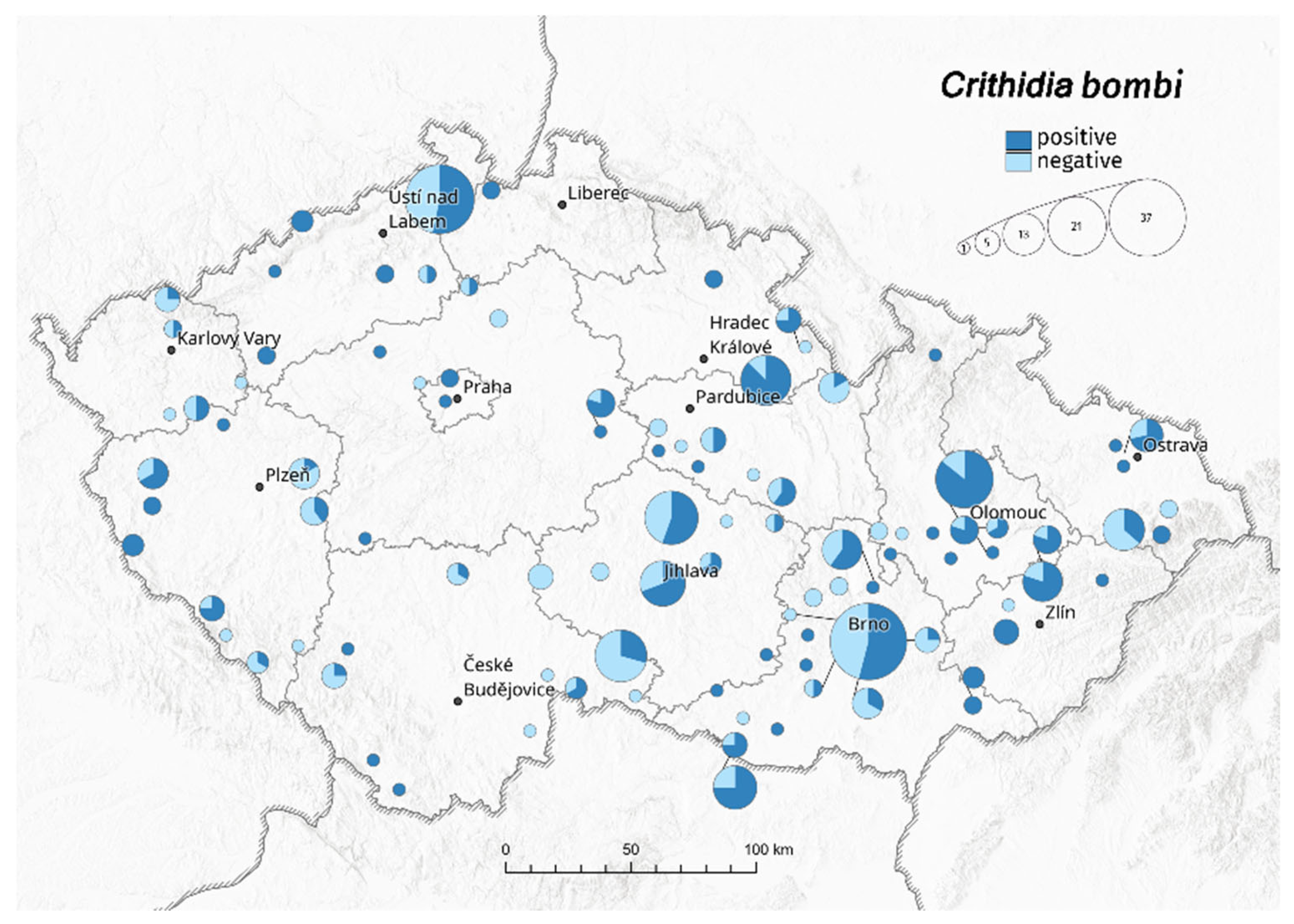

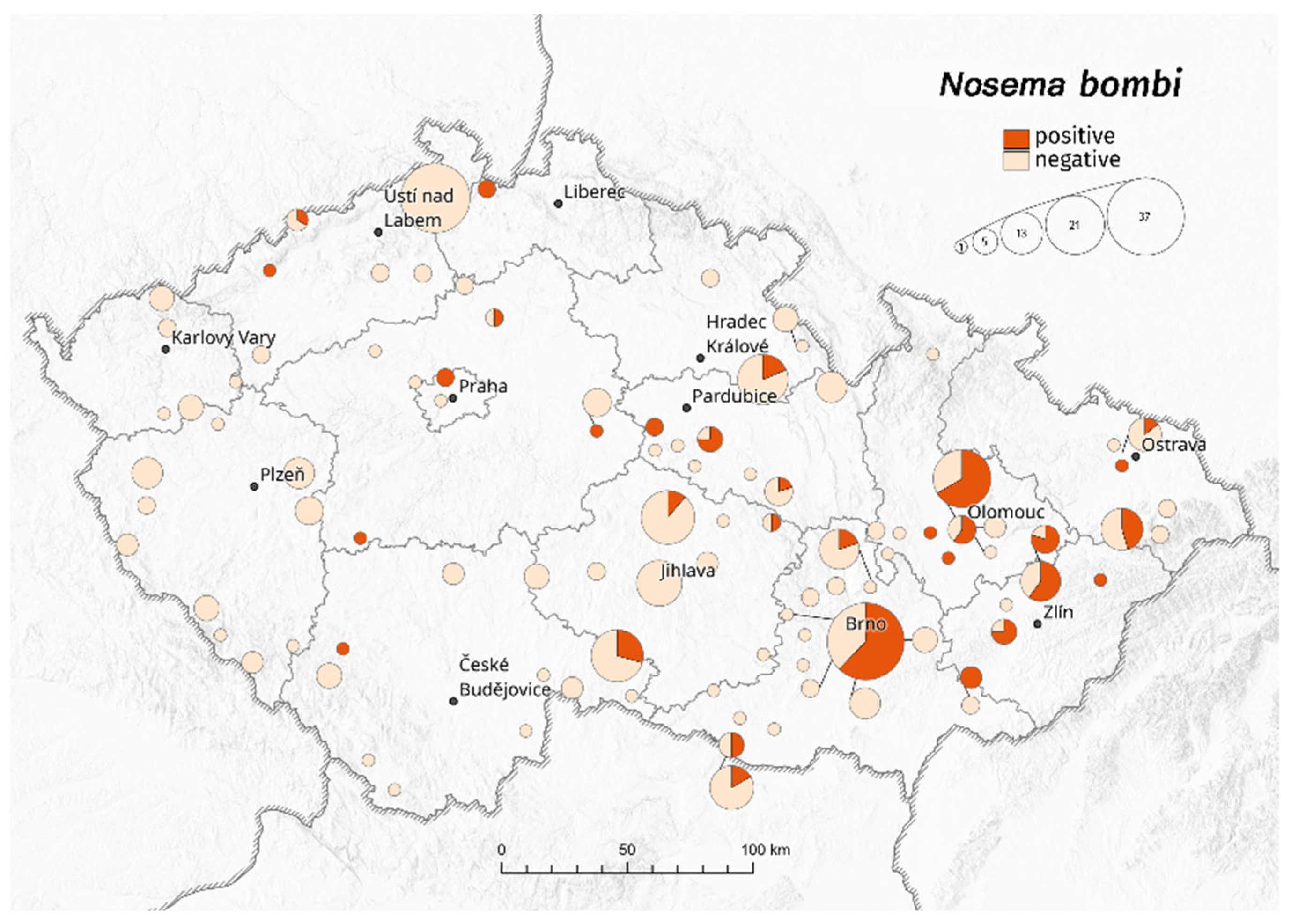


| Bombus terrestris/lucorum | Crithidia bombi | Apicystis bombi | Nosema bombi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Number of Locations | Workers | Males | W | % | M | % | W | % | M | % | W | % | M | % |
| 2015 | 26 | 103 | 7 | 72 | 70 | 1 | * | 8 | 8 | 0 | * | 26 | 25 | 0 | * |
| 2016 | 6 | 64 | 5 | 41 | 64 | 4 | * | 11 | 17 | 0 | * | 23 | 36 | 2 | * |
| 2017 | 3 | 61 | 2 | 30 | 49 | 1 | * | 2 | 3 | 1 | * | 29 | 48 | 0 | * |
| 2018 | 18 | 68 | 38 | 35 | 51 | 20 | 53 | 9 | 13 | 16 | 42 | 6 | 9 | 8 | 21 |
| 2019 | 32 | 61 | 55 | 31 | 51 | 16 | 29 | 2 | 3 | 5 | 9 | 7 | 11 | 11 | 20 |
| 2020 | 41 | 39 | 37 | 23 | 59 | 15 | 41 | 2 | 5 | 5 | 14 | 8 | 21 | 9 | 24 |
| Mean | 57 | 41 | 8 | 22 | 25 | 22 | |||||||||
| Total | 126 | 396 | 144 | 232 | 56 | 34 | 27 | 99 | 31 | ||||||
| Bombus lapidarius | Crithidia bombi | Apicystis bombi | Nosema bombi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Number of Locations | Workers | Males | W | % | M | % | W | % | M | % | W | % | M | % |
| 2015 | 10 | 34 | 0 | 17 | 50 | - | - | 1 | 3 | - | - | 1 | 3 | - | - |
| 2016 | 6 | 31 | 7 | 9 | 29 | 3 | * | 1 | 3 | 1 | * | 3 | 10 | 1 | * |
| 2017 | 2 | 16 | 0 | 5 | 31 | - | - | 1 | 6 | - | - | 6 | 38 | - | - |
| Mean | 37 | 4 | 17 | ||||||||||||
| Total | 17 | 81 | 7 | 31 | 3 | 3 | 1 | 10 | 1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Votavová, A.; Trněný, O.; Staveníková, J.; Dybová, M.; Brus, J.; Komzáková, O. Prevalence and Distribution of Three Bumblebee Pathogens from the Czech Republic. Insects 2022, 13, 1121. https://doi.org/10.3390/insects13121121
Votavová A, Trněný O, Staveníková J, Dybová M, Brus J, Komzáková O. Prevalence and Distribution of Three Bumblebee Pathogens from the Czech Republic. Insects. 2022; 13(12):1121. https://doi.org/10.3390/insects13121121
Chicago/Turabian StyleVotavová, Alena, Oldřich Trněný, Jana Staveníková, Magdaléna Dybová, Jan Brus, and Olga Komzáková. 2022. "Prevalence and Distribution of Three Bumblebee Pathogens from the Czech Republic" Insects 13, no. 12: 1121. https://doi.org/10.3390/insects13121121
APA StyleVotavová, A., Trněný, O., Staveníková, J., Dybová, M., Brus, J., & Komzáková, O. (2022). Prevalence and Distribution of Three Bumblebee Pathogens from the Czech Republic. Insects, 13(12), 1121. https://doi.org/10.3390/insects13121121






