Two New Species of Ripipterygidae (Orthoptera, Tridactyloidea) from Mid-Cretaceous of Myanmar with a Key to the Genera of Tridactyloidea in Amber †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
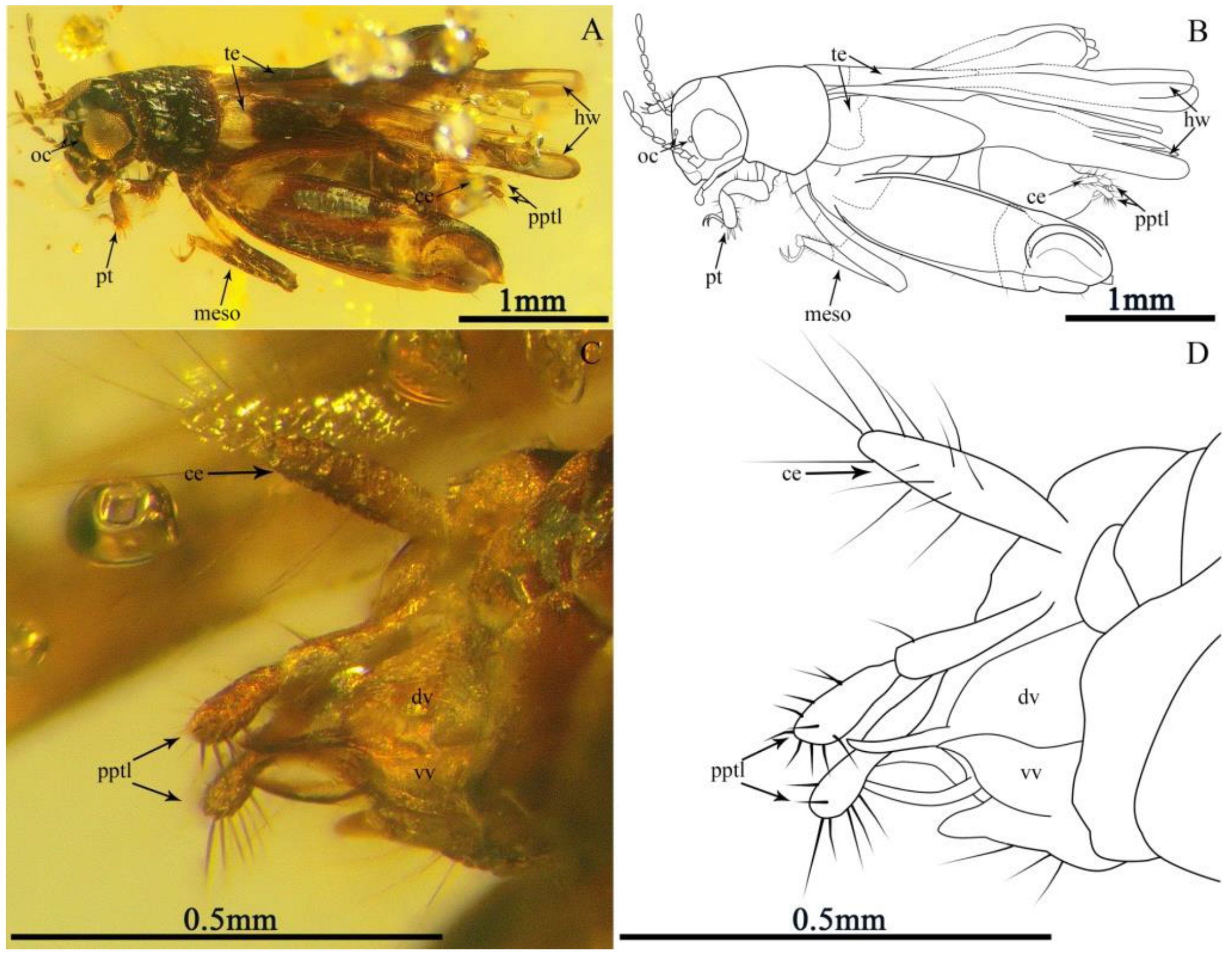
3.1. Systematic Palaeontology
3.1.1. Magnidactylus mirus sp. nov. Gu, Zheng, Cao et Yue
3.1.2. Magnidactylus gracilis sp. nov. Gu, Zheng, Cao et Yue
3.2. Key to Species for Magnidactylus
4. Discussion
Key to Genera for Tridactyloidea in Amber
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rentz, D.C.F. Orthoptera (grasshoppers, locusts, katydids, crickets). In The Insects of Australia: A Textbook for Students and Research Workers; Melbourne University Press: Melbourne, Australia, 1991; pp. 369–393. [Google Scholar]
- Heads, S.W. A new pygmy mole cricket in cretaceous amber from Burma (Orthoptera: Tridactylidae). Denisia 2009, 26, 75–82. [Google Scholar]
- Günther, K.K. Revision der familie Rhipipterygidae Chopard, 1949 (Saltatoria, Insecta). Mitt. Zool. Mus. Berl. 1969, 45, 259–425. [Google Scholar] [CrossRef]
- Günther, K.K. Einige Bemerkungen über die Gattungen der Familie Tridactylidae Brunner und zur Klassifi kation der Tridactylodea. Dtsche. Entomol. Z. 1979, 26, 255–264. [Google Scholar] [CrossRef]
- Günther, K.K. Die Tridactyloidea-Fauna Kolumbiens (Orthoptera, Caelifera). Dtsche. Entomol. Z. 1994, 41, 1–56. [Google Scholar]
- Heads, S.W. New Tridactyloidea in Miocene amber from the Dominican Republic (Orthoptera: Caelifera). Ann. Soc. Entomol. Fr. 2010, 46, 204–210. [Google Scholar] [CrossRef]
- Baena-Bejarano, N.; Heads, S.W. Three new species of the genus Ripipteryx from Colombia (Orthoptera, Ripipterygidae). ZooKeys 2015, 502, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Baena-Bejarano, N. Aspects of the natural history of Ripipteryx (Orthoptera: Ripipterygidae) species in Colombia. J. Insect Behav. 2015, 28, 44–54. [Google Scholar] [CrossRef]
- Baena-Bejarano, N.; Heads, S.W.; Taylor, S.J. Comments on the neglected nymphs of mud crickets in the genus Mirhipipteryx (Caelifera: Tridactyloidea: Ripipterygidae). Zootaxa 2018, 4486, 180–188. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. 2022. Available online: http://orthoptera.speciesfile.org (accessed on 13 September 2022).
- Xu, C.P.; Fang, Y.; Jarzembowski, E.A. A new pygmy mole cricket (Orthoptera: Tridactyloidea: Tridactylidae) from mid-Cretaceous Burmese amber. Cretac. Res. 2020, 111, 104371. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Yin, X.C.; Shih, C.k.; Gao, T.P.; Ren, D. Termite colonies from mid-Cretaceous Myanmardemonstrate their early eusocial lifestyle in damp wood. Natl. Sci. Rev. 2020, 7, 381–390. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhang, W.T.; Engel, M.S.; Sheng, X.Y.; Shih, C.K.; Ren, D. Early evolution of wing scales prior to the rise ofmoths and butterflies. Curr. Biol. 2022, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Grimaldi, D.A.; Harlow, G.E.; Wang, J.; Wang, J.; Yang, M.; Lei, W.; Li, Q.; Li, X. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Ragge, D.R. The Wing-Venation of the Orthoptera Saltatoria with Notes on Dictyopteran Wing-Venation; British Museum (Natural History): London, UK, 1955; p. 159. [Google Scholar]
- Azar, D.; Nel, A. First Tridactylidae from the Eocene French amber (Orthoptera: Tridactylidae). Alavesia 2008, 2, 169–175. [Google Scholar]
- Béthoux, O.; Nel, A. Venation pattern of Orthoptera. J. Orthoptera Res. 2001, 10, 195–199. [Google Scholar] [CrossRef]
- Béthoux, O.; Nel, A. Venation pattern and revision of Orthoptera sensunov. and sister groups. Phylogeny of Palaeozoic and Mesozoic Orthoptera sensunov. Zootaxa 2002, 96, 1–88. [Google Scholar] [CrossRef]
- Chopard, L. Some crickets from South America (Grylloidea and Tridactyloidea). Proc. U. S. Natl. Mus. 1956, 106, 241–293. [Google Scholar] [CrossRef]
- Woo, B. A new species of pygmy mole cricket (Orthoptera: Tridactylidae) from the Lake Wales ridge of Florida and new records of Ellipes eisneri from the northern Brooksville ridge. J. Orthoptera Res. 2021, 30, 131. [Google Scholar] [CrossRef]
- Gorochov, A.V. New and little-known orthopteroid insects (Polyneoptera) from fossil resins: Communication 4. Paleontol. J. 2010, 44, 657–671. [Google Scholar] [CrossRef]
- Gorochov, A.V.; Jarzembowski, E.A.; Coram, R.A. Grasshoppers and crickets (Insecta: Orthoptera) from the Lower Cretaceous of southern England. Cretac. Res. 2006, 27, 641–662. [Google Scholar] [CrossRef]
- Gorochov, A.V. New Fossil Orthoptera and Phasmoptera from the Mesozoic and Paleozoic of Mongolia. Tr. Sovm. Ross.-Mongol. Paleontol. Eksped. 1992, 41, 117–121. [Google Scholar]
- Xu, C.P.; Zhang, H.; Jarzembowski, E.A.; Fang, Y. The first Ripipterygidae (Orthoptera: Caelifera: Tridactyloidea) from mid-Cretaceous Burmese amber. Cretac. Res. 2020, 112, 104356. [Google Scholar] [CrossRef]
- Heads, S.W. A new species of Ripipteryx from the Ecuadorian Andes (Orthoptera: Tridactyloidea: Ripipterygidae). Zootaxa 2010, 2476, 23–29. [Google Scholar] [CrossRef]
- Heads, S.W.; Taylor, S.J. A new species of Ripipteryx from Belize with a key to the species of the Scrofulosa Group (Orthoptera, Ripipterygidae). ZooKeys 2012, 169, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Günther, K.K. Neue und wenig bekannte Tridactyloidea aus Mittel-und Südamerika (Orthoptera, Caelifera). Dtsche. Entomol. Z. 1989, 36, 347–379. [Google Scholar] [CrossRef]
- Poinar, G. Pygmy mole crickets (Orthoptera: Tridactylidae) in Dominican and Burmese amber. Hist. Biol. 2020, 32, 238–243. [Google Scholar] [CrossRef]
- Cao, C.Q.; Chen, S.Z.; Yin, Z. A new genus and a new species of pygmy mole cricket in Cretaceous amber from Burma (Orthoptera: Tridactylidae). Zootaxa 2019, 4559, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xu, C.; Zhang, L. Two new pygmy mole crickets from mid-Cretaceous Kachin amber of northern Myanmar (Orthoptera: Tridactyloidea: Tridactylidae). Cretac. Res. 2022, 129, 105002. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.-J.; Zheng, C.-J.; Ren, D.; Cao, C.-Q.; Yue, Y.-L. Two New Species of Ripipterygidae (Orthoptera, Tridactyloidea) from Mid-Cretaceous of Myanmar with a Key to the Genera of Tridactyloidea in Amber. Insects 2022, 13, 979. https://doi.org/10.3390/insects13110979
Gu J-J, Zheng C-J, Ren D, Cao C-Q, Yue Y-L. Two New Species of Ripipterygidae (Orthoptera, Tridactyloidea) from Mid-Cretaceous of Myanmar with a Key to the Genera of Tridactyloidea in Amber. Insects. 2022; 13(11):979. https://doi.org/10.3390/insects13110979
Chicago/Turabian StyleGu, Jun-Jie, Cheng-Jie Zheng, Dong Ren, Cheng-Quan Cao, and Yan-Li Yue. 2022. "Two New Species of Ripipterygidae (Orthoptera, Tridactyloidea) from Mid-Cretaceous of Myanmar with a Key to the Genera of Tridactyloidea in Amber" Insects 13, no. 11: 979. https://doi.org/10.3390/insects13110979
APA StyleGu, J.-J., Zheng, C.-J., Ren, D., Cao, C.-Q., & Yue, Y.-L. (2022). Two New Species of Ripipterygidae (Orthoptera, Tridactyloidea) from Mid-Cretaceous of Myanmar with a Key to the Genera of Tridactyloidea in Amber. Insects, 13(11), 979. https://doi.org/10.3390/insects13110979




