Efficacy Evaluation of Oregano Essential Oil Mixed with Bacillus thuringiensis israelensis and Diflubenzuron against Culex pipiens and Aedes albopictus in Road Drains of Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Tested
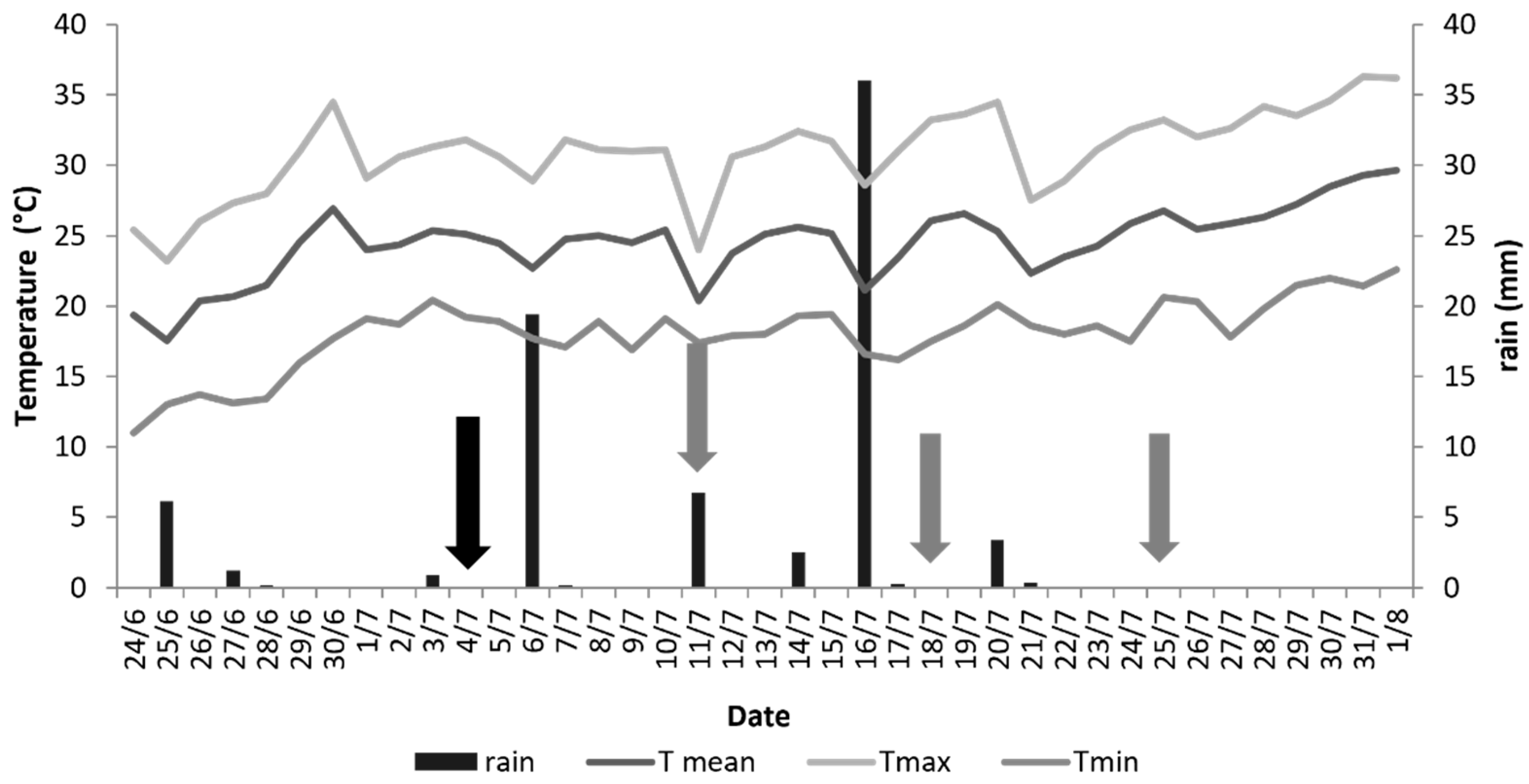
2.2. Experimental Setup
2.3. Data Analysis
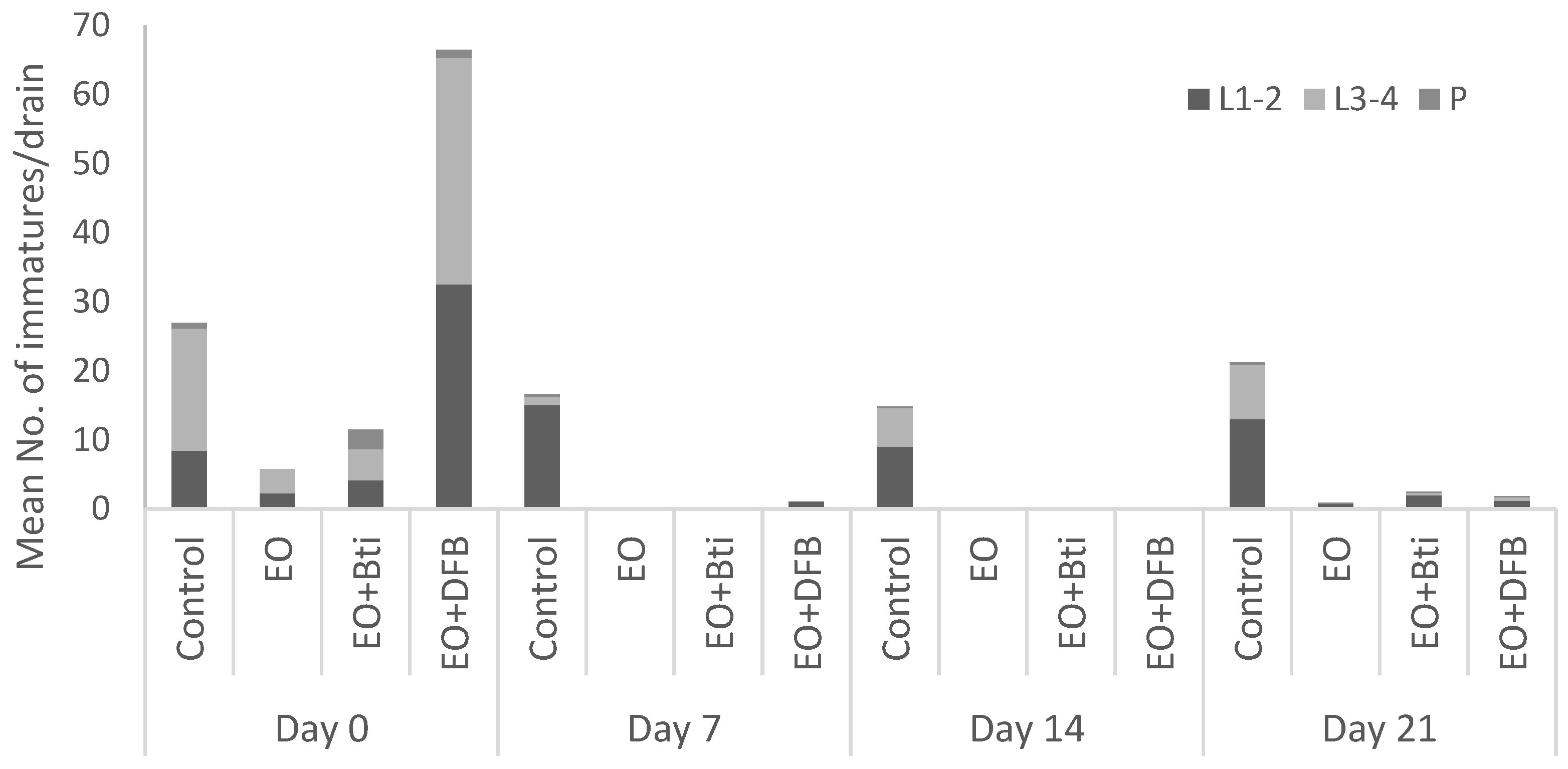
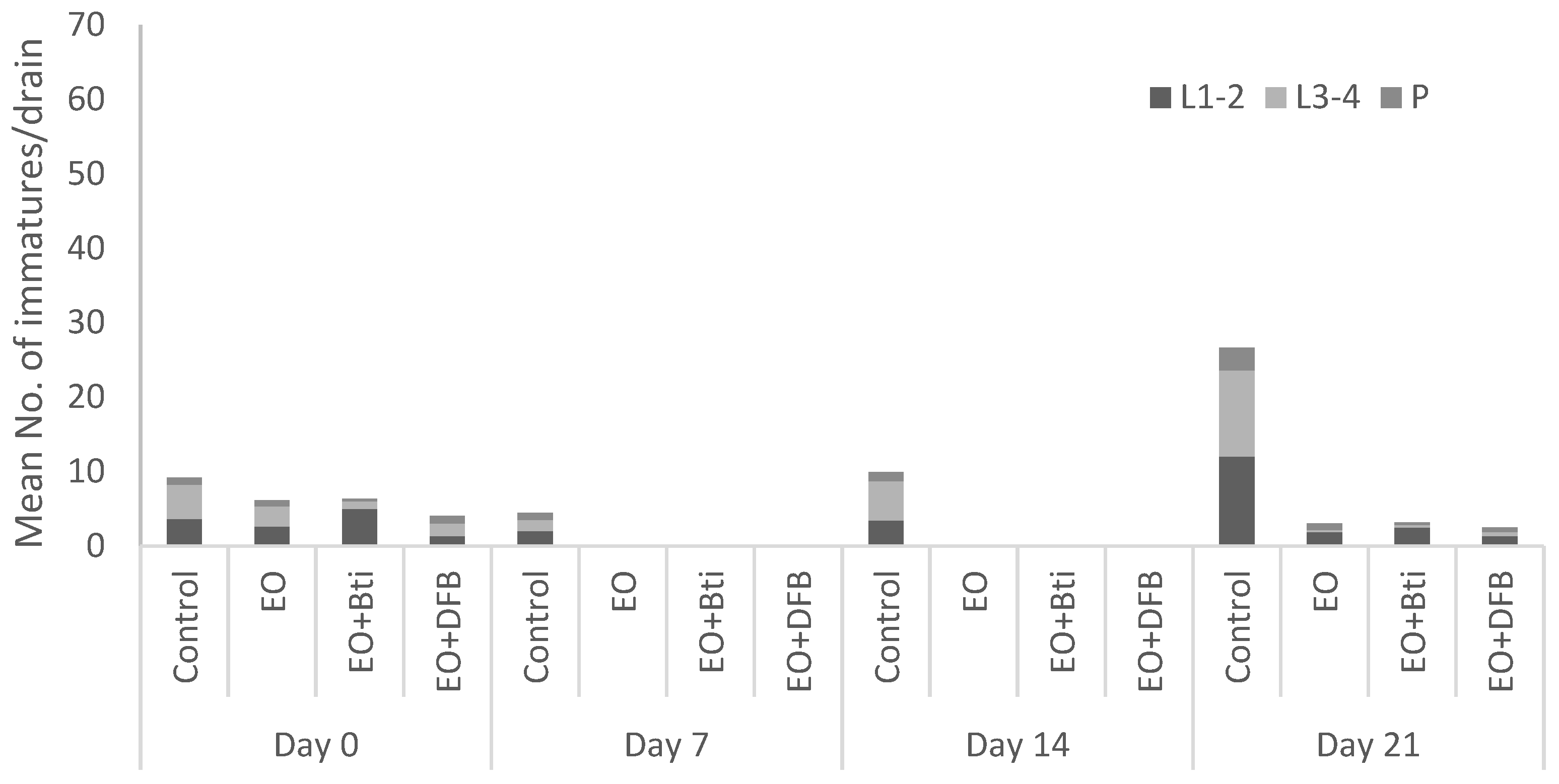
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; 577p. [Google Scholar]
- Medlock, J.M.; Hansford, K.M.; Versteirt, V.; Cull, B.; Kampen, H.; Fontenille, D.; Hendrickx, G.; Zeller, H.; Bortel, W.V.; Schaffner, F. An entomological review of invasive mosquitoes in Europe. Bull. Entomol. Res. 2015, 105, 637–663. [Google Scholar] [CrossRef]
- Adhami, J.; Reiter, P. Introduction and establishment of Aedes (Stegomyia) albopictus Skuse (Diptera: Culicidae) in Albania. J. Am. Mosq. Control Assoc. 1998, 14, 340–343. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). Aedes albopictus—Current Known Distribution in Europe, August 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/aedes-albopictus-current-known-distribution-march-2021 (accessed on 9 August 2022).
- Romiti, F.; Casini, R.; Magliano, A.; Ermenegildi, A.; De Liberato, C. Aedes albopictus abundance and phenology along an altitudinal gradient in Lazio region (central Italy). Parasit. Vectors 2022, 15, 92. [Google Scholar] [CrossRef]
- Bellini, R.; Michaelakis, A.; Petrić, D.; Schaffner, F.; Alten, B.; Angelini, P.; Aranda, C.; Becker, N.; Carrieri, M.; Di Luca, M.; et al. Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Med. Infect. Dis. 2020, 35, 101691. [Google Scholar] [CrossRef]
- Barzon, L.; Gobbi, F.; Capelli, G.; Montarsi, F.; Martini, S.; Riccetti, S.; Sinigaglia, A.; Pacenti, M.; Pavan, G.; Rassu, M.; et al. Autochthonous dengue outbreak in Italy 2020: Clinical, virological and entomological findings. J. Travel Med. 2021, 28, 130. [Google Scholar] [CrossRef]
- Bellini, R.; Zeller, H.; Van Bortel, W. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasit. Vectors 2014, 7, 323. [Google Scholar] [CrossRef]
- Rizzoli, A.; Bolzoni, L.; Chadwick, E.A.; Capelli, G.; Montarsi, F.; Grisenti, M.; de la Puente, J.M.; Muñoz, J.; Figuerola, J.; Soriguer, R.; et al. Understanding West Nile virus ecology in Europe: Culex pipiens host feeding preference in a hotspot of virus emergence. Parasit. Vectors 2015, 8, 213. [Google Scholar] [CrossRef]
- García-Carrasco, J.M.; Muñoz, A.R.; Olivero, J.; Segura, M.; Real, R. Predicting the spatio-temporal spread of West Nile virus in Europe. PLoS Negl. Trop. Dis. 2021, 15, e0009022. [Google Scholar] [CrossRef]
- Di Luca, M.; Toma, L.; Boccolini, D.; Severini, F.; La Rosa, G.; Minelli, G.; Bongiorno, G.; Montarsi, F.; Arnoldi, D.; Capelli, G.; et al. Ecological Distribution and CQ11 Genetic Structure of Culex pipiens Complex (Diptera: Culicidae) in Italy. PLoS ONE 2016, 11, e0146476. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S.; Balzarini, F. Epidemiology of West Nile Virus infections in humans, Italy, 2012–2020: A summary of available evidences. Trop. Med. Infect. Dis. 2021, 6, 61. [Google Scholar] [CrossRef]
- Killeen, G.F.; Fillinger, U.; Knols, B.G. Advantages of larval control for African malaria vectors: Low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malar. J. 2002, 1, 8. [Google Scholar] [CrossRef]
- Rey, J.R.; Walton, W.E.; Wolfe, R.J.; Connelly, C.R.; O’Connell, S.M.; Berg, J.; Sakolsky-Hoopes, G.E.; Laderman, A.D. North American wetlands and mosquito control. Int. J. Environ. Res. Public Health 2012, 9, 4537–4605. [Google Scholar] [CrossRef]
- Guzzetta, G.; Trentini, F.; Poletti, P.; Baldacchino, F.A.; Montarsi, F.; Capelli, G.; Rizzoli, A.; Rosà, R.; Merler, S.; Melegaro, A. Effectiveness and economic assessment of routine larviciding for prevention of chikungunya and dengue in temperate urban settings in Europe. PLoS Negl. Trop. Dis. 2017, 11, e0005918. [Google Scholar] [CrossRef]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef]
- Zhou, G.; Lo, E.; Githeko, A.K.; Afrane, Y.A.; Yan, G. Long-lasting microbial larvicides for controlling insecticide resistant and outdoor transmitting vectors: A cost-effective supplement for malaria interventions. Infect. Dis. Poverty 2020, 9, 162. [Google Scholar] [CrossRef]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; della Torre, A.; Montarsi, F.; Rizzoli, A. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Available online: https://echa.europa.eu/el/information-on-chemicals/biocidal-products (accessed on 11 July 2022).
- Caputo, B.; Ienco, A.; Manica, M.; Petrarca, V.; Rosà, R.; della Torre, A. New adhesive traps to monitor urban mosquitoes with a case study to assess the efficacy of insecticide control strategies in temperate areas. Parasit. Vectors 2015, 8, 134. [Google Scholar] [CrossRef]
- Bellini, R.; Albieri, A.; Carrieri, M.; Colonna, R.; Donati, L.; Magnani, M.; Pilani, R.; Veronesi, R.; Chiot, G.; Lanza, N. Efficacy and lasting activity of four IGRs formulations against mosquitoes in catch basins of northern Italy. Eur. Mosq. Bull. 2009, 27, 33–46. [Google Scholar]
- Baldacchino, F.; Bussola, F.; Arnoldi, D.; Marcantonio, M.; Montarsi, F.; Capelli, G.; Rosà, R.; Rizzoli, A. An integrated pest control strategy against the Asian tiger mosquito in northern Italy: A case study. Pest Manag. Sci. 2017, 73, 87–93. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007, 63, 628–633. [Google Scholar] [CrossRef]
- Pichler, V.; Caputo, B.; Valadas, V.; Micocci, M.; Horvath, C.; Virgillito, C.; Akiner, M.; Balatsos, G.; Bender, C.; Besnard, G.; et al. Geographic distribution of the V1016G knockdown resistance mutation in Aedes albopictus: A warning bell for Europe. Parasit. Vectors 2022, 15, 280. [Google Scholar] [CrossRef]
- Grigoraki, L.; Puggioli, A.; Mavridis, K.; Douris, V.; Montanari, M.; Bellini, R.; Vontas, J. Striking diflubenzuron resistance in Culex pipiens, the prime vector of West Nile Virus. Sci. Rep. 2017, 7, 11699. [Google Scholar] [CrossRef]
- Porretta, D.; Fotakis, E.A.; Mastrantonio, V.; Chaskopoulou, A.; Michaelakis, A.; Kioulos, I.; Weill, M.; Urbanelli, S.; Vontas, J.; Bellini, R. Focal distribution of diflubenzuron resistance mutations in Culex pipiens mosquitoes from Northern Italy. Acta Trop. 2019, 193, 106–112. [Google Scholar] [CrossRef]
- Piplani, M.; Bhagwat, D.P.; Singhvi, G.; Sankaranarayanan, M.; Balana-Fouce, R.; Vats, T.; Chander, S. Plant-based larvicidal agents: An overview from 2000 to 2018. Exp. Parasitol. 2019, 199, 92–103. [Google Scholar] [CrossRef]
- Sengül Demirak, M.S.; Canpolat, E. Plant-based bioinsecticides for mosquito control: Impact on insecticide resistance and disease transmission. Insects 2022, 13, 162. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Ntalli, N.; Koliopoulos, G.; Giatropoulos, A.; Menkissoglu-Spiroudi, U. Plant secondary metabolites against arthropods of medical importance. Phytochem. Rev. 2019, 18, 1255–1275. [Google Scholar] [CrossRef]
- Traboulsi, A.F.; Taoubi, K.; El-Haj, S.; Bessiere, J.M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef]
- Youssefi, M.R.; Tabari, M.A.; Esfandiari, A.; Kazemi, S.; Moghadamnia, A.A.; Sut, S.; Dall’Acqua, S.; Benelli, G.; Maggi, F. Efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the West Nile vector Culex pipiens. Molecules 2019, 24, 1867. [Google Scholar] [CrossRef]
- Mulla, M.S.; Su, T. Activity and biological effects of neem products against arthropods of medical and veterinary importance. J. Am. Mosq. Contr. Assoc. 1999, 15, 133–152. [Google Scholar]
- Murugan, K.; Thangamathi, P.; Jeyabalan, D. Interactive effect of botanicals and Bacillus thuringiensis subsp israelensis on Culex quinquefasciatus Say. J. Sci. Ind. Res. 2002, 61, 1068–1076. [Google Scholar]
- Shaalan, E.A.; Canyon, D.V.; Younes, M.W.; Abdel-Wahab, H.; Mansour, A.H. Synergistic efficacy of botanical blends with and without synthetic insecticides against Aedes aegypti and Culex annulirostris mosquitoes. J. Vector Ecol. 2005, 30, 284–288. [Google Scholar]
- Senthil Nathan, S.; Kalaivani, K. Combined effects of azadirachtin and nucleopolyhedrovirus (SpltNPV) on Spodoptera litura Fabricius (Lepidoptera: Noctuidae) larvae. Biol. Control 2006, 39, 1. [Google Scholar]
- Mahesh Kumar, P.; Kovendan, K.; Murugan, K. Integration of botanical and bacterial insecticide against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2013, 112, 761–771. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef]
- Evergetis, E.; Bellini, R.; Balatsos, G.; Michaelakis, A.; Carrieri, M.; Veronesi, R.; Papachristos, D.P.; Puggioli, A.; Kapsaski-Kanelli, V.-N.; Haroutounian, S.A. From bio-prospecting to field assessment: The case of carvacrol rich essential oil as a potent mosquito larvicidal and repellent agent. Front. Ecol. Evol. 2018, 6, 204. [Google Scholar] [CrossRef]
- Mulla, M.S.; Norland, R.L.; Fanara, D.M.; Darwazeh, H.A.; McKean, D.W. Control of chironomid midges in recreational lakes. J. Econ. Entomol. 1971, 64, 300–307. [Google Scholar] [CrossRef]
- Feng, R.; Isman, M.B. Selection for Resistance to Azadirachtin in the Green Peach Aphid, Myzus persicae. Experientia 1995, 51, 831–833. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Wraight, S.P.; Molloy, D.P.; Jamnback, H.; McCoy, P. Effects of temperature and instar on the efficacy of Bacillus thuringiensis var. israelensis and Bacillus sphaericus strain 1593 against Aedes stimulans larvae. J. Invertebr. Pathol. 1981, 38, 78–87. [Google Scholar] [CrossRef]
- Srivastava, R.; Bhalwar, R.; Tilak, V.W. A mathematical model for predicting the persistence and efficacy of Bacillus thuringiensis var. israelensis in relation to water pollution. Med. J. Armed Forces India 1998, 54, 107–110. [Google Scholar]
- Tetreau, G.; Stalinski, R.; Kersusan, D.; Veyrenc, S.; David, J.P.; Reynaud, S.; Després, L. Decreased toxicity of Bacillus thuringiensis subsp. israelensis to mosquito larvae after contact with leaf litter. Appl. Environ. Microbiol. 2012, 78, 5189–5195. [Google Scholar] [PubMed]
- Isman, M.B. Botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired byplant–herbivore chemical interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef]
| Time | Product | Cx. pipiens | Ae. albopictus | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean% | SEM | p Value | Mean % | SEM | p Value | Mean % | SEM | p Value | ||
| Day 7 | EO | 7 | 100.0 | 0.0 | 0.102 | 100.0 | 0.0 | 1.000 | 100.0 | 0.0 | 0.102 |
| EO+B.t.i. | 6 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| EO+DFB | 6 | 97.6 | 2.0 | 100.0 | 0.0 | 97.6 | 2.0 | ||||
| Day 14 | EO | 7 | 100.0 | 0.0 | 0.338 | 100.0 | 0.0 | 1.000 | 100.0 | 0.0 | 0.338 |
| EO+B.t.i. | 6 | 97.4 | 2.6 | 100.0 | 0.0 | 98.6 | 1.4 | ||||
| EO+DFB | 6 | 100.0 | 0.0 | 100.0 | 0.0 | 100.0 | 0.0 | ||||
| Day 21 | EO | 7 | 81.0 | 15.6 | 0.933 | 83.1 | 5.8 | 0.806 | 75.4 | 9.4 | 0.143 |
| EO+B.t.i. | 6 | 72.4 | 18.8 | 82.7 | 9.5 | 76.0 | 11.6 | ||||
| EO+DFB | 6 | 96.5 | 2.2 | 78.4 | 13.2 | 95.4 | 2.8 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giatropoulos, A.; Bellini, R.; Pavlopoulos, D.T.; Balatsos, G.; Karras, V.; Mourafetis, F.; Papachristos, D.P.; Karamaouna, F.; Carrieri, M.; Veronesi, R.; et al. Efficacy Evaluation of Oregano Essential Oil Mixed with Bacillus thuringiensis israelensis and Diflubenzuron against Culex pipiens and Aedes albopictus in Road Drains of Italy. Insects 2022, 13, 977. https://doi.org/10.3390/insects13110977
Giatropoulos A, Bellini R, Pavlopoulos DT, Balatsos G, Karras V, Mourafetis F, Papachristos DP, Karamaouna F, Carrieri M, Veronesi R, et al. Efficacy Evaluation of Oregano Essential Oil Mixed with Bacillus thuringiensis israelensis and Diflubenzuron against Culex pipiens and Aedes albopictus in Road Drains of Italy. Insects. 2022; 13(11):977. https://doi.org/10.3390/insects13110977
Chicago/Turabian StyleGiatropoulos, Athanasios, Romeo Bellini, Dionysios T. Pavlopoulos, George Balatsos, Vasileios Karras, Fotis Mourafetis, Dimitrios P. Papachristos, Filitsa Karamaouna, Marco Carrieri, Rodolfo Veronesi, and et al. 2022. "Efficacy Evaluation of Oregano Essential Oil Mixed with Bacillus thuringiensis israelensis and Diflubenzuron against Culex pipiens and Aedes albopictus in Road Drains of Italy" Insects 13, no. 11: 977. https://doi.org/10.3390/insects13110977
APA StyleGiatropoulos, A., Bellini, R., Pavlopoulos, D. T., Balatsos, G., Karras, V., Mourafetis, F., Papachristos, D. P., Karamaouna, F., Carrieri, M., Veronesi, R., Haroutounian, S. A., & Michaelakis, A. (2022). Efficacy Evaluation of Oregano Essential Oil Mixed with Bacillus thuringiensis israelensis and Diflubenzuron against Culex pipiens and Aedes albopictus in Road Drains of Italy. Insects, 13(11), 977. https://doi.org/10.3390/insects13110977











