Effects of Elevated CO2 Concentration on Host Adaptability and Chlorantraniliprole Susceptibility in Spodoptera frugiperda
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Insect Stocks
2.2. Effect of eCO2 on FAW Population Performance
2.3. Effect of eCO2 on the Chlorantraniliprole Susceptibility of the FAW
2.4. Effect of eCO2 on Wheat
2.5. RNA Isolation, Transcriptome Library Preparation and Sequencing
2.6. qRT-PCR
2.7. Data Analyses
3. Results
3.1. Effect of eCO2 on the Susceptibility of the FAW to Chlorantraniliprole
3.2. Effect of eCO2 on FAW Population Performance in Wheat
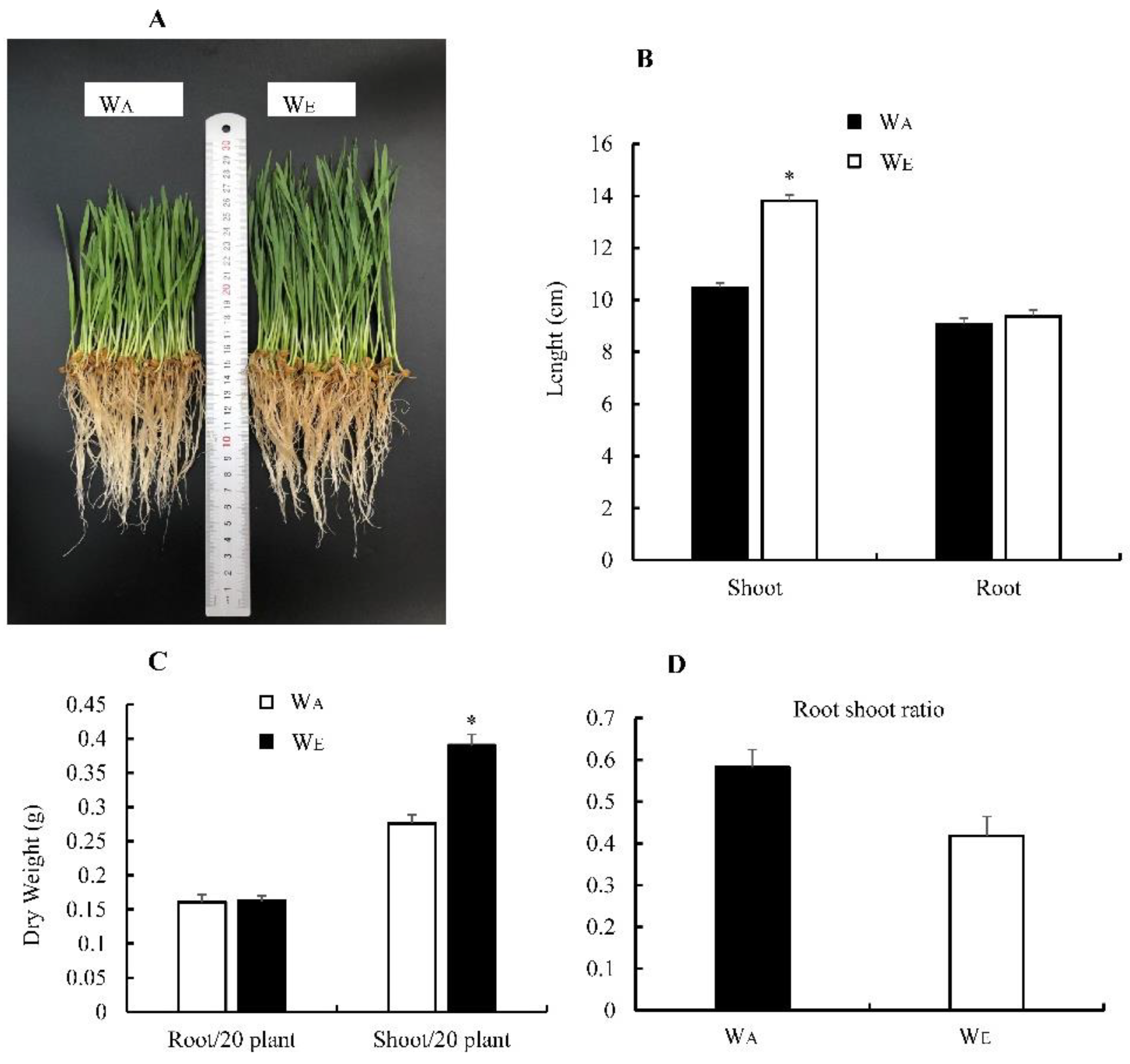
3.3. Effect of eCO2 on Wheat Biomass
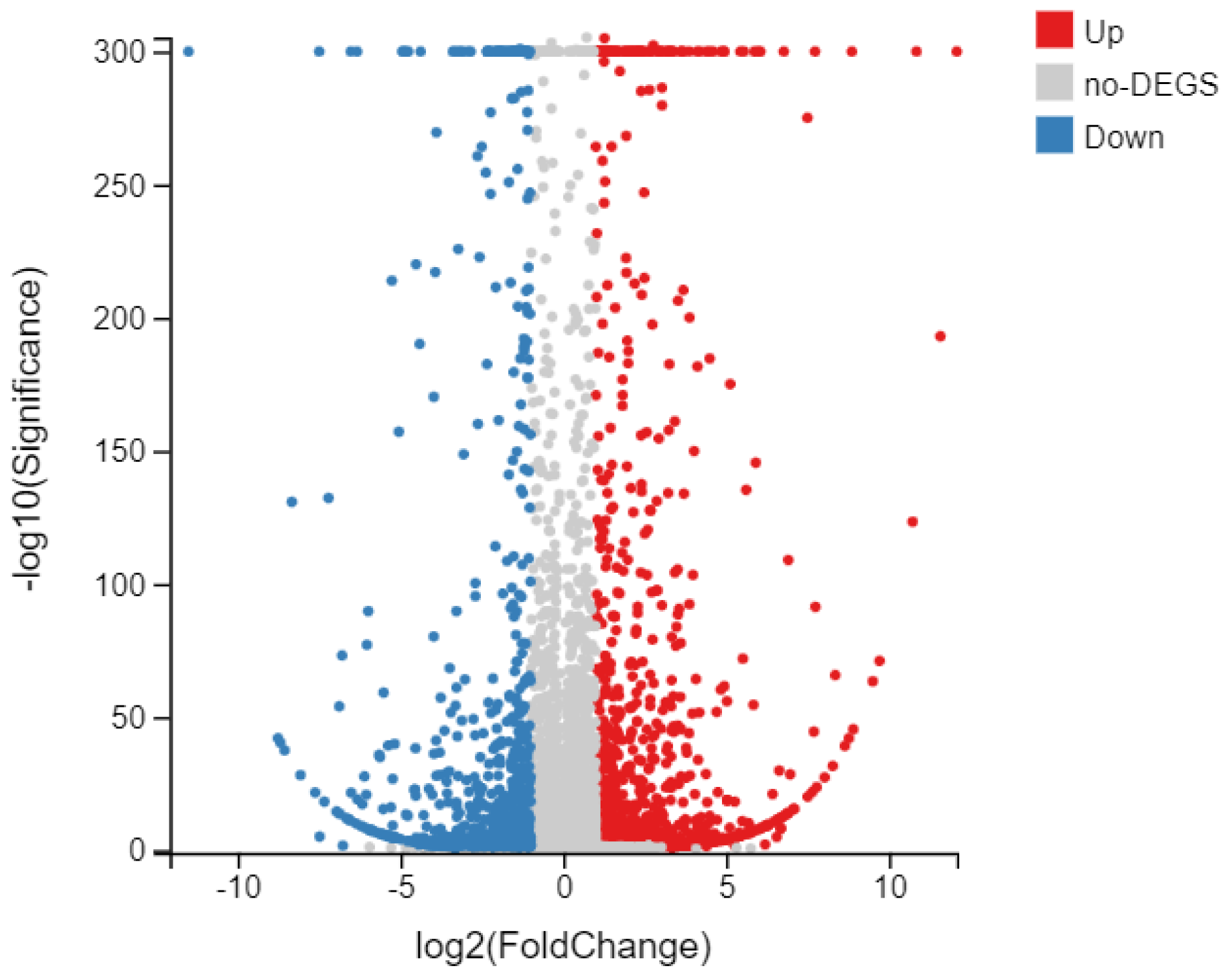
3.4. Effect of eCO2 on the DEGs of FAW
3.5. GO and KEGG Analyses
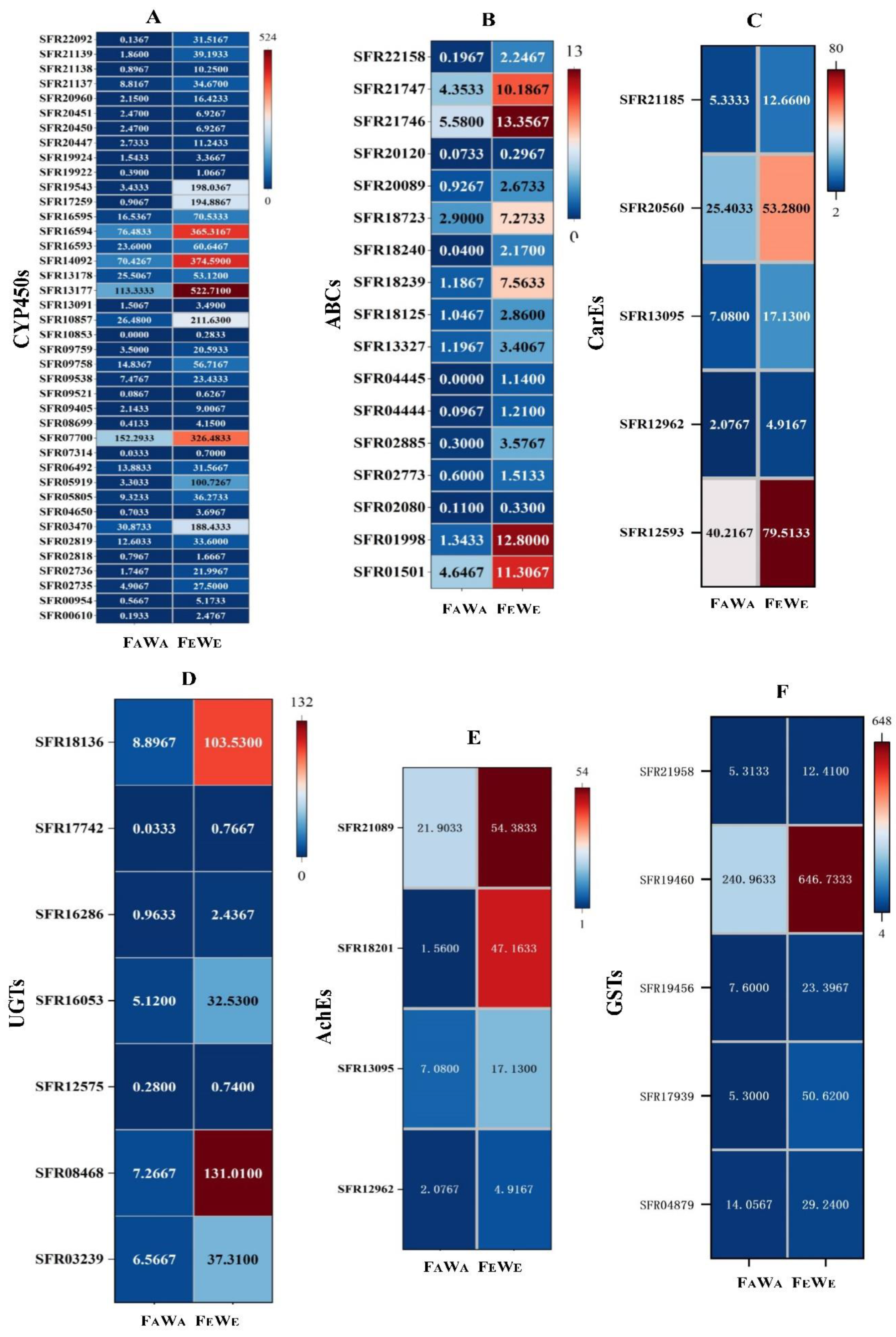
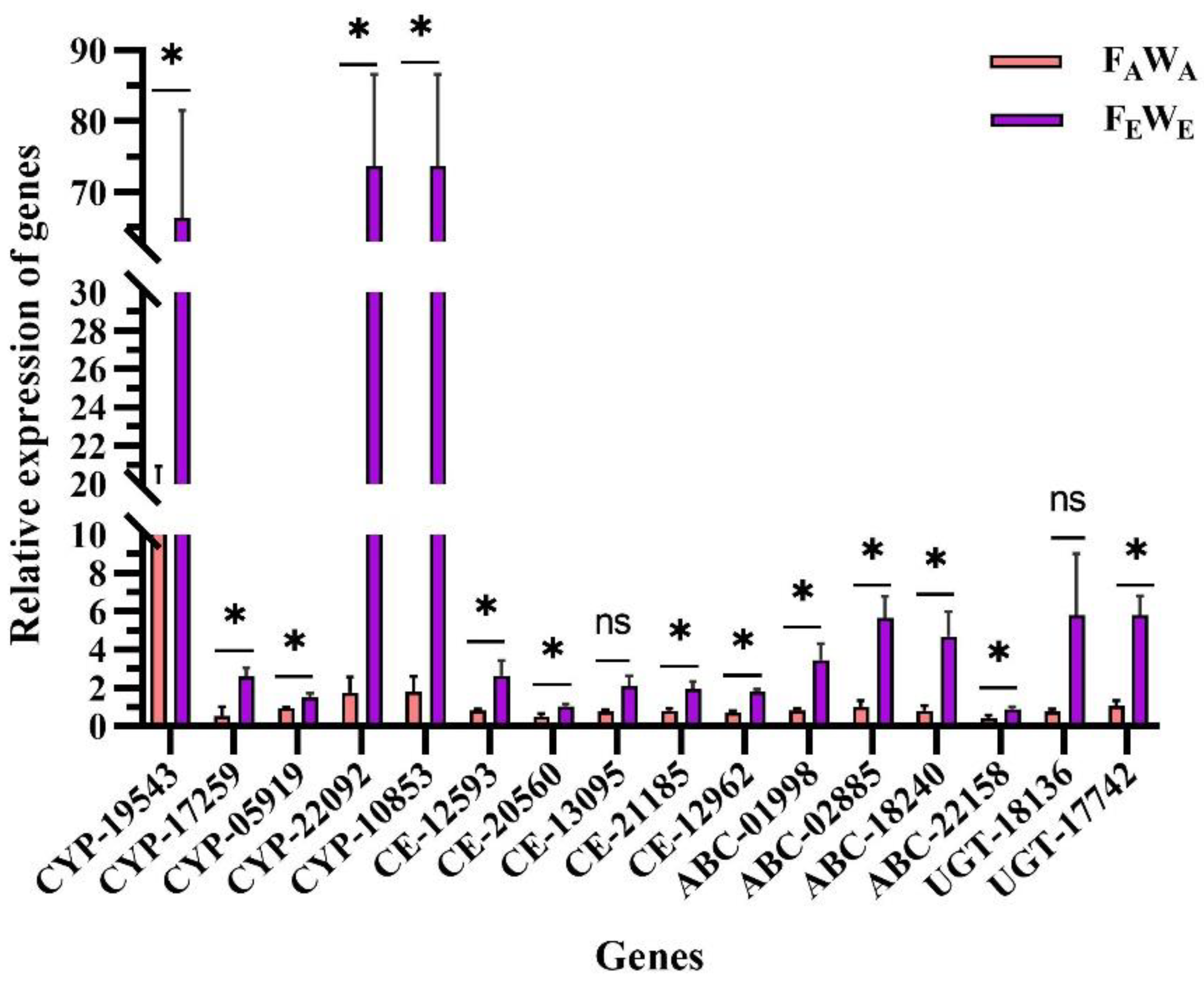
3.6. Detoxification Enzyme Gene Differentially Expression and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate change 2014: Synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. Available online: https://10013/epic.45156.d001 (accessed on 11 March 2022).
- Guerenstein, P.G.; Hildebrand, J.G. Roles and Effects of Environmental Carbon Dioxide in Insect Life. Annu. Rev. Èntomol. 2008, 53, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Zavala, J.A.; Nabity, P.D.; DeLucia, E.H. An Emerging Understanding of Mechanisms Governing Insect Herbivory Under Elevated CO2. Annu. Rev. Èntomol. 2013, 58, 79–97. [Google Scholar] [CrossRef]
- Shuqi, H.; Ying, L.; Lei, Q.; ZhiHua, L.; Chao, X.; Lu, Y.; Furong, G. The Influence of Elevated CO2 Concentration on the Fitness Traits of Frankliniella occidentalis and Frankliniella intonsa (Thysanoptera: Thripidae). Environ. Èntomol. 2017, 46, 722–728. [Google Scholar] [CrossRef] [PubMed]
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate Change: Resetting Plant-Insect Interactions. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Mattson, W.J. Herbivory in Relation to Plant Nitrogen Content. Annu. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Scriber, J.M.; Slansky, F., Jr. The Nutritional Ecology of Immature Insects. Annu. Rev. Èntomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zhang, X.; Li, L.; Lam, S.K.; Pan, G. Changes in plant C, N and P ratios under elevated [CO2] and canopy warming in a rice-winter wheat rotation system. Sci. Rep. 2019, 9, 5424. [Google Scholar] [CrossRef] [PubMed]
- Sparks, A.N. A Review of the Biology of the Fall Armyworm. Fla. Èntomol. 1979, 62, 82–87. [Google Scholar] [CrossRef]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. Available online: https://hdl.handle.net/10520/EJC-112bc26060 (accessed on 11 March 2022). [CrossRef]
- Gui, F.; Lan, T.; Zhao, Y.; Guo, W.; Dong, Y.; Fang, D.; Liu, H.; Li, H.; Wang, H.; Hao, R.; et al. Genomic and transcriptomic analysis unveils population evolution and development of pesticide resistance in fall armyworm Spodoptera frugiperda. Protein Cell 2020, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Huang, C.; Li, C.-Y.; Zhou, H.-X.; Ren, Y.-L.; Li, Z.-Y.; Xing, L.-S.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Wu, P.; Wu, F.; Fan, J.; Zhang, R. Potential economic impact of invasive fall armyworm on mainly affected crops in China. J. Pest Sci. 2021, 94, 1065–1073. [Google Scholar] [CrossRef]
- Calderon, M.; Leesch, J.G. Effect of Reduced Pressure and CO2 on the Toxicity of Methyl Bromide to Two Species of Stored-Product Insects1. J. Econ. Èntomol. 1983, 76, 1125–1128. [Google Scholar] [CrossRef]
- Fan, Z.; Qian, L.; Chen, Y.; Fan, R.; He, S.; Gao, Y.; Gui, F. Effects of elevated CO2 on activities of protective and detoxifying enzymes in Frankliniella occidentalis and F. intonsa under spinetoram stress. Pest Manag. Sci. 2021, 78, 274–286. [Google Scholar] [CrossRef]
- Rao, M.S.; Sreelakshmi, P.; Deekshita, K.; Vanaja, M.; Srinivas, I.; Maheswari, M.; Reddy, K.S.; Bhaskar, S.; Chary, G.R. Interactive effects of temperature and CO2 on efficacy of insecticides against Spodoptera litura Fab. in a global warming context. Phytoparasitica 2021, 49, 417–431. [Google Scholar] [CrossRef]
- Ge, L.-Q.; Wu, J.-C.; Sun, Y.-C.; Ouyang, F.; Ge, F. Effects of triazophos on biochemical substances of transgenic Bt rice and its nontarget pest Nilaparvata lugens Stål under elevated CO2. Pestic. Biochem. Physiol. 2013, 107, 188–199. [Google Scholar] [CrossRef]
- Qian, L.; He, S.; Liu, X.; Huang, Z.; Chen, F.; Gui, F. Effect of elevated CO2 on the interaction between invasive thrips, Frankliniella occidentalis, and its host kidney bean, Phaseolus vulgaris. Pest Manag. Sci. 2018, 74, 2773–2782. [Google Scholar] [CrossRef]
- Dai, L.; Gao, H.; Chen, H. Expression Levels of Detoxification Enzyme Genes from Dendroctonus armandi (Coleoptera: Curculionidae) Fed on a Solid Diet Containing Pine Phloem and Terpenoids. Insects 2021, 12, 926. [Google Scholar] [CrossRef]
- Liu, B.; Tang, M.; Chen, H. Activation of the ROS/CncC Signaling Pathway Regulates Cytochrome P450 CYP4BQ1 Responsible for (+)-α-Pinene Tolerance in Dendroctonus armandi. Int. J. Mol. Sci. 2022, 23, 11578. [Google Scholar] [CrossRef]
- Bolzan, A.; Padovez, F.E.O.; Nascimento, A.R.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Bai-Zhong, Z.; Xu, S.; Cong-Ai, Z.; Liu-Yang, L.; Ya-She, L.; Xing, G.; Dong-Mei, C.; Zhang, P.; Ming-Wang, S.; Xi-Ling, C. Silencing of Cytochrome P450 in Spodoptera frugiperda (Lepidoptera: Noctuidae) by RNA Interference Enhances Susceptibility to Chlorantraniliprole. J. Insect Sci. 2020, 20, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pes, M.P.; Melo, A.A.; Stacke, R.S.; Zanella, R.; Perini, C.R.; Silva, F.M.A.; Guedes, J.V.C. Translocation of chlorantraniliprole and cyantraniliprole applied to corn as seed treatment and foliar spraying to control Spodoptera frugiperda (Lepidoptera: Noctuidae). PLoS ONE 2020, 15, e0229151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, H.; Wu, J.; Zheng, F.; Xu, K.; Lin, Y.; Zhang, Z.; Xu, H. Drip application of chlorantraniliprole effectively controls invasive Spodoptera frugiperda (Lepidoptera: Noctuidae) and its distribution in maize in China. Crop Prot. 2020, 143, 105474. [Google Scholar] [CrossRef]
- Su, X.N.; Li, C.Y.; Huang, S.H.; Liu, W.L.; Zhang, Y.P.; Pan, Z.P. Optimization of artificial diet and rearing condition of fall armyworm, Spodoptera frugiperda (J. E. Smith). J. Environ. Entomol. 2019, 41, 992–998. (In Chinese) [Google Scholar]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Kolde, R. Package ‘Pheatmap’. 2019-01-04 13:50:12 UTC. Available online: https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf (accessed on 1 September 2021).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Matzrafi, M. Climate change exacerbates pest damage through reduced pesticide efficacy. Pest Manag. Sci. 2019, 75, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Teets, N.M.; Peyton, J.T.; Ragland, G.J.; Colinet, H.; Renault, D.; Hahn, D.A.; Denlinger, D.L. Combined transcriptomic and metabolomic approach uncovers molecular mechanisms of cold tolerance in a temperate flesh fly. Physiol. Genom. 2012, 44, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, X.; Shen, J.; Du, Y.; Xu, K.; Jiang, Y. Transcriptomic analysis reveals Apis mellifera adaptations to high temperature and high humidity. Ecotoxicol. Environ. Saf. 2019, 184, 109599. [Google Scholar] [CrossRef]
- Lessard, F.F. Effect of modified atmospheres on insects and mites infesting stored products. In Food Preservation by Modified Atmospheres; CRC Press: Boca Raton, FL, USA, 1990; pp. 21–38. Available online: https://www.researchgate.net/publication/305042923_Effect_of_modified_atmospheres_on_insects_and_mites_infesting_stored_products (accessed on 14 April 2022).
- Edwards, L.J. Carbon dioxide anaesthesia and succinic dehydrogenase in the corn earworm, Heliothis zea. J. Insect Physiol. 1968, 14, 1045–1048. [Google Scholar] [CrossRef]
- Friedlander, A.; Navarro, S. The effect of controlled atmospheres on carbohydrate metabolism in the tissue of Ephestia cautella (walker) pupae. Insect Biochem. 1979, 9, 79–83. [Google Scholar] [CrossRef]
- Zhou, S.; Criddle, R.S.; Mitcham, E.J. Metabolic response of Platynota stultana pupae during and after extended exposure to elevated CO2 and reduced O2 atmospheres. J. Insect Physiol. 2001, 47, 401–409. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, H.; Ge, F. Plant–Aphid Interactions Under Elevated CO2: Some Cues from Aphid Feeding Behavior. Front. Plant Sci. 2016, 7, 502. [Google Scholar] [CrossRef]
- Liu, J.; Fang, X.; Deng, Q.; Han, T.; Huang, W.; Li, Y. CO2 enrichment and N addition increase nutrient loss from decomposing leaf litter in subtropical model forest ecosystems. Sci. Rep. 2015, 5, srep07952. [Google Scholar] [CrossRef]
- Manimanjari, D.; Rao, M.S.; Swathi, P.; Rao, C.A.R.; Vanaja, M.; Maheswari, M. Temperature- and CO2-dependent life table parameters of Spodoptera litura (Noctuidae: Lepidoptera) on sunflower and prediction of pest scenarios. J. Insect Sci. 2014, 14, 297. [Google Scholar] [CrossRef][Green Version]
- Satishchandra, N.K.; Vaddi, S.; Naik, S.O.; Chakravarthy, A.K.; Atlihan, R. Effect of Temperature and CO2 on Population Growth of South American Tomato Moth, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on Tomato. J. Econ. Èntomol. 2018, 111, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Högy, P.; Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 2008, 48, 580–591. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO 2 reduces the nitrogen concentration of plant tissues. Glob. Chang. Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Elevated Carbon Dioxide Increases Contents of Flavonoids and Phenolic Compounds, and Antioxidant Activities in Malaysian Young Ginger (Zingiber officinale Roscoe.) Varieties. Molecules 2010, 15, 7907–7922. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.F.; Zangerl, A.R.; Dermody, O.; Bilgin, D.D.; Casteel, C.L.; Zavala, J.A.; DeLucia, E.H.; Berenbaum, M.R. Impact of Elevated Levels of Atmospheric CO2 and Herbivory on Flavonoids of Soybean (Glycine max Linnaeus). J. Chem. Ecol. 2010, 36, 35–45. [Google Scholar] [CrossRef]
- Bidart-Bouzat, M.G.; Mithen, R.; Berenbaum, M.R. Elevated CO2 influences herbivory-induced defense responses of Arabidopsis thaliana. Oecologia 2005, 145, 415–424. [Google Scholar] [CrossRef]
- Schonhof, I.; Kläring, H.-P.; Krumbein, A.; Schreiner, M. Interaction Between Atmospheric CO2 and Glucosinolates in Broccoli. J. Chem. Ecol. 2006, 33, 105–114. [Google Scholar] [CrossRef]
- Lu, K.; Cheng, Y.; Li, Y.; Li, W.; Zeng, R.; Song, Y. Phytochemical Flavone Confers Broad-Spectrum Tolerance to Insecticides in Spodoptera litura by Activating ROS/CncC-Mediated Xenobiotic Detoxification Pathways. J. Agric. Food Chem. 2021, 69, 7429–7445. [Google Scholar] [CrossRef]
- Lu, K.; Li, Y.; Cheng, Y.; Li, W.; Zeng, B.; Gu, C.; Zeng, R.; Song, Y. Activation of the ROS/CncC and 20-Hydroxyecdysone Signaling Pathways Is Associated with Xanthotoxin-Induced Tolerance to λ-Cyhalothrin in Spodoptera litura. J. Agric. Food Chem. 2021, 69, 13425–13435. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, Q.; Li, Q.; Wang, R.; Xu, C.; Wang, H.; Ran, C.; Song, Y.; Zeng, R. Identification of a cytochrome P450 CYP6AB60 gene associated with tolerance to multi-plant allelochemicals from a polyphagous caterpillar tobacco cutworm (Spodoptera litura). Pestic. Biochem. Physiol. 2019, 154, 60–66. [Google Scholar] [CrossRef]
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Èntomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.R.; Johnson, R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2020, 43, 103–107. [Google Scholar] [CrossRef]
- Qian, L.; Chen, F.; Liu, J.; He, S.; Liu, J.; Li, Z.; Gui, F. Effects of elevated CO2 on life-history traits of three successive generations of Frankliniella occidentalis and F. intonsa on kidney bean, Phaseolus vulgaris. Èntomol. Exp. Appl. 2017, 165, 50–61. [Google Scholar] [CrossRef]
- Yin, J.; Sun, Y.; Wu, G.; Ge, F. Effects of elevated CO2 associated with maize on multiple generations of the cotton bollworm, Helicoverpa armigera. Èntomol. Exp. Appl. 2010, 136, 12–20. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, L.; Yang, Q.; Wang, Z.; He, K. Direct Effects of Elevated CO2 Levels on the Fitness Performance of Asian Corn Borer (Lepidoptera: Crambidae) for Multigenerations. Environ. Èntomol. 2015, 44, 1250–1257. [Google Scholar] [CrossRef]


| Generation | Treatment | Concentration Response Regression Equation | χ2 | p | LC50 (mg L−1) 95% CI |
|---|---|---|---|---|---|
| 1st | FAWA | y = 4.2826 + 0.7447x | 51.97 | 0.0001 | 9.19 (1.57−59.67) |
| FEWE | y = 2.6994 + 1.7117x | 33.90 | 0.0001 | 22.08 (9.00−64.63) | |
| 2nd | FAWA | y = 3.4718 + 1.5021x | 40.16 | 0.0001 | 10.41 (4.25−27.20) |
| FEWE | y = 3.9791 + 0.7667x | 8.71 | 0.0334 | 21.45 (13.49−39.63) | |
| 3rd | FAWA | y = 4.2871 + 0.5890x | 23.20 | 0.0001 | 16.82 (13.44−51.72) |
| FEWE | y = 3.5639 + 1.0336x | 7.99 | 0.0463 | 24.52 (12.96−71.98) |
| Pathway ID | Pathway Name | p Value | Q Value |
|---|---|---|---|
| ko00563 | Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 0.000000001 | 0.0000000581 |
| ko00965 | Betalain biosynthesis | 0.000000001 | 0.0000000581 |
| ko00950 | Isoquinoline alkaloid biosynthesis | 0.000000016 | 0.0000006040 |
| ko00790 | Folate biosynthesis | 0.000015700 | 0.0004409770 |
| ko00350 | Tyrosine metabolism | 0.000021100 | 0.0004722640 |
| ko03450 | Non-homologous end-joining | 0.000074600 | 0.0013931500 |
| ko03420 | Nucleotide excision repair | 0.000169718 | 0.0027154880 |
| ko00310 | Lysine degradation | 0.000531000 | 0.0074340010 |
| ko00052 | Galactose metabolism | 0.000914600 | 0.0113816900 |
| ko00590 | Arachidonic acid metabolism | 0.001703890 | 0.0173487000 |
| ko00561 | Glycerolipid metabolism | 0.002804281 | 0.0261732900 |
| ko03060 | Protein export | 0.005070793 | 0.0436868300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Sun, Z.; Li, Y.; Hao, R.; Chen, Y.; Chen, B.; Qin, X.; Tao, X.; Gui, F. Effects of Elevated CO2 Concentration on Host Adaptability and Chlorantraniliprole Susceptibility in Spodoptera frugiperda. Insects 2022, 13, 1029. https://doi.org/10.3390/insects13111029
Lu Z, Sun Z, Li Y, Hao R, Chen Y, Chen B, Qin X, Tao X, Gui F. Effects of Elevated CO2 Concentration on Host Adaptability and Chlorantraniliprole Susceptibility in Spodoptera frugiperda. Insects. 2022; 13(11):1029. https://doi.org/10.3390/insects13111029
Chicago/Turabian StyleLu, Zhihui, Zhongxiang Sun, Yahong Li, Ruoshi Hao, Yaping Chen, Bin Chen, Xiaoping Qin, Xuan Tao, and Furong Gui. 2022. "Effects of Elevated CO2 Concentration on Host Adaptability and Chlorantraniliprole Susceptibility in Spodoptera frugiperda" Insects 13, no. 11: 1029. https://doi.org/10.3390/insects13111029
APA StyleLu, Z., Sun, Z., Li, Y., Hao, R., Chen, Y., Chen, B., Qin, X., Tao, X., & Gui, F. (2022). Effects of Elevated CO2 Concentration on Host Adaptability and Chlorantraniliprole Susceptibility in Spodoptera frugiperda. Insects, 13(11), 1029. https://doi.org/10.3390/insects13111029






