Genome-Wide Identification and Expression Profiling of Candidate Sex Pheromone Biosynthesis Genes in the Fall Armyworm (Spodoptera frugiperda)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Tissue Collection
2.2. RNA Isolation, cDNA Library Construction, Illumina Sequencing
2.3. Identification and Analysis of Sex Pheromone Biosynthesis-Related Genes
2.4. cDNA Synthesis and Full-Length cDNA Cloning
2.5. Phylogenetic Analysis
2.6. Quantitative RT-PCR and Data Analysis
3. Results
3.1. Identification and Localization of the Candidate Sex Pheromone Biosynthesis Genes in the S. frugiperda Genome
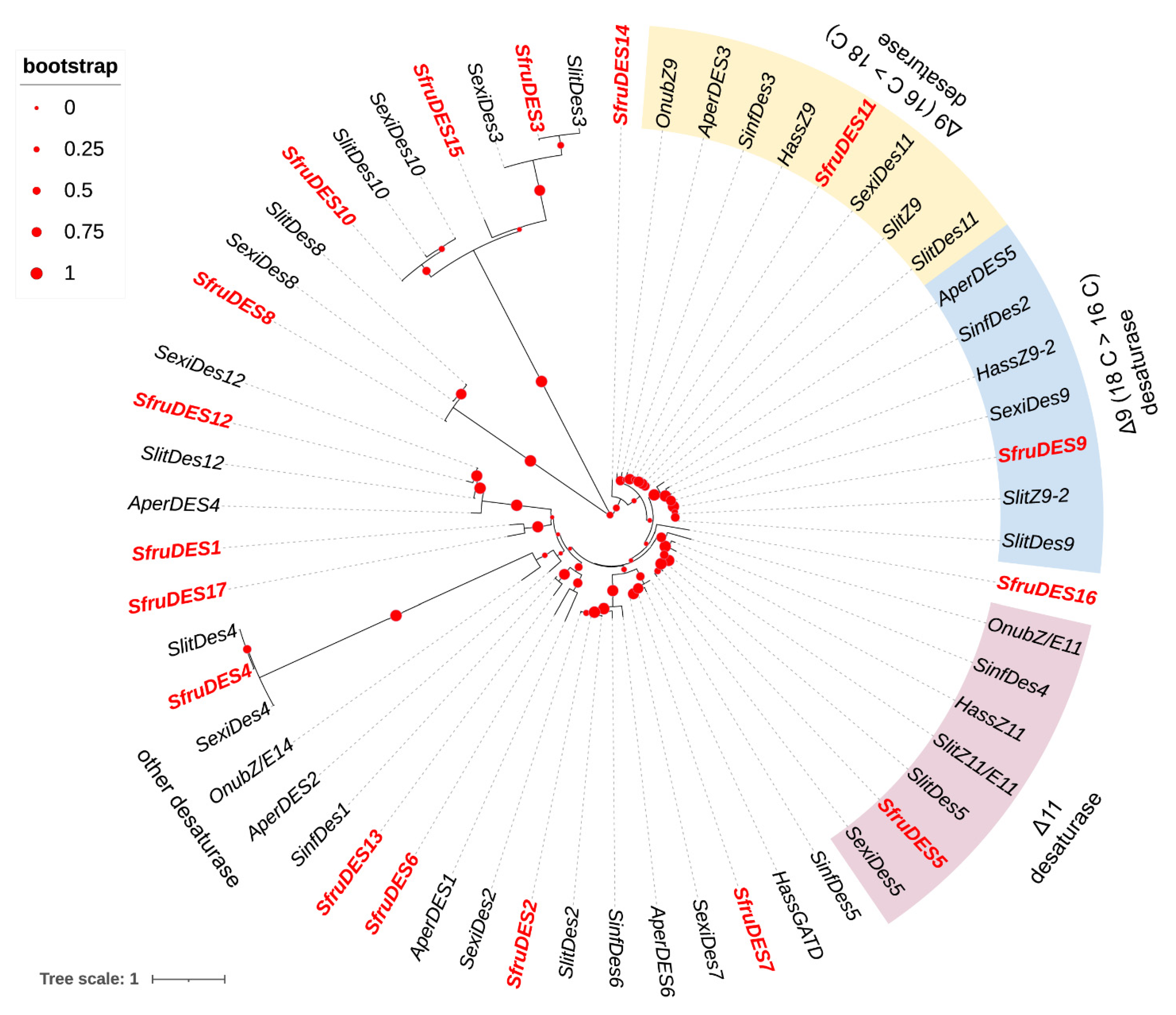
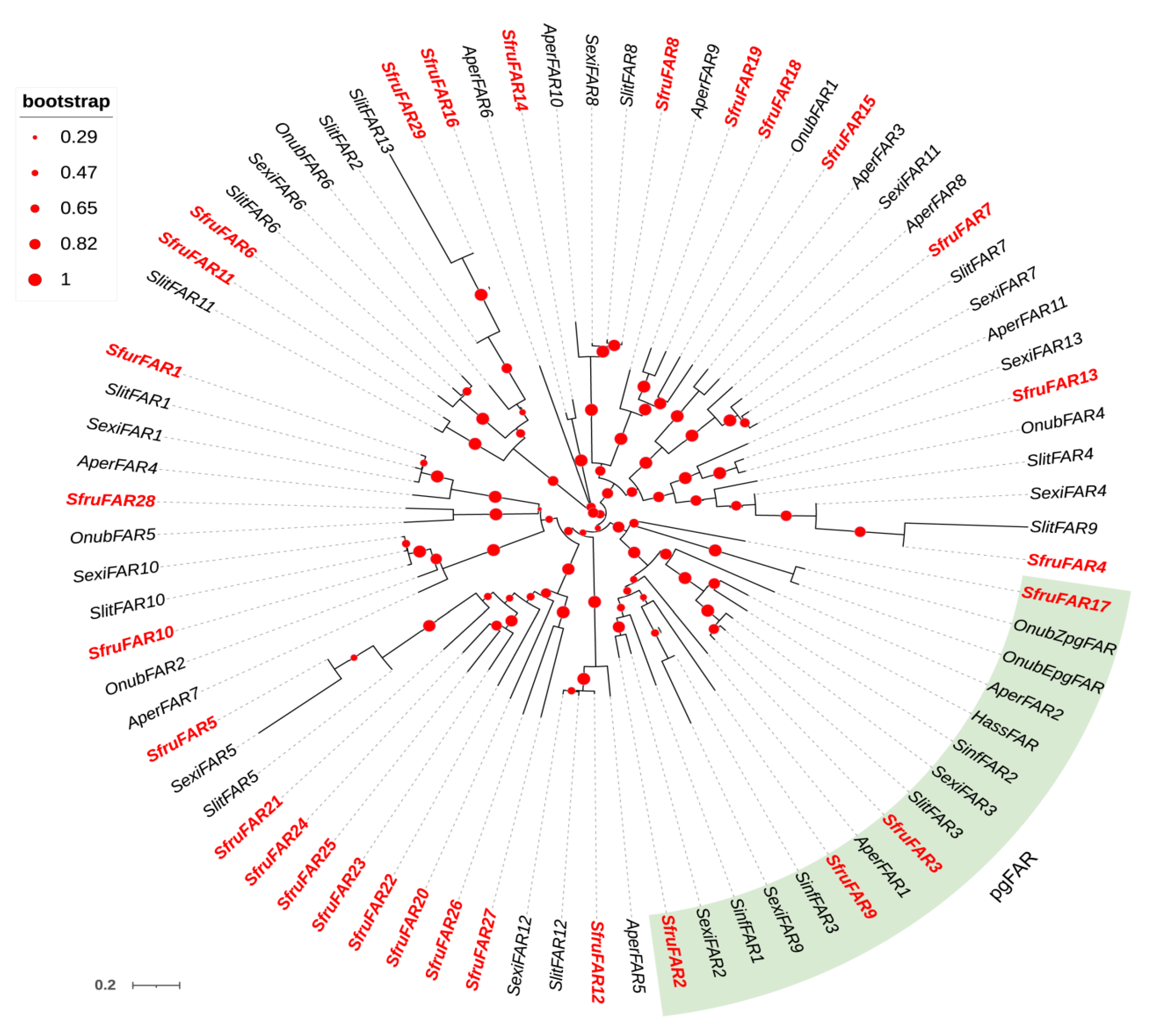
3.2. Phylogenetic Analyses of DESs and FARs
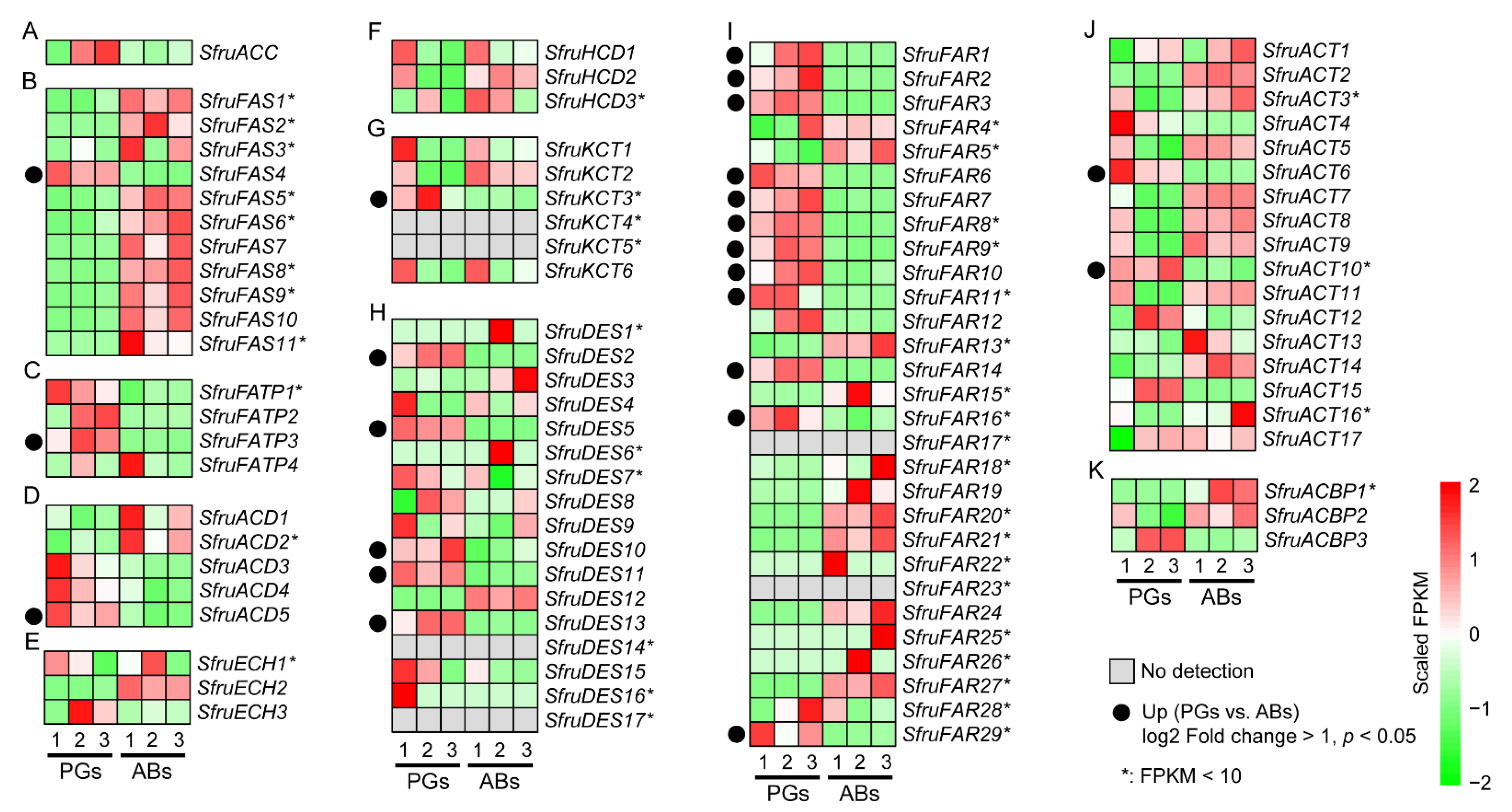
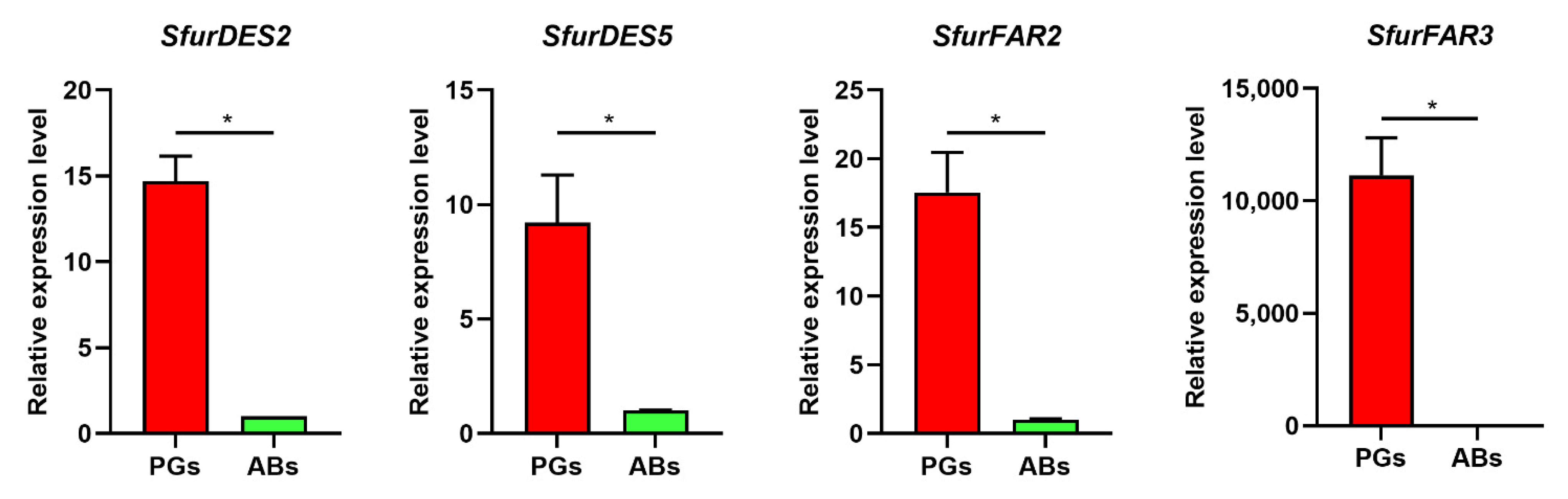
3.3. Expression Profile of Sex Pheromone Biosynthesis Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jurenka, R. Regulation of pheromone biosynthesis in moths. Curr. Opin. Insect Sci. 2017, 24, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Thanasirungkul, W.; Aslam, A.; Niu, F.; Guo, H.-R.; Chi, D.-F. Genes involved in the Type I pheromone biosynthesis pathway and chemoreception from the sex pheromone gland transcriptome of Dioryctria abietella. Comp. Biochem. Physiol. Part D: Genom. Proteom. 2021, 40, 100892. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Xia, Y.-H.; Zhu, J.-Y.; Li, S.-Y.; Dong, S.-L. Putative Pathway of Sex Pheromone Biosynthesis and Degradation by Expression Patterns of Genes Identified from Female Pheromone Gland and Adult Antenna of Sesamia inferens (Walker). J. Chem. Ecol. 2014, 40, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-B.; Yin, N.-N.; Long, J.-M.; Zhang, Y.-K.; Liu, N.-Y.; Zhu, J.-Y. Transcriptome analysis of the pheromone glands in Noorda blitealis reveals a novel AOX group of the superfamily Pyraloidea. J. Asia-Pac. Èntomol. 2021, 24, 110–119. [Google Scholar] [CrossRef]
- Ando, T.; Yamakawa, R. Analyses of lepidopteran sex pheromones by mass spectrometry. Trends Anal. Chem. 2011, 30, 990–1002. [Google Scholar] [CrossRef]
- Ando, T.; Inomata, S.; Yamamoto, M. Lepidopteran Sex Pheromones. Topics in Current Chemistry; Springer: Cham, Switzerland, 2004; pp. 51–96. [Google Scholar]
- Jurenka, R. Insect Pheromone Biosynthesis. Topics in Current Chemistry; Springer: Cham, Switzerland, 2004; pp. 97–131. [Google Scholar]
- Zhang, Y.-N.; Zhu, X.-Y.; Fang, L.-P.; He, P.; Wang, Z.-Q.; Chen, G.; Sun, L.; Ye, Z.-F.; Deng, D.-G.; Li, J.-B. Identification and Expression Profiles of Sex Pheromone Biosynthesis and Transport Related Genes in Spodoptera litura. PLoS ONE 2015, 10, e0140019. [Google Scholar] [CrossRef]
- Lin, X.; Wang, B.; Du, Y. Key genes of the sex pheromone biosynthesis pathway in female moths are required for pheromone quality and possibly mediate olfactory plasticity in conspecific male moths in Spodoptera litura. Insect Mol. Biol. 2018, 27, 8–21. [Google Scholar] [CrossRef]
- Yang, Y.C.; Tao, J.; Zong, S.X. Identification of putative Type-I sex pheromone biosynthesis-related genes expressed in the female pheromone gland of Streltzoviella insularis. PLoS ONE 2020, 15, e0227666. [Google Scholar] [CrossRef]
- Moto, K.; Yoshiga, T.; Yamamoto, M.; Takahashi, S.; Okano, K.; Ando, T.; Nakata, T.; Matsumoto, S. Pheromone gland-specific fatty-acyl reductase of the silkmoth, Bombyx mori. Proc. Natl. Acad. Sci. USA 2003, 100, 9156–9161. [Google Scholar] [CrossRef]
- Ohnishi, A.; Hashimoto, K.; Imai, K.; Matsumoto, S. Functional Characterization of the Bombyx mori Fatty Acid Transport Protein (BmFATP) within the Silkmoth Pheromone Gland. J. Biol. Chem. 2009, 284, 5128–5136. [Google Scholar] [CrossRef]
- Jing, W.; Huang, C.; Li, C.Y.; Zhou, H.X.; Ren, Y.L.; Li, Z.Y.; Xing, L.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Suby, S.B.; Soujanya, P.L.; Yadava, P.; Patil, J.; Subaharan, K.; Prasad, G.S.; Babu, K.S.; Jat, S.L.; Yathish, K.R.; Vadassery, J.; et al. Invasion of Fall Armyworm (Spodoptera frugiperda) in India: Nature, Distribution, Management and Potential Impact. Curr. Sci. 2020, 119, 44–51. [Google Scholar] [CrossRef]
- Tepa-Yotto, G.; Chinwada, P.; Rwomushana, I.; Goergen, G.; Subramanian, S. Integrated management of Spodoptera frugiperda 6 d years post detection in Africa: A review. Curr. Opin. Insect Sci. 2022, 50, 100928. [Google Scholar] [CrossRef]
- Jia, H.-R.; Guo, J.-L.; Wu, Q.-L.; Hu, C.-X.; Li, X.-K.; Zhou, X.-Y.; Wu, K.-M. Migration of invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) across the Bohai Sea in northern China. J. Integr. Agric. 2021, 20, 685–693. [Google Scholar] [CrossRef]
- Wu, F.F.; Zhang, L.; Liu, Y.Q.; Cheng, Y.X.; Su, J.Y.; Sappington, T.W.; Jiang, X.F. Population Development, Fecundity, and Flight of Spodoptera frugiperda (Lepidoptera: Noctuidae) Reared on Three Green Manure Crops: Implications for an Ecologically Based Pest Management Approach in China. J. Econ. Èntomol. 2022, 115, 693. [Google Scholar] [CrossRef]
- Cruz, I.; de Lourdes, M.; Figueiredo, C.; da Silva, R.B.; da Silva, I.F.; Paula, C.D.; Foster, J.E. Using sex pheromone traps in the decision-making process for pesticide application against fall armyworm (Spodoptera frugiperda [Smith] [Lepidoptera: Noctuidae]) larvae in maize. Int. J. Pest Manag. 2012, 58, 83–90. [Google Scholar] [CrossRef]
- Meagher, R.L.; Nagoshi, R.N.; Armstrong, J.S.; Niogret, J.; Epsky, N.D.; Flanders, K.L. Captures and Host Strains of Fall Armyworm (Lepidoptera: Noctuidae) Males in Traps Baited with Different Commercial Pheromone Blends. Fla. Èntomol. 2013, 96, 729–740. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Tumlinson, J.H.; McNeil, J.N. Field Evaluation of Commercial Pheromone Formulations and Traps Using a More Effective Sex Pheromone Blend for the Fall Armyworm (Lepidoptera: Noctuidae)1. J. Econ. Èntomol. 1985, 78, 1364–1369. [Google Scholar] [CrossRef]
- Rojas, J.C.; Virgen, A.; Malo, E.A. Seasonal and nocturnal flight activity of Spodoptera frugiperda males (Lepidoptera: Noctuidae) monitored by pheromone traps in the Coast of Chiapas, Mexico. Fla. Èntomol. 2004, 87, 496–503. [Google Scholar] [CrossRef]
- Cruz-Esteban, S. Antennal sensitivity to female sex pheromone compounds of Spodoptera frugiperda males (Lepidoptera: Noctuidae) and associated field behaviour. Physiol. Èntomol. 2020, 45, 140–146. [Google Scholar] [CrossRef]
- Sekul, A.A.; Sparks, A.N. Sex Pheromone of the Fall Armyworm Moth: Isolation, Identification, and Synthesis. J. Econ. Èntomol. 1967, 60, 1270–1272. [Google Scholar] [CrossRef]
- Tumlinson, J.H.; Mitchell, E.R.; Teal, P.E.A.; Heath, R.R.; Mengelkoch, L.J. Sex pheromone of fall armyworm, Spodoptera frugiperda (J.E. Smith). J. Chem. Ecol. 1986, 12, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Batista-Pereira, L.G.; Stein, K.; De Paula, A.F.; Moreira, J.A.; Cruz, I.; Figueiredo, M.D.L.C.; Perri, J., Jr.; Corrêa, A.G. Isolation, Identification, Synthesis, and Field Evaluation of the Sex Pheromone of the Brazilian Population of Spodoptera frugiperda. J. Chem. Ecol. 2006, 32, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.T.; Marr, M.; Schöfl, G.; Lorenz, S.; Svatos, A.; Heckel, D.G. Host strain specific sex pheromone variation in Spodoptera frugiperda. Front. Zool. 2008, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Meagher, R.L.; Nagoshi, R.N. Attraction of Fall Armyworm Males (Lepidoptera: Noctuidae) to Host Strain Females. Environ. Èntomol. 2013, 42, 751–757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, N.J.; Mo, B.T.; Guo, H.; Yang, J.; Tang, R.; Wang, C.Z. Revisiting the sex pheromone of the fall armyworm Spodoptera frugiperda, a new invasive pest in South China. Insect Sci. 2021, 29, 865–878. [Google Scholar] [CrossRef]
- Unbehend, M.; Hänniger, S.; Meagher, R.L.; Heckel, D.G.; Groot, A.T. Pheromonal Divergence Between Two Strains of Spodoptera frugiperda. J. Chem. Ecol. 2013, 39, 364–376. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, L.-W.; Chen, D.-S.; Sun, L.; Li, Z.-Q.; Ye, Z.-F.; Zheng, M.-Y.; Li, J.-B.; Zhu, X.-Y. Molecular identification of differential expression genes associated with sex pheromone biosynthesis in Spodoptera exigua. Mol. Genet. Genom. 2017, 292, 795–809. [Google Scholar] [CrossRef]
- Li, R.-T.; Ning, C.; Huang, L.-Q.; Dong, J.-F.; Li, X.; Wang, C.-Z. Expressional divergences of two desaturase genes determine the opposite ratios of two sex pheromone components in Helicoverpa armigera and Helicoverpa assulta. Insect Biochem. Mol. Biol. 2017, 90, 90–100. [Google Scholar] [CrossRef]
- Ding, B.-J.; Löfstedt, C. Analysis of the agrotis segetum pheromone gland transcriptome in the light of Sex pheromone biosynthesis. BMC Genom. 2015, 16, 711. [Google Scholar] [CrossRef]
- Gu, S.-H.; Wu, K.-M.; Guo, Y.-Y.; Pickett, J.A.; Field, L.M.; Zhou, J.-J.; Zhang, Y.-J. Identification of genes expressed in the sex pheromone gland of the black cutworm Agrotis ipsilon with putative roles in sex pheromone biosynthesis and transport. BMC Genom. 2013, 14, 636. [Google Scholar] [CrossRef]
- Vogel, H.; Heidel, A.J.; Heckel, D.G.; Groot, A.T. Transcriptome analysis of the sex pheromone gland of the noctuid moth Heliothis virescens. BMC Genom. 2010, 11, 29. [Google Scholar] [CrossRef]
- Dou, X.Y.; Liu, S.J.; Ahn, S.-J.; Choi, M.-Y.; Jurenka, R. Transcriptional comparison between pheromone gland-ovipositor and tarsi in the corn earworm moth Helicoverpa zea. Comp. Biochem. Physiol. Part D: Genom. Proteom. 2019, 31, 100604. [Google Scholar] [CrossRef]
- Liu, J.Q.; Li, S.L.; Li, W.S.; Peng, L.; Chen, Z.W.; Xiao, Y.D.; Gu, H.Z.; Zhang, J.W.; Cheng, T.C.; Goldsmith, M.R.; et al. Genome-wide annotation and comparative analysis of cuticular protein genes in the noctuid pest Spodoptera litura. Insect Biochem. Mol. Biol. 2019, 110, 90–97. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhang, F.; Tay, W.T.; Robin, C.; Shi, Y.; Guan, F.; Yang, Y.H.; Wu, Y.D. Population genomics provides insights into lineage divergence and local adaptation within the cotton bollworm. Mol. Ecol. Resour. 2022, 22, 1875–1891. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Zhang, S.; Luo, J.-Y.; Wang, C.-Y.; Lv, L.-M.; Dong, S.-L.; Cui, J.-J. Transcriptome comparison of the sex pheromone glands from two sibling Helicoverpa species with opposite sex pheromone components. Sci. Rep. 2015, 5, 9324. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Gong, Q.; Fang, S.-M.; Liu, Y.-Q.; Zhang, Z.; Yu, Q.-Y. Identification of genes involved in sex pheromone biosynthesis and metabolic pathway in the Chinese oak silkworm, Antheraea pernyi. Int. J. Biol. Macromol. 2020, 163, 1487–1497. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, J.Y.; Ruan, H.Y.; Yang, C.L.; Zhang, C.Y. Expression stability of candidate RT-qPCR housekeeping genes in Spodoptera frugiperda (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2021, 108, e21831. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Tillman, J.A.; Seybold, S.J.; Jurenka, R.A.; Blomquist, G.J. Insect pheromones—An overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 1999, 29, 481–514. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.T.; Dekker, T.; Heckel, D.G. The Genetic Basis of Pheromone Evolution in Moths. Annu. Rev. Èntomol. 2016, 61, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Alabaster, A.; Isoe, J.; Zhou, G.L.; Lee, A.; Murphy, A.; Day, W.A.; Miesfeld, R.L. Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell formation and blood meal digestion in Aedes aegypti. Insect Biochem. Mol. Biol. 2011, 41, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-Y.; Jurenka, R.A. C75, a Fatty Acid Synthase Inhibitor, Inhibits Feeding Activity and Pheromone Production in a Moth, Helicoverpa zea. J. Asia-Pac. Èntomol. 2006, 9, 43–48. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Hwarari, D.T.; Liang, X.-H.; Ding, J.-H.; Yan, M.W.; Wu, F.A.; Wang, J.; Sheng, S. Fatty acid synthases and desaturases are essential for the biosynthesis of alpha-linolenic acid and metamorphosis in a major mulberry pest, Glyphodes pyloalis walker (Lepidoptera: Pyralidae). Pest Manag. Sci. 2022, 78, 2629–2642. [Google Scholar] [CrossRef]
- Qian, S.G.; Fujii, T.; Ito, K.; Nakano, R.; Ishikawa, Y. Cloning and functional characterization of a fatty acid transport protein (FATP) from the pheromone gland of a lichen moth, Eilema japonica, which secretes an alkenyl sex pheromone. Insect Biochem. Mol. Biol. 2011, 41, 22–28. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Zhou, Z.-F.; Jia, J.-Q.; Gui, Z.-Z. Cloning, expression and functional analysis of a delta 6-desaturase gene from the silkworm, Bombyx mori L. J. Asia-Pacific Èntomol. 2016, 19, 581–587. [Google Scholar] [CrossRef]
- Hagström, K.; Albre, J.; Tooman, L.K.; Thirmawithana, A.H.; Corcoran, J.; Löfstedt, C.; Newcomb, R.D. A Novel Fatty Acyl Desaturase from the Pheromone Glands of Ctenopseustis obliquana and C. herana with Specific Z5-Desaturase Activity on Myristic Acid. J. Chem. Ecol. 2014, 40, 63–70. [Google Scholar] [CrossRef]
- Xia, Y.-H.; Zhang, Y.-N.; Ding, B.-J.; Wang, H.-L.; Löfstedt, C. Multi-Functional Desaturases in Two Spodoptera Moths with ∆11 and ∆12 Desaturation Activities. J. Chem. Ecol. 2019, 45, 378–387. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Zhang, X.-Q.; Zhu, G.H.; Zheng, M.-Y.; Yan, Q.; Zhu, X.-Y.; Xu, J.-W.; Zhang, Y.-Y.; He, P.; Sun, L.; et al. A Δ9 desaturase (SlitDes11) is associated with the biosynthesis of ester sex pheromone components in Spodoptera litura. Pestic. Biochem. Physiol. 2019, 156, 152–159. [Google Scholar] [CrossRef]
- Fujii, T.; Ito, K.; Tatematsu, M.; Shimada, T.; Katsuma, S.; Ishikawa, Y. Sex pheromone desaturase functioning in a primitive Ostrinia moth is cryptically conserved in congeners’ genomes. Proc. Natl. Acad. Sci. USA 2011, 108, 7102–7106. [Google Scholar] [CrossRef]
- Hagström, A.K.; Liénard, M.A.; Groot, A.T.; Hedenström, E.; Löfstedt, C. Semi–Selective Fatty Acyl Reductases from Four Heliothine Moths Influence the Specific Pheromone Composition. PLoS ONE 2012, 7, e37230. [Google Scholar] [CrossRef]
- Jurenka, R.A.; Roelofs, W.L. Characterization of the acetyltransferase used in pheromone biosynthesis in moths: Specificity for the Z isomer in tortricidae. Insect Biochem. 1989, 19, 639–644. [Google Scholar] [CrossRef]
- Fujii, T.; Ito, K.; Katsuma, S.; Nakano, R.; Shimada, T.; Ishikawa, Y. Molecular and functional characterization of an acetyl-CoA acetyltransferase from the adzuki bean borer moth Ostrinia scapulalis (Lepidoptera: Crambidae). Insect Biochem. Mol. Biol. 2010, 40, 74–78. [Google Scholar] [CrossRef]
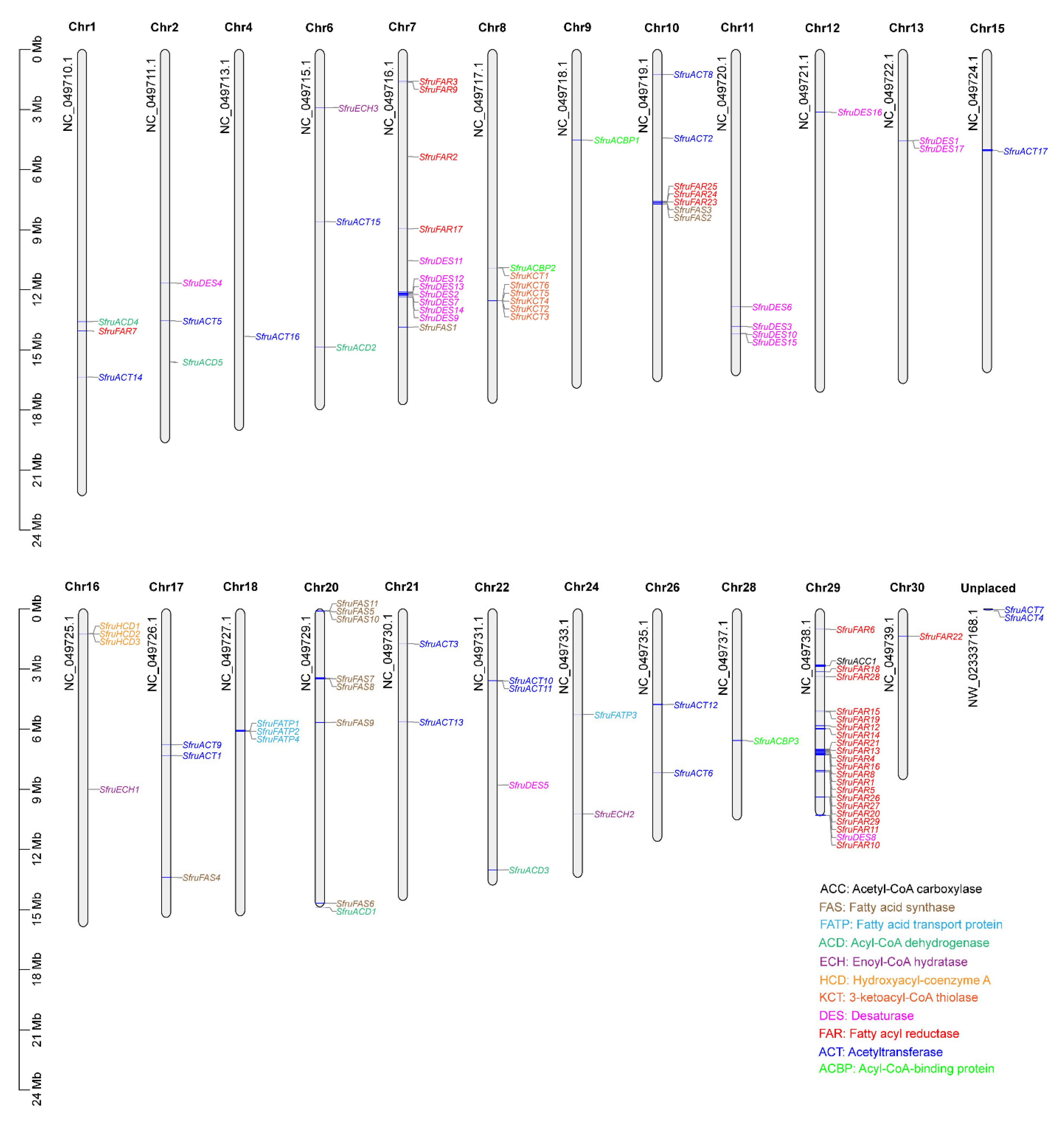
| Gene Name | Gene ID | ORF (aa) | Gene_chr | Gene Start | Gene End | Best BlastX Match | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene Name | Acc.no. | Species | E Value | Identity (%) | ||||||
| Acetyl-CoA carboxylase (ACC) | ||||||||||
| SfruACC1 | LOC118280410 | 2385 | NC_049738.1 | 2,772,652 | 2,888,149 | acetyl-CoA carboxylase | XP_022824105.1 | Spodoptera litura | 0.0 | 99.20 |
| Fatty acid synthase (FAS) | ||||||||||
| SfruFAS1 | LOC118265122 | 1503 | NC_049716.1 | 13,860,534 | 13,873,617 | fatty acid synthase-like | XP_035448777.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS2 | LOC118267601 | 2337 | NC_049719.1 | 7,708,838 | 7,745,487 | fatty acid synthase-like | XP_035437568.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS3 | LOC118267741 | 2328 | NC_049719.1 | 7,644,074 | 7,683,559 | fatty acid synthase-like | XP_035437791.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS4 | LOC118272899 | 1286 | NC_049726.1 | 13,387,368 | 13,397,181 | fatty acid synthase | AGR49310.1 | Agrotis ipsilon | 0.0 | 85.25 |
| SfruFAS5 | LOC118274970 | 2384 | NC_049729.1 | 88,602 | 113,004 | fatty acid synthase-like | XP_035448683.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS6 | LOC118275042 | 1580 | NC_049729.1 | 14,669,977 | 14,692,392 | fatty acid synthase-like | XP_035448779.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS7 | LOC118274634 | 2422 | NC_049729.1 | 3,425,920 | 3,481,831 | fatty acid synthase-like | XP_035448162.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS8 | LOC118274778 | 2348 | NC_049729.1 | 3,486,227 | 3,504,960 | fatty acid synthase-like | XP_035448370.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS9 | LOC118274999 | 2276 | NC_049729.1 | 5,659,108 | 5,676,788 | fatty acid synthase-like | XP_035448720.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAS10 | LOC118274601 | 2321 | NC_049729.1 | 117,349 | 133,649 | fatty acid synthase-like | XP_047033130.1 | Helicoverpa zea | 0.0 | 68.91 |
| SfruFAS11 | LOC118274969 | 294 | NC_049729.1 | 84,898 | 87,981 | fatty acid synthase-like | XP_035448682.1 | Spodoptera frugiperda | 0.0 | 100 |
| Fatty acid transport protein (FATP) | ||||||||||
| SfruFATP1 | LOC118273519 | 700 | NC_049727.1 | 6,041,998 | 6,085,508 | fatty acid transport protein | ARD71229.1 | Spodoptera exigua | 0.0 | 96.43 |
| SfruFATP2 | LOC118273025 | 651 | NC_049727.1 | 6,086,086 | 6,101,822 | fatty acid transport protein | ARD71230.1 | Spodoptera exigua | 0.0 | 98.00 |
| SfruFATP3 | LOC118277430 | 661 | NC_049733.1 | 5,268,282 | 5,285,638 | fatty acid transport protein | ARD71231.1 | Spodoptera exigua | 0.0 | 96.37 |
| SfruFATP4 | LOC118273026 | 643 | NC_049727.1 | 6,103,562 | 6,131,706 | fatty acid transport protein | ARD71232.1 | Spodoptera exigua | 0.0 | 98.29 |
| Acyl-CoA dehydrogenase (ACD) (β-oxidation enzyme) | ||||||||||
| SfruACD1 | LOC118274515 | 408 | NC_049729.1 | 14,890,759 | 14,893,193 | short-chain specific acyl-CoA dehydrogenase, mitochondrial | XP_022831790.1 | Spodoptera litura | 0.0 | 99.02 |
| SfruACD2 | LOC118264809 | 410 | NC_049715.1 | 14,866,408 | 14,875,265 | acyl-CoA dehydrogenase | QZC92122.1 | Dioryctria abietella | 0.0 | 72.68 |
| SfruACD3 | LOC118276297 | 418 | NC_049731.1 | 13,012,363 | 13,027,495 | short/branched-chain specific acyl-CoA dehydrogenase, mitochondrial | XP_022827910.1 | Spodoptera litura | 0.0 | 99.28 |
| SfruACD4 | LOC118265626 | 422 | NC_049710.1 | 13,581,976 | 13,599,130 | putative medium-chain specific acyl-CoA dehydrogenase | AID66670.1 | Agrotis segetum | 0.0 | 96.45 |
| SfruACD5 | LOC118278275 | 626 | NC_049711.1 | 15,605,655 | 15,609,993 | very long-chain specific acyl-CoA dehydrogenase, mitochondrial | XP_047020249.1 | Helicoverpa zea | 0.0 | 89.97 |
| Enoyl-CoA hydratase (ECH) (β-oxidation enzyme) | ||||||||||
| SfruECH1 | LOC118271967 | 278 | NC_049725.1 | 8,995,073 | 8,998,854 | enoyl-CoA hydratase domain-containing protein 3, mitochondrial-like | XP_035444142.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruECH2 | LOC118277211 | 343 | NC_049733.1 | 10,231,633 | 10,237,455 | putative enoyl-CoA hydratase | AID66686.1 | Agrotis segetum | 0.0 | 77.42 |
| SfruECH3 | LOC118264501 | 298 | NC_049715.1 | 2,819,343 | 2,822,884 | enoyl-CoA hydratase domain-containing protein 2, mitochondrial-like | XP_047024252.1 | Helicoverpa zea | 5 × 10−180 | 89.23 |
| Hydroxyacyl-coenzyme A (HCD) (β-oxidation enzyme) | ||||||||||
| SfruHCD1 | LOC118272082 | 313 | NC_049725.1 | 1,232,063 | 1,234,251 | hydroxyacyl-coenzyme A dehydrogenase, mitochondrial-like | XP_035451823.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruHCD2 | LOC118272083 | 307 | NC_049725.1 | 1,235,137 | 1,239,192 | putative 3-hydroxyacyl-CoA dehydrogenase | AID66695.1 | Agrotis segetum | 0.0 | 94.14 |
| SfruHCD3 | LOC118272085 | 80 | NC_049725.1 | 1,234,695 | 1,242,746 | hydroxyacyl-coenzyme A dehydrogenase, mitochondrial-like | XP_022823313.1 | Spodoptera litura | 3 × 10−49 | 100 |
| 3-ketoacyl-CoA thiolase (KCT) (β-oxidation enzyme) | ||||||||||
| SfruKCT1 | LOC118266029 | 396 | NC_049717.1 | 10,904,839 | 10,911,237 | 3-ketoacyl-CoA thiolase, mitochondrial-like | XP_035434809.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruKCT2 | LOC118266244 | 400 | NC_049717.1 | 12,544,565 | 12,548,651 | 3-ketoacyl-CoA thiolase, mitochondrial-like | XP_035435538.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruKCT3 | LOC118266444 | 398 | NC_049717.1 | 12,548,954 | 12,551,900 | 3-ketoacyl-CoA thiolase, mitochondrial-like | XP_035435800.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruKCT4 | LOC118266245 | 394 | NC_049717.1 | 12,538,512 | 12,544,434 | 3-ketoacyl-CoA thiolase, mitochondrial-like | XP_035435539.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruKCT5 | LOC118266304 | 396 | NC_049717.1 | 12,535,796 | 12,538,103 | 3-ketoacyl-CoA thiolase, mitochondrial-like | XP_035435600.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruKCT6 | LOC118265763 | 396 | NC_049717.1 | 12,527,729 | 12,534,164 | 3-ketoacyl-CoA thiolase, mitochondrial-like | XP_035434809.1 | Spodoptera frugiperda | 0.0 | 100 |
| Desaturase (DES) | ||||||||||
| SfruDES1 | LOC118269923 | 379 | NC_049722.1 | 4,551,252 | 4,554,527 | acyl-CoA desaturase 1-like | XP_035441201.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruDES2 | LOC118264925 | 375 | NC_049716.1 | 12,167,231 | 12,217,589 | desaturase | AAQ74260.1 | Spodoptera littoralis | 0.0 | 96.54 |
| SfruDES3 | LOC118268438 | 444 | NC_049720.1 | 13,813,813 | 13,831,130 | desaturase | ARD71179.1 | Spodoptera exigua | 0.0 | 91.22 |
| SfruDES4 | LOC118274250 | 336 | NC_049711.1 | 11,647,117 | 11,651,688 | desaturase | ARD71180.1 | Spodoptera exigua | 0.0 | 98.21 |
| SfruDES5 | LOC118276125 | 339 | NC_049731.1 | 8,780,609 | 8,784,814 | delta 11 desaturase | AGH12217.1 | Spodoptera litura | 0.0 | 91.15 |
| SfruDES6 | LOC118268131 | 322 | NC_049720.1 | 12,847,464 | 12,851,906 | acyl-CoA Delta(11) desaturase-like | XP_035438325.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruDES7 | LOC118264926 | 371 | NC_049716.1 | 12,252,078 | 12,286,714 | stearoyl-CoA desaturase 5-like | XP_035433487.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruDES8 | LOC118280447 | 321 | NC_049738.1 | 9,365,125 | 9,401,887 | putative desaturase des8 | ALJ30231.1 | Spodoptera litura | 0.0 | 97.51 |
| SfruDES9 | LOC118264929 | 354 | NC_049716.1 | 12,362,286 | 12,378,777 | desaturase | ARD71183.1 | Spodoptera exigua | 0.0 | 98.02 |
| SfruDES10 | LOC118268107 | 390 | NC_049720.1 | 14,173,733 | 14,179,282 | desaturase | ARD71184.1 | Spodoptera exigua | 2 × 10−151 | 95.43 |
| SfruDES11 | LOC118264952 | 377 | NC_049716.1 | 10,539,212 | 10,545,018 | acyl-CoA Delta(11) desaturase-like | XP_035433515.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruDES12 | LOC118264931 | 358 | NC_049716.1 | 12,104,709 | 12,122,940 | desaturase | ARD71185.1 | Spodoptera exigua | 0.0 | 92.12 |
| SfruDES13 | LOC118264927 | 369 | NC_049716.1 | 12,146,558 | 12,164,738 | acyl-CoA Delta(11) desaturase-like | XP_035433488.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruDES14 | LOC118264933 | 340 | NC_049716.1 | 12,346,231 | 12,349,171 | acyl-CoA Delta(11) desaturase-like | XP_035433497.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruDES15 | LOC118268104 | 453 | NC_049720.1 | 14,186,781 | 14,189,958 | Desaturase | KOB71313.1 | Operophtera brumata | 0.0 | 64.52 |
| SfruDES16 | LOC118269215 | 360 | NC_049721.1 | 3,116,248 | 3,129,873 | acyl-CoA Delta(11) desaturase-like | XP_035440106.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruDES17 | LOC118269922 | 396 | NC_049722.1 | 4,554,971 | 4,557,910 | acyl-CoA Delta(11) desaturase-like | XP_035441200.1 | Spodoptera frugiperda | 0.0 | 100 |
| Fatty acyl reductase (FAR) | ||||||||||
| SfruFAR1 | LOC118280370 | 535 | NC_049738.1 | 7,174,208 | 7,214,859 | fatty acyl reductase | ARD71186.1 | Spodoptera exigua | 0.0 | 96.07 |
| SfruFAR2 | LOC118265382 | 462 | NC_049716.1 | 5,347,090 | 5,355,248 | fatty acyl reductase | ARD71187.1 | Spodoptera exigua | 0.0 | 82.93 |
| SfruFAR3 | LOC118265592 | 454 | NC_049716.1 | 1,589,115 | 1,598,988 | putative fatty acyl reductase FAR3 | ALJ30237.1 | Spodoptera litura | 0.0 | 93.83 |
| SfruFAR4 | LOC118280550 | 498 | NC_049738.1 | 7,051,936 | 7,079,859 | fatty acyl-CoA reductase 13 | AKD01774.1 | Helicoverpa armigera | 0.0 | 88.76 |
| SfruFAR5 | LOC118280458 | 525 | NC_049738.1 | 7,234,148 | 7,242,711 | putative fatty acyl reductase FAR5 | ALJ30239.1 | Spodoptera litura | 0.0 | 94.26 |
| SfruFAR6 | LOC118280491 | 520 | NC_049738.1 | 1,004,955 | 1,024,481 | fatty acyl reductase | ARD71191.1 | Spodoptera exigua | 0.0 | 85.38 |
| SfruFAR7 | LOC118263342 | 520 | NC_049710.1 | 14,027,024 | 14,057,897 | fatty acyl reductase | ARD71192.1 | Spodoptera exigua | 0.0 | 89.62 |
| SfruFAR8 | LOC118280282 | 526 | NC_049738.1 | 7,122,361 | 7,154,556 | fatty acyl-CoA reductase 1-like | XP_035456141.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR9 | LOC118265401 | 490 | NC_049716.1 | 1,634,217 | 1,641,393 | fatty acyl-CoA reductase 1-like | XP_035434130.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR10 | LOC118280227 | 624 | NC_049738.1 | 10,266,635 | 10,318,251 | putative fatty acyl reductase FAR10 | ALJ30244.1 | Spodoptera litura | 0.0 | 95.83 |
| SfruFAR11 | LOC118280439 | 510 | NC_049738.1 | 8,122,362 | 8,131,120 | fatty acyl-CoA reductase 2 | AKD01763.1 | Helicoverpa armigera | 0.0 | 56.24 |
| SfruFAR12 | LOC118280219 | 523 | NC_049738.1 | 5,814,548 | 5,844,700 | fatty acyl-CoA reductase 1-like | XP_047038603.1 | Helicoverpa zea | 0.0 | 68.84 |
| SfruFAR13 | LOC118280549 | 520 | NC_049738.1 | 7,017,686 | 7,037,406 | putative fatty acyl-CoA reductase CG5065 | XP_035456531.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR14 | LOC118280222 | 528 | NC_049738.1 | 5,956,001 | 6,001,728 | fatty acyl-CoA reductase 8 | QLI61998.1 | Streltzoviella insularis | 0.0 | 86.39 |
| SfruFAR15 | LOC118280297 | 512 | NC_049738.1 | 5,092,043 | 5,105,984 | fatty acyl reductase 15 | ATJ44470.1 | Helicoverpa armigera | 0.0 | 78.52 |
| SfruFAR16 | LOC118280293 | 531 | NC_049738.1 | 7,103,707 | 7,121,330 | fatty acyl reductase 16 | ATJ44527.1 | Helicoverpa assulta | 0.0 | 89.29 |
| SfruFAR17 | LOC118265535 | 450 | NC_049716.1 | 8,947,008 | 8,959,114 | putative fatty acyl-CoA reductase CG5065 | XP_035434352.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR18 | LOC118280263 | 511 | NC_049738.1 | 3,127,028 | 3,152,947 | fatty acyl reductase 12 | ATJ44526.1 | Helicoverpa assulta | 0.0 | 75.15 |
| SfruFAR19 | LOC118280403 | 513 | NC_049738.1 | 5,124,444 | 5,137,543 | fatty acyl-CoA reductase wat-like | XP_035456297.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR20 | LOC118280192 | 523 | NC_049738.1 | 7,287,590 | 7,295,954 | fatty acyl-CoA reductase wat-like | XP_035455970.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR21 | LOC118280547 | 542 | NC_049738.1 | 6,979,095 | 6,990,649 | fatty acyl reductase 13 | ATJ44468.1 | Helicoverpa armigera | 0.0 | 66.73 |
| SfruFAR22 | LOC118280723 | 537 | NC_049739.1 | 1,366,062 | 1,372,472 | fatty acyl-CoA reductase wat-like | XP_035456945.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR23 | LOC118267621 | 535 | NC_049719.1 | 7,599,750 | 7,608,971 | fatty acyl-CoA reductase wat-like | XP_035437588.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR24 | LOC118267780 | 538 | NC_049719.1 | 7,588,680 | 7,597,048 | fatty acyl-CoA reductase 1-like | XP_035438040.1 | Spodoptera frugiperda | 0.0 | 69.60 |
| SfruFAR25 | LOC118267895 | 548 | NC_049719.1 | 7,574,852 | 7,582,604 | fatty acyl-CoA reductase 1-like | XP_035438040.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR26 | LOC118280456 | 538 | NC_049738.1 | 7,251,597 | 7,260,794 | fatty acyl-CoA reductase wat-like | XP_035456388.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR27 | LOC118280298 | 530 | NC_049738.1 | 7,271,670 | 7,283,596 | fatty acyl-CoA reductase wat-like | XP_035456154.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruFAR28 | LOC118280321 | 539 | NC_049738.1 | 3,362,799 | 3,380,634 | fatty acyl reductase 5 | ATJ44463.1 | Helicoverpa armigera | 0.0 | 88.27 |
| SfruFAR29 | LOC118280434 | 538 | NC_049738.1 | 8,055,508 | 8,085,920 | fatty acyl reductase | AID66647.1 | Agrotis segetum | 0.0 | 80.76 |
| Acetyltransferase (ACT) | ||||||||||
| SfruACT1 | LOC118272936 | 252 | NC_049726.1 | 7,310,246 | 7,316,764 | N-alpha-acetyltransferase 60-like | XP_035445570.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruACT2 | LOC118267998 | 176 | NC_049719.1 | 4,426,976 | 4,431,010 | probable N-acetyltransferase san | XP_022826510.1 | Spodoptera litura | 1 × 10−127 | 100 |
| SfruACT3 | LOC118275415 | 199 | NC_049730.1 | 1,741,133 | 1,742,303 | N-alpha-acetyltransferase 80-like | XP_035449252.1 | Spodoptera frugiperda | 8 × 10−143 | 100 |
| SfruACT4 | LOC118281885 | 107 | NW_023337168.1 | 95,711 | 96,749 | N-alpha-acetyltransferase 38-B | XP_022826548.1 | Spodoptera litura | 8 × 10−66 | 91.35 |
| SfruACT5 | LOC118272777 | 710 | NC_049711.1 | 13,544,018 | 13,554,256 | N-alpha-acetyltransferase 35, NatC auxiliary subunit | XP_028155763.1 | Ostrinia furnacalis | 0.0 | 91.85 |
| SfruACT6 | LOC118278521 | 469 | NC_049735.1 | 8,174,301 | 8,181,092 | putative acetyltransferase ACT9 | ALJ30256.1 | Spodoptera litura | 6 × 10−178 | 99.25 |
| SfruACT7 | LOC118281877 | 294 | NW_023337168.1 | 41,788 | 43,450 | N-alpha-acetyltransferase 30-like | XP_035458539.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruACT8 | LOC118267522 | 173 | NC_049719.1 | 1,242,030 | 1,243,082 | N-alpha-acetyltransferase 20 | XP_022826734.1 | Spodoptera litura | 9 × 10−127 | 100 |
| SfruACT9 | LOC118272523 | 180 | NC_049726.1 | 6,776,065 | 6,778,854 | N-alpha-acetyltransferase 10 | XP_022830428.1 | Spodoptera litura | 6 × 10−132 | 99.44 |
| SfruACT10 | LOC118276179 | 396 | NC_049731.1 | 3,555,474 | 3,572,627 | acetyltransferase | ARD71213.1 | Spodoptera exigua | 0.0 | 98.49 |
| SfruACT11 | LOC118276445 | 512 | NC_049731.1 | 3,602,442 | 3,605,363 | fatty alcohol acetyltransferase | AIN34682.1 | Agrotis segetum | 0.0 | 88.85 |
| SfruACT12 | LOC118278776 | 479 | NC_049735.1 | 4,751,837 | 4,791,014 | acetyltransferase | ARD71206.1 | Spodoptera exigua | 0.0 | 99.79 |
| SfruACT13 | LOC118275943 | 245 | NC_049730.1 | 5,635,234 | 5,651,704 | N-alpha-acetyltransferase 40-like | XP_035449986.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruACT14 | LOC118267183 | 480 | NC_049710.1 | 16,346,539 | 16,357,150 | acetyltransferase 18 | ATJ44585.1 | Helicoverpa assulta | 0.0 | 77.90 |
| SfruACT15 | LOC118264603 | 505 | NC_049715.1 | 8,586,407 | 8,600,294 | acetyltransferase | ARD71205.1 | Spodoptera exigua | 0.0 | 90.69 |
| SfruACT16 | LOC118262813 | 195 | NC_049713.1 | 14,325,493 | 14,326,654 | N-acetyltransferase 9-like | XP_035430320.1 | Spodoptera frugiperda | 1 × 10−194 | 100 |
| SfruACT17 | LOC118271269 | 869 | NC_049724.1 | 4,989,091 | 5,073,434 | N-alpha-acetyltransferase 15 | XP_035442996.1 | Spodoptera frugiperda | 0.0 | 99.88 |
| Acyl-CoA-binding protein (ACBP) | ||||||||||
| SfruACBP1 | LOC118267206 | 85 | NC_049718.1 | 4,519,313 | 4,523,840 | putative acyl-CoA-binding protein | XP_021185372.1 | Helicoverpa armigera | 2 × 10−52 | 94.12 |
| SfruACBP2 | LOC118266031 | 470 | NC_049717.1 | 10,902,558 | 10,904,528 | acyl-CoA-binding domain-containing protein 6-like | XP_035434810.1 | Spodoptera frugiperda | 0.0 | 100 |
| SfruACBP3 | LOC118279722 | 265 | NC_049737.1 | 6,551,474 | 6,584,040 | acyl-CoA-binding domain-containing protein 5-like isoform X4 | XP_035455327.1 | Spodoptera frugiperda | 0.0 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, C.; Kang, Z.; Zhang, B.; Fang, Y.; Wang, R.; Li, F.; Zhao, H.; Luo, C. Genome-Wide Identification and Expression Profiling of Candidate Sex Pheromone Biosynthesis Genes in the Fall Armyworm (Spodoptera frugiperda). Insects 2022, 13, 1078. https://doi.org/10.3390/insects13121078
Qu C, Kang Z, Zhang B, Fang Y, Wang R, Li F, Zhao H, Luo C. Genome-Wide Identification and Expression Profiling of Candidate Sex Pheromone Biosynthesis Genes in the Fall Armyworm (Spodoptera frugiperda). Insects. 2022; 13(12):1078. https://doi.org/10.3390/insects13121078
Chicago/Turabian StyleQu, Cheng, Zhiwei Kang, Biyun Zhang, Yong Fang, Ran Wang, Fengqi Li, Haipeng Zhao, and Chen Luo. 2022. "Genome-Wide Identification and Expression Profiling of Candidate Sex Pheromone Biosynthesis Genes in the Fall Armyworm (Spodoptera frugiperda)" Insects 13, no. 12: 1078. https://doi.org/10.3390/insects13121078
APA StyleQu, C., Kang, Z., Zhang, B., Fang, Y., Wang, R., Li, F., Zhao, H., & Luo, C. (2022). Genome-Wide Identification and Expression Profiling of Candidate Sex Pheromone Biosynthesis Genes in the Fall Armyworm (Spodoptera frugiperda). Insects, 13(12), 1078. https://doi.org/10.3390/insects13121078









