Simple Summary
The lesser chestnut weevil (Curcilio sayi) is an emergent pest of chestnuts in the United States that can cause multifaceted damage and has limited management options. We explored the damage caused by C. sayi in a commercial chestnut orchard. Additionally, we evaluated potential management options for biological control. We found that C. sayi emerged from infested chestnuts more than four weeks post harvest and, in some cases, single nuts can host more than 10 C. sayi larvae. We also found that nut weight continues to decline even after C. sayi larvae have emerged from the chestnuts. Specific strains of entomopathogenic nematodes increase the mortality of C. sayi larvae. Biological control using entomopathogenic fungi and entomopathogenic nematodes could be complementary approaches to managing this pest and reduce C. sayi populations and chance of damage.
Abstract
The lesser chestnut weevil, Curculio sayi (Gyllenhal), can cause irreparable damage to chestnuts through direct consumption and/or introduction of secondary pathogens. With the resurgence of blight resistant American Chestnut plantings both for commercial production and for habitat restoration, C. sayi has become a similarly resurgence pest. Here, we investigated the nature and extent of C. sayi larval damage on individual nuts and collected harvests with an eye toward the quantifying impacts. Next, we explored management options using biological control including entomopathogenic fungi and entomopathogenic nematodes. Nut damage from C. sayi can be extensive with individual nuts hosting several larvae, larvae emerging from nuts several weeks post harvest, and nut weight loss even after C. sayi have emerged from the nut. Applications of entomopathogenic fungi reduced chances of chestnut infestation, while certain strains of entomopathogenic nematodes increased the probability of C. sayi larval mortality. Understanding C. sayi damage and exploring biological control management options could be a useful tool in the effective management of this resurgent pest.
1. Introduction
American Chestnut (Castanea dentata) was once the foundation species for forests throughout Appalachia along the east coast of the United States. Occupying at least 50% of the basal area across 800,000 square kilometers, American Chestnut trees were once estimated to have numbered more than 4 billion in the early 1900s [1]. These trees produced a prodigious amount of chestnuts. In 1911, a single West Virginia train station shipped 155,000 lbs of chestnuts with a retail market value of nearly $750,000 USD today [2]. These chestnuts were a staple of animal and human diets; nutritious chestnuts were used to fatten livestock before the market and were an ubiquitous commodity in early American life [2,3,4]
With the introduction of chestnut blight (Cryphonectria parasitica) in the early 1900s, American Chestnut trees suffered a precipitous and rapid decline. By 1960, American Chestnut trees were almost completely absent from the canopies of eastern forests [1]. It was only in the past decades that blight resistant hybrids made the resurgence of chestnut production possible. The development of these blight resistant hybrids have opened opportunities for commercial expansion of chestnut as a high value specialty crop. Although blight resistant hybrids have largely overcome the challenges imposed by chestnut blight, commercial resurgence of American Chestnut Hybrids in recent years has been accompanied by the resurgence of another organism: the lesser chestnut weevil (Curculio sayi).
The lesser chestnut weevil was documented extensively in the early 1900s as being a prominent pest of chestnuts. Reports mentioned that large losses in chestnut production occurred due to infestation by C. sayi larvae [5]. Infestation by C. sayi was recognized to range widely with rates between 50–75% considered normal [6]. Infestation rates as high as 100% were also reported [6]. The extent of these pest problems made it a public safety concern with federal and state governments seizing large quantities of chestnuts because they contained large amounts of C. sayi larvae and excreta [6].
While C. sayi numbers decreased alongside its host, the American Chestnut, this weevil is once again becoming a prominent pest of commercial chestnut plantings, rapidly emerging in as little as two years to high levels of infestation [7]. With bivoltine populations in the southern United States and univoltine populations in the north, we now realize that C. sayi damage takes two primary forms [7].
The first is physical damage. Consumer confidence in chestnuts takes an incredible blow when larvae emerge on the counter at home. Even if the larvae are killed in post-harvest heat treatments, excessive damage can also substantially reduce nut-meat. The second is damage through facilitation of infections. C. sayi larvae are associated with Aspergillus fungi [8] which produce the diarrheagenic toxin emodin [9].
Despite these negative impacts, options for control are limited. Few commercial products are labeled for use against C. sayi and those commercial pesticides that are used have potentially toxic environmental effects [10]. Post harvest control methods for treating larvae in collected chestnuts abound but, even if they are effective, they only treat the symptoms. Growers still lose production and it is unclear whether post-harvest treatments can effectively address fungi. Biological control has been implemented to control other related Curculio weevils, but their efficacy with C. sayi is unknown [11,12,13,14,15].
To explore options for biological control, entomopathogenic fungi, entomopathogenic nematodes, and their combination were evaluated in commercial chestnuts over two years. Because emerging C. sayi adults tend to climb to the chestnut canopy in the spring, we thought that topical trunk applications of entomopathogenic fungi could be effective at targeting the adult lifestage. Because C. sayi larvae burrow into the soil to pupate and overwinter, we thought that entomopathogenic nematodes could be effective at targeting the larval life stage. Both these biological control options, alone and combined, have been used to control Curculio elephas but never used against C. sayi [12,14,15,16,17]. In addition, damage from C. sayi larvae was quantified to address and document anecdotal reports of damage that varied widely.
2. Materials and Methods
To evaluate the damage and management of C. sayi, we used a commercial chestnut stand at the Rose Valley Farm (43°09026.500 N, 76°55021.100 W) in upstate New York. This commercial stand was composed of a blend of mature (15+ years old) American Chestnut Hybrids planted approximately 7 m on center and forming a complete canopy after leafing out. Catkins (flowering) occurred in mid to late June and nut drop began in late September to early October. The soil type was Elnora Loamy Fine Sand.
2.1. Chestnut Weevil Damage
To evaluate the damage caused by C. sayi, chestnuts were collected within 24 h of nut drop and brought to the lab in two different cohorts.
For the first cohort, fifty chestnuts from each of four treatments (for a total of 200 chestnuts, treatments described below) were placed individually into 50 mL Falcon Tubes. Each of these nuts were evaluated every 24 h and the number of larvae that had emerged from the nuts counted.
For the second cohort, chestnuts sufficient to fill a 1 L plastic planting container were collected from the control treatment (described below). These planting containers were perforated on the bottom to allow for larval egress after emergence from the chestnuts. Ten replicates of these containers were collected. Every 24 h for 45 days these containers were evaluated. The number of larvae that had emerged were counted and weighed. The total weight of the nuts in the container were also weighed.
2.2. Entomopathogenic Nematode Strain Evaluation under Lab Conditions
To evaluate the ability of entomopathogenic nematode strains to control C. sayi larvae, we evaluated the mortality of late instar C. sayi larvae exposed to thirteen different strains of entomopathogenic nematodes collected from the east coast of the United States. Late instar C. sayi larvae were collected as they emerged from harvested chestnuts and placed individually into wells of ELISA plates lined with 1 cm diameter filter paper (Whatman #1(St. Louis, MO, USA)). Each of these wells received 200 µL of liquid; controls received water only while treatments received a nematode solution containing 1000 infective juveniles of the appropriate strain per mL for approximately 200 nematodes per larvae. Treatments were replicated 24 times and controls replicated 96 times across ELISA plates. C. sayi mortality was monitored for 10 days.
2.3. Chestnut Weevil Management
To evaluate the viability of biological control techniques for the management of C. sayi, we evaluated both the application of entomopathogenic nematodes (EPN) to the soil and application of entomopathogenic fungi (EPF) to the trunks of chestnut trees. To do so, we utilized a two factor, full factorial design with four treatments: EPN Only, EPF Only, Both EPN and EPF, and the Control (neither EPN nor EPF). We utilized a randomized controlled block design and treated individual monitoring locations (approximately four trees) as replicates.
2.3.1. Chestnut Weevil Biological Control Applications
Entomopathogenic nematodes were applied to the soil using a 15L backpack sprayer (Chapin 61800, Chapin Manufacturing, Batavia, NY, USA) at a rate of 120 million per acre. Two complementary species of entomopathogenic nematode were used: Steinernema feltiae and Heterorhabditis bacteriophora both collected from upstate NY and reared in Galleria mellonella larvae following previously established methods [18,19]. These species were chosen for their established hardiness in similar environments. Infective juveniles (IJs) of both species were collected after emergence from their hosts and used within seven days. The two species were mixed at a 1:1 ratio to a concentration of 2000 IJs/ml. Prior to each application, samples were taken in the field from the backpack sprayer before and after passing through the sprayer to ensure the viability for application. EPNs were applied three times each field season on May 23rd, June 6th, and July 1st in both 2019 and 2020. Plots not receiving the nematode treatment instead received the same amount of water delivered via a separate backpack sprayer, but without nematodes.
Entomopathogenic fungi were applied to the trunks of each tree in the treatment in a 0.5 m band entirely circling the circumference of the tree, terminating approximately 1 m above ground level using a large paintbrush similar to whitewashing. Beauveria bassiana, commercially available in Mycotrol ESO (Bioworks, Victor, NY, USA) was used as the entomopathogenic fungi at the recommended rate (1 quart/acre) on June 17th in both 2019 and 2020. The efficacy of this product was confirmed by sampling the solution immediately prior to the field application then subsequent application to G. mellonella larvae in the lab. Plots not receiving the EPF treatment instead received the same amount of the solution but without B. bassiana.
The efficacy of these treatments was evaluated using pyramid and trunk traps to monitor adult C. sayi populations. Pyramid traps (Tedders Pyramid Trap, GL-5000-06, Great Lakes IPM, Vestaburg, MI, USA) are designed to attract newly emerging C. sayi adults as they climb adjacent vertical objects. These traps were staked to the ground approximately 0.5 m from adjacent tree trunks. Trunk Traps (Circle Trunk Trap, Small GL-4000-06, Great Lakes IPM, Vestaburg, MI, USA) were affixed to the tree trunks at breast height (1.35 m) such that the mesh screen of the trap directly abutted the trunk of the tree. These traps are designed to arrest and C. sayi adults ascending the trunks of the trees. These traps were emplaced in late April before the start of each season and monitored biweekly.
2.3.2. Microcosms
Microcosms consisted of 20, five gallon (18.93L) plastic buckets into which 15–20 large diameter (about 7cm) holes were drilled at the sides and bottom of the bucket and then covered with a fine mesh screen (1000 micron). These microcosms were placed adjacent to the commercial chestnut orchard by excavating 20 individual holes for each microcosm, placing the buckets in the holes, then backfilling the soil into each bucket. The soil was allowed to resettle for one week then 25 C. sayi late instar larvae (that had emerged from collected chestnuts within 48 h) were placed into each microcosm and sealed inside with a mesh cap on the buried buckets. This arrangement ensured that the C. sayi could not escape the microcosms but that other microorganisms and water could move in and out via the soil.
Each of the four treatments described above were replicated five times in a randomized controlled block design. Each microcosm in each treatment received 500 mL of solution. Control microcosms received 500 mL of water. Entomopathogenic nematode-only treatments received a 500 mL of a solution containing S. feltiae and H. bacteriophora in a 1:1 ratio at 1000 IJs/ml for each species (2000 IJs/ml total). Entomopathogenic fungi only treatments received 500 mL of Mycotrol ESO B. bassiana solution (made by mixing 3.75 mL of Mycotrol ESO in water for a 500 mL total volume). Treatments receiving both entomopathogenic nematodes and entomopathogenic fungi received the same amount of total volume with both EPN and EPF treatments mixed. The application of treatments occurred one week following introduction of the C. sayi larvae.
Microcosms were initially emplaced in late October 2019 with the introduction of C. sayi larvae and application of treatments accomplished by late November 2019. Microcosms remained unperturbed until November 2020 when they were removed, taken to the lab, and carefully excavated. As a first step in the excavation, the covering screen was removed and the surface examined for emerged C. sayi adults. Following this initial examination, the soil was carefully excavated and delicately passed through a fine mesh seive to extract any remaining C. sayi adults, pupae, or larvae.
2.4. Analysis
Nut damage by emerging C. sayi larvae was visualized using histograms and density distributions then modeled using generalized additive models.
Mortality of C. sayi larvae resulting from nematode infection was evaluated using logistic regression considering the interaction between nematode strain and days post inoculation as factors. Best fit models were chosen after consideration of all potential interactions, residual analysis, goodness of fit metrics, and likelihood ratio tests. Post-hoc treatment comparisons were conducted using the Dunnett method of contrasting treatments with the control.
The efficacy of C. sayi management strategies was evaluated using poisson regression models for trap catch and emergence from microcosms. Logistic regression was used to evaluate nut infestation from respective treatments. Best fit models were chosen after consideration of all potential interactions, residual analysis, goodness of fit metrics, and likelihood ratio tests. Post-hoc treatment comparisons were conducted using custom contrasts for desired comparisons and the Bonferroni correction for adjusting the family-wise error rate.
All analysis was conducted in R version 4.2.1 [20] using RStudio [21] as an integrated development environment (IDE). The following packages facilitated the analysis, modeling, and visualization of results: tidyverse for data preparation and plotting [22]; car and emmeans for model evaluation and post-hoc comparisons [23,24]; ggpubr, here, and cowplot for assistance in plotting and figure generation [25,26,27].
3. Results
3.1. Chestnut Weevil Damage
Larval C. sayi emerged from individual chestnuts at varying rates and over an extended period of time. Out of the chestnuts with emergent larvae, eight had only one larva emerging in the time under observation (Figure 1A). A few chestnuts had more than five larvae emerging with three individual chestnuts hosting more than 10 C. sayi larvae that emerged under the period of observation. More than three quarters of chestnuts (75.8%) with larval emergence had more than one larva emerging over the observation period.
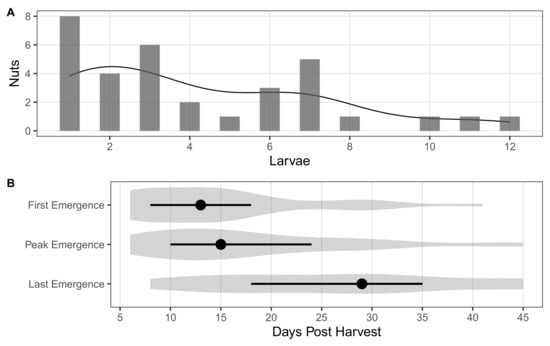
Figure 1.
Larval C. sayi emergence from individual chestnuts. (A) Number of C. sayi larvae emerging from each individual chestnut. Each bar indicates the number of nuts having that many C. sayi larvae emerge. The line indicates the smoothed density distribution of that emergence. (B) Time to emergence by C. sayi larvae. First emergence denotes the amount of time to when the first C. sayi larva was observed leaving the nut. Peak emergence denotes the time to when the most number of larvae were observed leaving the nut on a given day. Last emergence denotes the time to when the last C. sayi larva was observed emerging from the chestnut. Shaded grey areas denote density distributions for each metric. Points denote the median. Error bars denote the first and third quartiles.
Larval C. sayi began emerging within a few days of the field collection of nuts dropped in the previous 24 h (Figure 1B). Median first emergence of larval C. sayi occurred between 10 and 15 days after harvest with median peak emergence occurring at 15 days. The median last observed emergence of C. sayi was at close to 30 days post harvest with the last C. sayi emerging at 45 days after harvest.
In examining cohorts of chestnuts collected in planting containers after dropping in the previous 24 h, the numbers of emerging larvae peaked at six days post harvest (Figure 2A). Following the peak, larval emergence tailed off with the last observed larval emergence occurring 31 days post harvest. Cumulative weight of the larvae emerging from collected chestnuts flatlined at 16 days post harvest (Figure 2B) and remained mostly flat for the remaining period of observation. The nut weight of collected nuts declined almost linearly over the period of observation (Figure 2C), even after 16 days post harvest.
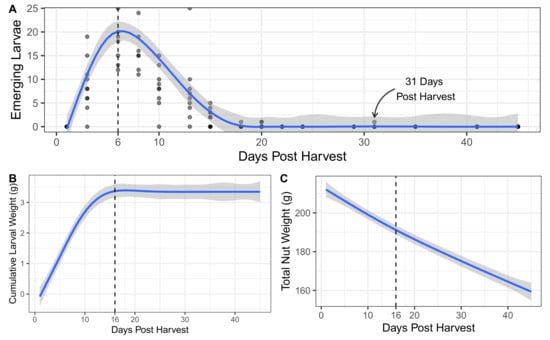
Figure 2.
Larval C. sayi damage on groups of chestnuts. (A) Number of emerging larvae from 1 L planting containers of chestnuts. (B) Cumulative weight of all emerging larvae emerging from each 1 L planting container of chestnut. (C) Total weight of chestnuts in each 1 L planting container of chestnuts. For all plots: points denote individual observations; blue lines and shaded areas denote generalized additive model smoothed fit and 95% confidence intervals, respectively.
3.2. Entomopathogenic Nematode Strain Evaluation under Lab Conditions
Entomopathogenic nematode strain, days post inoculation, and their interaction significantly explained the observed C. sayi mortality (Treatment: = 131.2, = 13, 0.001; Days: = 13.2, = 1, p = 0.0003; Interaction: = 114.2, = 13, 0.001). Exposure to many nematode strains resulted in a significantly higher probability of mortality (Figure 3) compared to the controls.
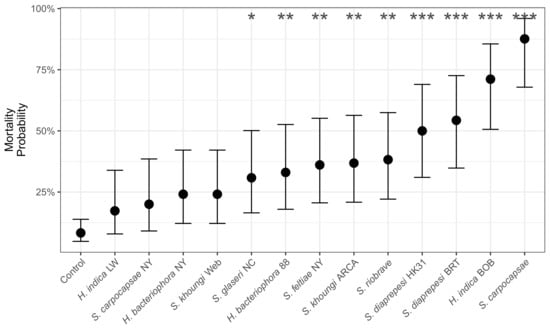
Figure 3.
Probability of C. sayi mortality resulting from exposure to entomopathogenic nematode strains. H. bacteriophora NY and S. feltiae NY were the strains used in the field and microcosm trials. Mortality probability is calculated as of four days post inoculation. Points and errorbars denote mean and 95% confidence intervals, respectively. Asterisks denote significant differences at (*), (**), and (***) compared to controls, not among strains.
3.3. Chestnut Weevil Management
Biological control treatments significantly explained the differences in C. sayi responses. Biological control treatments and year (but not their interaction) significantly explained adult C. sayi trap catch (Treatment: = 14.7, = 3, p = 0.002; Year: = 208.9, = 1, 0.001). Entomopathogenic fungi (EPF) significantly reduced adult C. sayi trap catch as a main effect and compared to controls (p = 0.007, 0.03; Figure 4A). Adult C. sayi trap catch was significantly less (0.0001) in 2020 compared to 2019 (Figure 4B).
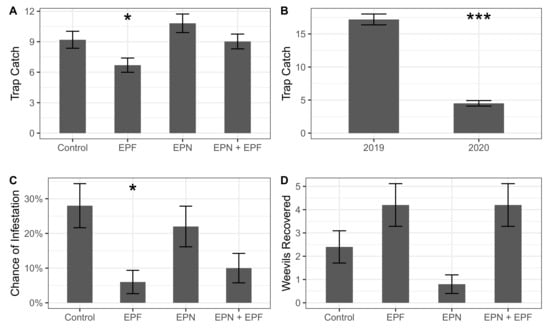
Figure 4.
Biological control of C. sayi. (A) Effect of biological control treatments on C. sayi adult trap catch in a commercial chestnut orchard. Bars and error bars denote rate of trap catch in each treatment on a per trap basis and standard error, respectively. Single asterisk denotes a significant () difference from the control treatment. (B) C. sayi adult trap catch in each year of this study. Bars and error bars denote rate of trap catch in each year on a per trap basis and standard error, respectively. Multiple asterisks denote significant () difference from 2019. (C) Likelihood of a nut from a given treatment being infested by C. sayi larvae. Bars and error bars denote the chance of a nut from a given treatment being infested with C. sayi larvae and the standard error, respectively. Single asterisk denotes the significant () difference from the control treatment. (D) C. sayi recovered from microcosms by treatment. Bars and error bars denote weevils recovered by treatment and standard error, respectively. Single asterisk denotes significant () difference from EPN+EPF treatment and marginally significant () difference from the control.
Biological control treatments significantly explained the probability of larval C. sayi emergence from chestnuts ( = 11.96, = 3, p = 0.008). Chestnuts from the treatment receiving only entomopathogenic fungi had a significantly lower chance of weevil infestation both as a main effect and compared to the control treatment (p = 0.006, 0.02; Figure 4C).
Biological control treatments also significantly explained the number of weevils recovered from microcosms ( = 16.27, = 3, p = 0.001). Treatments receiving entomopathogenic nematodes only had a significantly lower (p = 0.007) number of weevils recovered compared to the EPN + EPF treatment (Figure 4D). The average (±SD, SEM) recovery rate of weevils from microcosms across all treatments was 12% (±14%, 3%) or approximately 3 (±3.4, 0.75) weevils.
4. Discussion
4.1. Chestnut Weevil Damage
Curculio sayi larvae emergence exceeded expectations. While early reports of C. sayi emergence documented the ability for multiple larvae to emerge from a single nut [5,6,28], many modern anecdotes focused on the one larva, one nut rule of thumb. This is not the case. While some of the chestnuts with observed larval emergence did only have one larva, three quarters of the chestnuts with observed larval emergence had two or more larvae. One single chestnut contained 12 larvae.
This abundance of C. sayi larvae in a single nut could suggest that females do not have a mechanism for identifying chestnuts already with eggs. It is also unclear if there are advantages or disadvantages to having more than one larva in a single nut. There were not obvious differences in the health of the larvae emerging from the nuts with 10 or more larvae compared with the nuts containing a single larvae, although we did not accompany each larvae to adulthood.
Emergence of C. sayi was also distributed in time. While peak emergence (when most of the larvae in a nut emerged) tended to occur between 10 and 24 days post harvest, the emergence of the first weevil from an individual chestnut occurred as early as a few days following harvest and as late as 42 days post harvest. This long time period for larval emergence was corroborated in observations of planting containers of chestnuts with the last larva emerging 31 days post harvest.
The distributed nature of C. sayi emergence suggests that post harvest control strategies may need to be adapted. A practice that involves waiting for two weeks (14 days) to determine which nuts are not infested does not seem adequate. Even nuts with no emergence after 14 days may still contain active larvae that could emerge potentially even three weeks later.
Waiting six weeks (42 days) to evaluate larval emergence and identify healthy nuts is also not an ideal option. Irrespective of time to market considerations, nut weight appears to decline linearly with time. This is true even after most larvae have left the nuts. Peak emergence of C. sayi in buckets of chestnuts occurred at six days post harvest and dropped to low levels by day 16 when the cumulative weight of the larvae flatlined. Despite very little emergence occurring after day 16, nut weight continued its almost linear decline.
This decline suggests that other factors are at play in decreasing nut weight, not solely C. sayi consumption of nut meat. A potential culprit is a bacterial/fungal complex associated with C. sayi larval tunnels inside the nuts. Feeding by C. sayi larvae open channels in the chestnut, which are colonized by potentially harmful microorganisms such as Aspergillus fungi [8,9,29,30]. These microorganisms are present long after C. sayi larvae have emerged from the nut. Their presence could be a contributing factor to the continued decline of nut weight over time. Independent of the causes, the more time between harvest and market delivery of chestnuts, the less chestnut material is available.
4.2. Chestnut Weevil Management
Because of the losses caused by C. sayi, management techniques that address burgeoning populations are critical. Applications of entomopathogenic fungi to trunks of mature chestnut trees significantly reduced the adult C. sayi trap catch compared to the control.
This reduction in trap catch could potentially be attributed to two possible factors. First, the applications of entomopathogenic fungi to the trunks of chestnut trees could have potentially served as a repellent to adult C. sayi moving to the tree canopy. Second, applications of entomopathogenic fungi could have had an effect of C. sayi mortality. While not immediately effective in this regard, insects coming into contact with entomopathogenic fungi can be colonized by this fungus and die in a matter of days from a systemic fungal infection.
Irrespective of the mechanism, the reduction in adult C. sayi trap catch was also reflected in the chance of weevil infestation in collected chestnuts. Chestnuts collected from the EPF treatment had a lower chance of being infested with C. sayi larvae than other treatments. This suggests that the effect of application of entomopathogenic fungi, translates into a reduction in weevil infestation of harvested chestnuts.
Treatments receiving both entomopathogenic nematodes and entomopathogenic fungi did not respond the same as entomopathogenic fungi only treatments. While these treatments were comparably lower in terms of adult C. sayi trap catch and chance of infestation, in each case, treatments of entomopathogenic fungi lowered the respective response compared to the entomopathogenic nematode-only treatments.
The lack of response of the entomopathogenic nematode treatments in the field was surprising given that many entomopathogenic nematode strains significantly increased the probability of C. sayi larvae. Although the strains chosen in this study were selected more for their ability to survive northern winters as opposed to virulence alone, they still increased the probability of larval mortality in laboratory trials. In particular, in one of the species we used, (S. feltiae) can significantly increase the probability of mortality compared with controls. While it is entirely possible that applications of entomopathogenic nematodes had no effect on C. sayi populations, it is also possible that the effect might be lagged. The drastic drop in C. sayi populations between years might be the result of the efficacy of entomopathogenic nematodes, which are are highly effective mobile agents that seek out weevil larvae. A similar effect has been observed in applications of entomopathogenic nematodes to control the pecan weevil (Curculio caryae); repeated applications of entomopathogenic nematodes resulted in the improved control of C. caryae in year two [31].
Because chestnut weevils in general, and likely C. sayi as well, seem to demonstrate a staggered approach to cohort development with potentially multi-year periods in the soil as eclosed adults, timing in using entomopathogenic nematodes could be critical [7,32,33]. Entomopathogenic nematodes applied at the beginning of the season might be helpful in establishing populations of these nematodes in the soil, but at the beginning of the season, most C. sayi in the soil are in nematode-resistant pupal cells, as more nematode resistant adults [7,34].
In contrast, applications of entomopathogenic nematodes at the end of the season might be more effective. At the end of the season, C. sayi are emerging from chestnuts and burrowing into the soil as larvae prior to establishing protections from soil organisms like entomopathogenic nematodes. The efficacy of these applications would not appear, however, until the next season, when the impact of this mortality on the emerging cohort would become apparent. If entomopathogenic nematodes were effective, we would expect this to become apparent over the course of two to three years as we began to see in this study and as has been observed in other field settings [35,36]. In addition, our microcosm experiments also indicated that applications of entomopathogenic nematodes could reduce populations of C. sayi when introduced to the soil as larvae.
5. Conclusions
Together, entomopathogenic nematodes and entomopathogenic fungi could present an option for the biological control of C. sayi infestations in commercial chestnut orchards. This dual approach has been evaluated in other similar systems with the greater chestnut weevil (C. elephas) [14]. Applications of entomopathogenic fungi could reduce chestnut infestations initially, while applications of entomopathogenic nematodes could reduce C. sayi populations over the longer term. There might even be a synergistic effect to this approach that emerges over the course of three to five years. This is the focus of future work.
Author Contributions
Conceptualization, C.C.F. and D.S.W.; methodology, C.C.F. and D.S.W.; formal analysis, C.C.F. and D.S.W.; investigation, C.C.F. and D.S.W.; resources, C.C.F. and D.S.W.; data curation, C.C.F. and D.S.W.; writing—original draft preparation, C.C.F. and D.S.W.; writing—review and editing, C.C.F. and D.S.W.; visualization, C.C.F. and D.S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NE SARE grant number ONE19-353.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and code supporting this publication will be made available via GitHub following publication.
Acknowledgments
Bo Holladay, Ramom Vasconcelos Pereira, Matthew Barrett, and Michael Mueller assisted with field and microcosm trials. David Stern and all members of Rose Valley Farm provided the resources and motivation for this work and provided invaluable assistance. Deborah McCullough provided a sounding board for the preliminary results of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jacobs, D.F. Toward development of silvical strategies for forest restoration of American chestnut (Castanea dentata) using blight-resistant hybrids. Biol. Conserv. 2007, 137, 497–506. [Google Scholar] [CrossRef]
- Kuhlman, E. The devastation of American chestnut by blight. In Proceedings of the American Chestnut Symposium; MacDonald, W.L., Ed.; West Virginia University Press: Morgantown, WV, USA, 1978. [Google Scholar]
- Hepting, G.H. Death of the American chestnut. J. For. Hist. 1974, 18, 60–67. [Google Scholar] [CrossRef]
- Thoreau, H.D. Walden; Yale University Press: New Haven, CT, USA, 1906. [Google Scholar]
- Chittenden, F.H. The Nut Weevils; Number 99; US Department of Agriculture, Bureau of Entomology: San Marcos, CA, USA, 1908.
- Brooks, F.E.; Cotton, R.T. The Chestnut Curculios; Number 130; US Department of Agriculture: San Marcos, CA, USA, 1929.
- Filgueiras, C.C.; Willett, D.S. Phenology and Monitoring of the Lesser Chestnut Weevil (Curculio sayi). Insects 2022, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Payne, J. Mycoflora and market quality of chestnuts treated with hot water to control the chestnut weevil. Plant Dis. 1980, 64, 999–1001. [Google Scholar] [CrossRef][Green Version]
- Wells, J.M.; Cole, R.J.; Kirksey, J.W. Emodin, a toxic metabolite of Aspergillus Wentii Isol. Weevil-Damaged Chestnuts. Appl. Microbiol. 1975, 30, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Lizotte, E. Chestnut Weevil: A Potential Pest of Michigan Chestnuts. 2014. Available online: https://www.canr.msu.edu/news/chestnut_weevil_a_potential_pest_of_michigan_chestnuts (accessed on 14 September 2022).
- Smith, M.; Georgis, R.; Nyczepir, A.; Miller, R. Biological control of the pecan weevil, Curculio caryae (Coleoptera: Curculionidae), with entomopathogenic nematodes. J. Nematol. 1993, 25, 78. [Google Scholar] [PubMed]
- Paparatti, B.; Speranza, S. Biological Control of Chestnut Weevil (Curculio elephas Gyll.; Coleoptera, Curculionidae) with the Entomopathogen Fungus Beauveria bassiana (Balsamo) Vuill. (Deuteromycotina, Hyphomycetes) SP1. In Proceedings of the II International Symposium on Chestnut, Bordeaux, France, 1 July 1999. [Google Scholar]
- Paparatti, B.; Speranza, S. Biological control of Hazelnut weevil (Curculio nucum L. Coleoptera, Curculionidae) using the entomopathogenic fungus Beauveria bassiana (Balsamo) Vuill. (Deuteromycotina, Hyphomycetes). In Proceedings of the VI International Congress on Hazelnut 686, Tarragona-Reus, Spain, 14 June 2004; pp. 407–412. [Google Scholar]
- Torrini, G.; Benvenuti, C.; Binazzi, F.; Marianelli, L.; Paoli, F.; Sabbatini Peverieri, G.; Roversi, P.F. Entomopathogenic fungi and nematodes against larvae of the chestnut weevil, Curculio elephas (Coleoptera: Curculionidae): A laboratory evaluation. Int. J. Pest Manag. 2018, 64, 287–293. [Google Scholar] [CrossRef]
- Asan, C.; Hazir, S.; Cimen, H.; Ulug, D.; Taylor, J.; Butt, T.; Karagoz, M. An innovative strategy for control of the chestnut weevil Curculio elephas (Coleoptera: Curculionidae) using Metarhizium brunneum. Crop Prot. 2017, 102, 147–153. [Google Scholar] [CrossRef]
- Karagoz, M.; Gulcu, B.; Hazir, S.; Kaya, H.K. Laboratory evaluation of Turkish entomopathogenic nematodes for suppression of the chestnut pests, Curculio elephas (Coleoptera: Curculionidae) and Cydia splendana (Lepidoptera: Tortricidae). Biocontrol Sci. Technol. 2009, 19, 755–768. [Google Scholar] [CrossRef]
- Kepenekci, I.; Gokce, A.; Gaugler, R. Virulence of three species of entomopathogenic nematodes to the chestnut weevil, Curculio elephas (Coleoptera: Curculionidae). Nematropica 2004, 199–204. [Google Scholar]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Manual of Techniques in Insect Pathology; Elsevier: Amsterdam, The Netherlands, 1997; pp. 281–324. [Google Scholar]
- White, G. A method for obtaining infective nematode larvae from cultures. Science 1927, 66, 302–303. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC.: Boston, MA, USA, 2020. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.7.4-1. 2022. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 14 September 2022).
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://cran.microsoft.com/snapshot/2021-09-26/web/packages/ggpubr/index.html (accessed on 14 September 2022).
- Müller, K. Here: A Simpler Way to Find Your Files. R Package Version 1.0.1. 2020. Available online: https://rdrr.io/cran/here/ (accessed on 14 September 2022).
- Wilke, C.O. cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. R Package Version 1.1.1. 2020. Available online: https://cran.r-project.org/web/packages/cowplot/cowplot.pdf (accessed on 14 September 2022).
- Johnson, W.T. On the Biology and Control of the North American Chestnut Weevils. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 1956. [Google Scholar]
- Prencipe, S.; Siciliano, I.; Contessa, C.; Botta, R.; Garibaldi, A.; Gullino, M.L.; Spadaro, D. Characterization of Aspergillus section Flavi isolated from fresh chestnuts and along the chestnut flour process. Food Microbiol. 2018, 69, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, S.; Siciliano, I.; Gatti, C.; Gullino, M.L.; Garibaldi, A.; Spadaro, D. Chestnut drying is critical in determining Aspergillus flavus growth and aflatoxin contamination. Toxins 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Shapiro-Ilan, D.; Gardner, W.A. Improved control of Curculio caryae (Coleoptera: Curculionidae) through multi-stage pre-emergence applications of Steinernema carpocapsae. J. Entomol. Sci. 2012, 47, 27–34. [Google Scholar] [CrossRef]
- Menu, F.; Debouzie, D. Coin-flipping plasticity and prolonged diapause in insects: Example of the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 1993, 93, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Menu, F. Strategies of emergence in the chestnut weevil Curculio elephas (Coleoptera: Curculionidae). Oecologia 1993, 96, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Keesey, I.W. The Seasonal Occurrence, Soil Distribution and Flight Characteristics of Curculio sayi (Coleoptera: Curculionidae) in Mid-Missouri; University of Missouri-Columbia: Columbia, MO, USA, 2007. [Google Scholar]
- Shapiro-Ilan, D.I.; Hazir, S.; Glazer, I. 12 Entomopathogenic Nematodes as Models for Inundative Biological Control. Nematodes Model Org. 2022, 293. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Shapiro-Ilan, D.I.; Hiltpold, I. Advances in the use of entomopathogenic nematodes (EPNs) as biopesticides in suppressing crop insect pests. In Biopesticides for Sustainable Agriculture; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 195–232. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).