Resistance Mechanisms of Sitobion miscanthi (Hemiptera: Aphididae) to Malathion Revealed by Synergist Assay
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Chemicals
2.3. Bioassays
2.4. Selection
2.5. Cross-Resistance Pattern and Synergist Study
2.6. Biochemical Assays
2.6.1. Carboxylesterase Activity Assays
2.6.2. Glutathione S-Transferase Assays
2.6.3. Acetylcholinesterase Assays
2.7. Protein Assays
2.8. Statistical Analysis
3. Results
3.1. Selection
3.2. Cross-Resistance Pattern
3.3. Synergism
3.4. Biochemical Assays
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, X.L.; Peng, X.; Qiao, X.F.; Chen, M.H. Life cycle and population genetics of bird cherry–oat aphids Rhopalosiphum padi in China: An important pest on wheat crops. J. Pest. Sci. 2016, 90, 1–14. [Google Scholar] [CrossRef]
- George, K.S.; Gair, R. Crop loss assessment on winter wheat attacked by the grain aphid, Sitobion avenae (F.) 1974–1977. Plant Pathol. 2010, 28, 143–149. [Google Scholar] [CrossRef]
- Hu, X.S.; Liu, Y.J.; Wang, Y.H.; Wang, Z.; Yu, X.L.; Wang, B.; Zhang, G.S.; Liu, X.F.; Hu, Z.Q.; Zhao, H.Y.; et al. Resistance of wheat accessions to the English grain aphid Sitobion avenae. PLoS ONE 2016, 11, e0156158. [Google Scholar] [CrossRef]
- Gong, P.; Li, X.; Gao, H.; Wang, C.; Li, M.; Zhang, Y.; Li, X.; Liu, E.; Zhu, X. Field evolved resistance to pyrethroids, neonicotinoids, organophosphates and macrolides in Rhopalosiphum padi (Linnaeus) and Sitobion avenae (Fabricius) from China. Chemosphere 2020, 269, 128747. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Q.; Qin, Y.; Yin, H.; Zhang, S.; Li, Q.; Zhang, Y.; Fan, J.; Chen, J. A chromosome-level draft genome of the grain aphid Sitobion miscanthi. Gigascience 2019, 8, giz101. [Google Scholar] [CrossRef]
- Chen, M.H.; Han, Z.J.; Qiao, X.F.; Qu, M.J. Resistance mechanisms and associated mutations in acetylcholinesterase genes in Sitobion avenae (Fabricius). Pestic. Biochem. Physiol. 2007, 87, 189–195. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, M.; Huang, Y.N.; Yang, Z.L.; Su, S.; Chen, M.H. Characterisation of imidacloprid resistance in the bird cherry–oat aphid, Rhopalosiphum padi, a serious pest on wheat crops. Pest Manag. Sci. 2018, 74, 1457–1465. [Google Scholar] [CrossRef]
- Wei, C.; Huang, S.N.; Fan, X.L.; Sun, X.P.; Wang, W.L.; Liu, Z.W.; Chen, G.Q. A study on the resistance of grain aphid Sitobion avenae Fab to pesticides. Acta Entomol. Sin. 1988, 31, 148–156. [Google Scholar]
- Gao, X.W.; Zheng, B.Z.; Cao, B.J. Resistance in Myzus persicae to organophosphorus and carbamate insecticides in China. J. Plant Prot. 1992, 19, 369–371. [Google Scholar]
- Liu, Z.W.; Han, Z.J. The roles of carboxylesterase and AChE insensitivity in malathion resistance development in brown planthopper. Acta Entomol. Sin. 2003, 46, 3. [Google Scholar]
- Scott, J.G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 1999, 29, 757–777. [Google Scholar] [CrossRef]
- Yang, X.; Xie, W.; Wang, S.L.; Wu, Q.J.; Pan, H.P.; Li, R.M.; Yang, N.N.; Liu, B.M.; Xu, B.Y.; Zhou, X.M.; et al. Two cytochrome P450 genes are involved in imidacloprid resistance in field populations of the whitefly, Bemisia tabaci, in China. Pestic. Biochem. Physiol. 2013, 107, 343–350. [Google Scholar] [CrossRef]
- Low, V.L.; Chen, C.D.; Lim, P.E.; Lee, H.L.; Lim, Y.A.L.; Tan, T.K.; Sofian-Azirun, M. First molecular genotyping of insensitive acetylcholinesterase associated with malathion resistance in Culex quinquefasciatus Say populations in Malaysia. Pest Manag. Sci. 2013, 69, 1362–1368. [Google Scholar] [CrossRef]
- Edi, C.V.; Djogbenou, L.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.; Poupardin, R.; Jones, C.M.; Essandoh, J.; Ketoh, G.K.; Paine, M.J.; et al. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014, 10, e1004236. [Google Scholar] [CrossRef]
- Tian, F.J.; Li, C.F.; Wang, Z.B.; Liu, J.L.; Zeng, X.N. Identification of detoxification genes in imidacloprid-resistant Asian citrus psyllid (Hemiptera: Lividae) and their expression patterns under stress of eight insecticides. Pest Manag. Sci. 2019, 75, 1400–1410. [Google Scholar] [CrossRef]
- Voudouris, C.C.; Kati, A.N.; Sadikoglou, E.; Williamson, M.; Skouras, P.J.; Dimotsiou, O.; Georgiou, S.; Fenton, B.; Skavdis, G.; Margaritopoulos, J.T. Insecticide resistance status of Myzus persicae in Greece: Long-term surveys and new diagnostics for resistance mechanisms. Pest Manag. Sci. 2016, 72, 671–683. [Google Scholar] [CrossRef]
- Stará, J.; Kocourek, F. Seven-year monitoring of pyrethroid resistance in the pollen beetle (Brassicogethes aeneus F.) during implementation of insect resistance management. Pest Manag. Sci. 2018, 74, 200–209. [Google Scholar] [CrossRef]
- Lu, Y.H.; Yang, T.; Gao, X.W. Establishment of baseline susceptibility data to various insecticides for aphids Rhopalosiphum padi (Linnaeus) and Sitobion avenae (Fabricius) (Homoptera: Aphididae) by the method of residual film in glass tube. Acta Entomol. Sin. 2009, 52, 52–58. [Google Scholar]
- Chen, X.K.; Xia, X.M.; Wang, H.Y.; Qiao, K.; Wang, K.Y. Cross–resistance to clothianidin and acetamiprid in the imidacloprid resistant strain of Aphis gossypii (Hemiptera: Aphididae) and the related enzyme mechanisms. Acta Entomol. Sin. 2013, 56, 1143–1151. [Google Scholar]
- Vanasperen, K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 1962, 8, 401–414, IN3, 415–416. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferase AA from rat liver. Arch. Biochem. Biophys. 1976, 175, 710–716. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman: Harlow, UK, 1996. [Google Scholar]
- Pan, Y.O.; Guo, H.L.; Gao, X.W. Carboxylesterase activity, cDNA sequence, and gene expression in malathion susceptible and resistant strains of the cotton aphid, Aphis gossypii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 152, 266–270. [Google Scholar] [CrossRef]
- Xu, T.Y.; Zhang, S.; Liu, Y.; Ma, L.; Li, X.Q.; Zhang, Y.X.; Fan, Y.J.; Song, D.L.; Gao, X.W. Slow resistance evolution to neonicotinoids in field populations of wheat aphids revealed by insecticide resistance monitoring in China. Pest Manag. 2022, 78, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Gao, X.W. Inhibiting effects of common insecticides on carboxylesterase activity in Sitobion avenae and Rhopalosiphum padi (Hemiptera: Aphididae) and their synergism to beta-cypermethrin. Acta Entomol. Sin. 2016, 59, 1151–1158. [Google Scholar]
- Zhu, K.Y.; Gao, J.R. Kinetic properties and variability of esterases in organophosphate-susceptible and -resistant greenbugs, Schizaphis graminum (Homoptera: Aphididae). Pestic. Biochem. Phys. 1998, 62, 135–145. [Google Scholar] [CrossRef]
- Alon, M.; Alon, F.; Nauen, R.; Morin, S. Organophosphates’ resistance in the B-biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1-type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochem. Mol. Biol. 2008, 38, 940–949. [Google Scholar] [CrossRef]
- Wang, L.L.; Lu, X.P.; Meng, L.W.; Huang, Y.; Wei, D.; Jiang, H.B.; Smagghe, G.; Wang, J.J. Functional characterization of an alpha-esterase gene involving malathion detoxification in Bactrocera dorsalis (Hendel). Pestic. Biochem. Physiol. 2016, 130, 44–51. [Google Scholar] [CrossRef]
- Lan, W.S.; Cong, J.; Jiang, H.; Jiang, S.R.; Qiao, C.L. Expression and characterization of carboxylesterase E4 gene from peach-potato aphid (Myzus persicae) for degradation of carbaryl and malathion. Biotechnol. Lett. 2005, 27, 1141–1146. [Google Scholar] [CrossRef]
- Wang, I.L.; Feng, Z.J.; Li, T.; Lu, X.P.; Zhao, J.J.; Niu, J.Z.; Smagghe, G.; Wang, J.J. Inheritance, Realized heritability, and biochemical mechanisms of malathion resistance in Bactrocera dorsalis (Diptera: Tephritidae). J. Econ. Entomol. 2016, 109, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Snodgrass, G.L.; Chen, M.S. Comparative study on glutathione S-transferase activity, cDNA, and gene expression between malathion susceptible and resistant strains of the tarnished plant bug, Lygus lineolaris. Pestic. Biochem. Physiol. 2007, 87, 62–72. [Google Scholar] [CrossRef]
- Liu, Z.W.; Han, S.J. Cross resistance of malathion resistant strain of Nilaparvata lugens and its biochemical aspects. J. Nanjing Agric. Univ. 2004, 27, 55–58. [Google Scholar]
- Nabeshima, T.; Kozaki, T.; Tomita, T.; Kono, Y. An amino acid substitution on the second acetylcholinesterase in the pirimicarb-resistant strains of the peach potato aphid, Myzus persicae. Biochem. Biophys. Res. Commun. 2003, 307, 15–22. [Google Scholar] [CrossRef]
- Li, F.; Han, Z.J. Mutations in acetylcholinesterase associated with insecticide resistance in the cotton aphid, Aphis gossypii Glover. Insect Biochem. Mol. Biol. 2005, 35, 1309. [Google Scholar] [CrossRef]
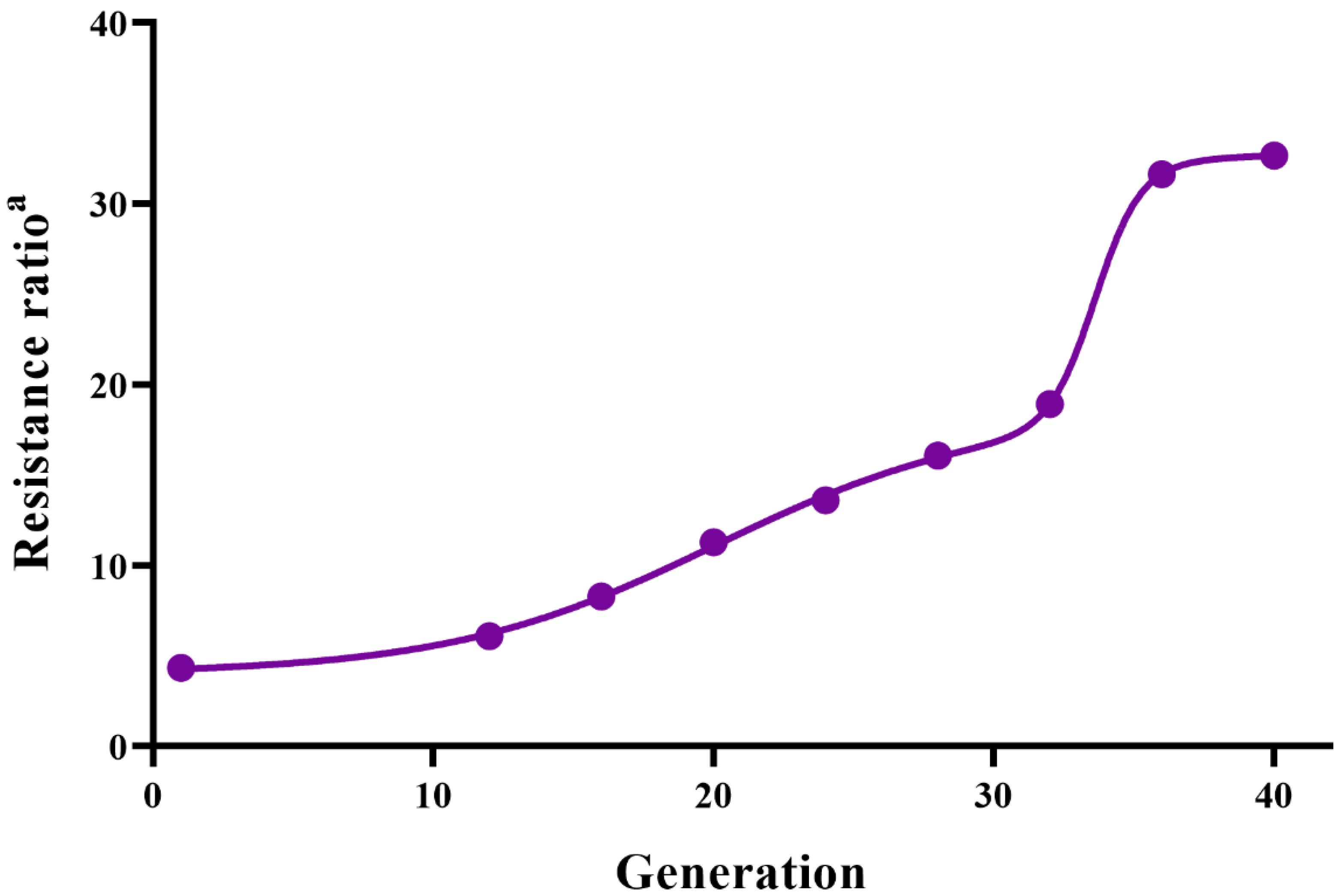
| Number of Generations for Selection | Estimate of Mean Response per Generation | Estimate of Mean Selection Differential per Generation | h2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Initial LC50 (Log) | Final LC50 (Log) | R | p | i | Initial Slope | Final Slope | δp | S | |
| 40 | 1.09 | 1.97 | 0.02 | 22.2 | 1.34 | 1.70 | 1.38 | 0.65 | 0.87 | 0.02 |
| Insecticides | Strains | Slop ± SE | LC50 mg L−1 (95% Confidence Limit) | Resistance Ratio 2 |
|---|---|---|---|---|
| malathion | MRS | 1.383 ± 0.204 | 90.373 (66.752–141.708) | 33.0 |
| MSS | 1.965 ± 0.249 | 2.743 (2.240–3.283) | ||
| omethoate | MRS | 1.259 ± 0.199 | 67.786 (48.908–112.036) | 13.5 |
| MSS | 1.737 ± 0.221 | 5.004 (3.943–6.360) | ||
| methomyl | MRS | 1.821 ± 0.228 | 4.875 (3.840–6.155) | 2.9 |
| MSS | 1.887 ± 0.219 | 1.680 (1.339–2.068) | ||
| beta-cypermethrin | MRS | 1.781 ± 0.223 | 64.910 (51.452–83.560) | 4.8 |
| MSS | 2.889 ± 0.486 | 13.564 (10.846–16.186) | ||
| imidacloprid | MRS | 1.421 ± 0.212 | 15.710 (11.858–22.360) | 1.6 |
| MSS | 1.639 ± 0.213 | 9.786 (7.654–12.559) |
| Strain | Compound | Slope ± SE | LC50 mg L−1 (95% Confidence Limit) | SR 2 | SRR 3 |
|---|---|---|---|---|---|
| MSS | malathion | 1.97 ± 0.25 | 2.74 (2.24–3.28) | 1 | 1 |
| +PBO | 1.84 ± 0.22 | 2.71 (2.13–3.35) | 1.01 | 1 | |
| +DEF | 2.05 ± 0.24 | 2.11 (1.66–2.57) | 1.30 | 1 | |
| +DEM | 2.06 ± 0.23 | 2.42 (1.93–2.94) | 1.14 | 1 | |
| +TPP | 1.55 ± 0.21 | 1.66 (0.54–2.81) | 1.65 | 1 | |
| MRS | malathion | 1.38 ± 0.20 | 90.37 (66.75–141.71) | 1 | 1 |
| +PBO | 1.47 ± 0.20 | 71.49 (54.94–102.26) | 1.26 | 1.25 | |
| +DEF | 1.68 ± 0.20 | 17.65 (14.24–22.58) | 5.12 | 3.94 | |
| +DEM | 1.59 ± 0.21 | 52.43 (41.75–68.91) | 1.72 | 1.51 | |
| +TPP | 2.43 ± 0.243 | 8.09 (6.79–9.52) | 11.17 | 6.77 |
| Strains | CarE Activity (nmol mg Protein−1 min−1) | Km (M) | Vmax (μmol mg Protein−1 min−1) |
|---|---|---|---|
| MSS | 66.57 ± 2.80 a | 4.46 × 10−4 ± 1.87 × 10−4 a | 1.81 × 10−1 ± 2.74 × 10−2 a |
| MRS | 233.56 ± 11.89 b | 2.35 × 10−4 ± 6.28 × 10−5 b | 4.47 × 10−1 ± 3.66 × 10−2 b |
| Strains | GSTs Activity (nmol mg Protein−1 min−1) | AChE Activity (pmol mg Protein−1 min−1) |
|---|---|---|
| MSS | 677.85 ± 58.64 a | 42.73 ± 1.88 a |
| MRS | 1175.78 ± 38.72 b | 75.04 ± 5.86 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Lou, K.; Song, D.; Zhu, B.; Liang, P.; Gao, X. Resistance Mechanisms of Sitobion miscanthi (Hemiptera: Aphididae) to Malathion Revealed by Synergist Assay. Insects 2022, 13, 1043. https://doi.org/10.3390/insects13111043
Xu T, Lou K, Song D, Zhu B, Liang P, Gao X. Resistance Mechanisms of Sitobion miscanthi (Hemiptera: Aphididae) to Malathion Revealed by Synergist Assay. Insects. 2022; 13(11):1043. https://doi.org/10.3390/insects13111043
Chicago/Turabian StyleXu, Tianyang, Kai Lou, Dunlun Song, Bin Zhu, Pei Liang, and Xiwu Gao. 2022. "Resistance Mechanisms of Sitobion miscanthi (Hemiptera: Aphididae) to Malathion Revealed by Synergist Assay" Insects 13, no. 11: 1043. https://doi.org/10.3390/insects13111043
APA StyleXu, T., Lou, K., Song, D., Zhu, B., Liang, P., & Gao, X. (2022). Resistance Mechanisms of Sitobion miscanthi (Hemiptera: Aphididae) to Malathion Revealed by Synergist Assay. Insects, 13(11), 1043. https://doi.org/10.3390/insects13111043






