Survey of Candidatus Liberibacter Solanacearum and Its Associated Vectors in Potato Crop in Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling Methods for Insects
2.3. CaLsol Detection in Psyllids and Plants
3. Results
3.1. Occasional Surveys
3.1.1. Detection of the Disease and the Bacterium in Plants
3.1.2. Psyllid Species Associated with Potato Crop in Spain
3.2. Regular Surveys
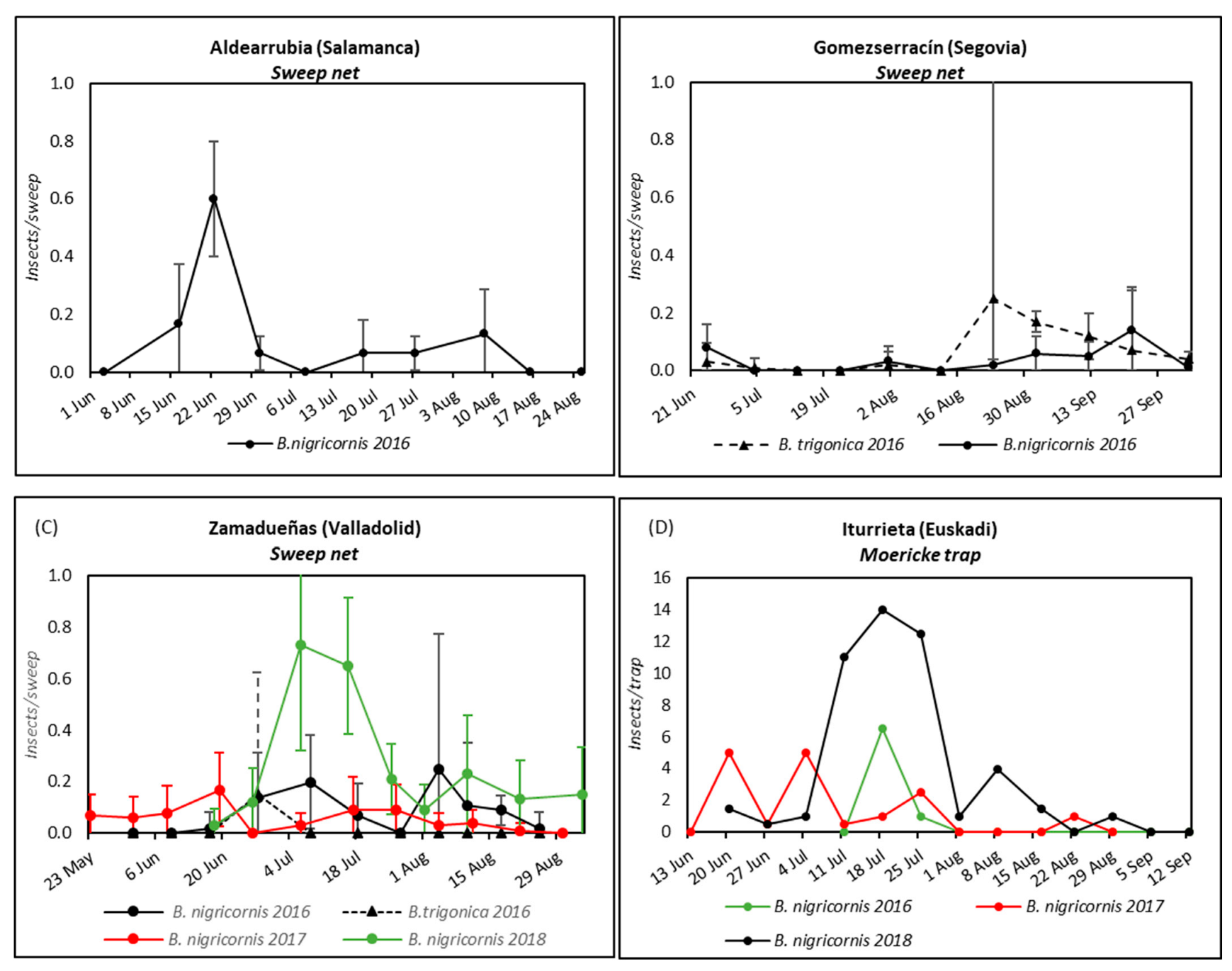
3.2.1. Study of the Abundance of the Psyllid Species during the Potato Cropping Season
3.2.2. Study of the Psyllids as Vector of CaLsol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munyaneza, J. Zebra Chip Disease, Candidatus Liberibacter, and Potato Psyllid: A Global Threat to the Potato Industry. Am. J. Potato Res. 2015, 92, 230–235. [Google Scholar] [CrossRef]
- American Phytopathological Society New Variety of Zebra Chip Disease Threatens Potato Production in Southwestern Oregon. ScienceDaily. 2019. Available online: https://www.sciencedaily.com/releases/2019/04/190418153644.htm (accessed on 21 August 2022).
- Greenway, G.A.; Rondon, S. Economic Impacts of Zebra Chip in Idaho, Oregon, and Washington. Am. J. Potato Res. 2018, 95, 362–367. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Cebrián, M.C.; Villaescusa, F.J.; de Mendoza, A.H.; Ferrándiz, J.C.; Sanjuán, S.; Font, M.I. First Report of Candidatus Liberibacter Solanacearum in Carrot in Mainland Spain. Plant Dis. 2012, 96, 582. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, M.; Garnier, S.; Boirin, V.; Merieau, M.; Leguay, A.; Renaudin, I.; Renvoisé, J.-P.; Gentit, P. First Report of Candidatus Liberibacter Solanacearum in Carrot in France. Plant Dis. 2014, 98, 839. [Google Scholar] [CrossRef]
- Munyaneza, J.; Fisher, T.W.; Sengoda, V.G.; Garczynski, S.F.; Nissinen, A.; Lemmetty, A. First Report of Candidatus Liberibacter Solanacearum Associated with Psyllid-Affected Carrots in Europe. Plant Dis. 2010, 94, 639. [Google Scholar] [CrossRef]
- EPPO PM 7/143 (1) Candidatus Liberibacter Solanacearum. EPPO Bull. 2020, 50, 49–68. [CrossRef]
- Alfaro-Fernández, A.; Siverio, F.; Cebrián, M.C.; Villaescusa, F.J.; Font, M.I. Candidatus Liberibacter Solanacearum Associated with Bactericera trigonica Affected Carrots in the Canary Islands. Plant Dis. 2012, 96, 581. [Google Scholar] [CrossRef]
- Teresani, G.; Bertolini, E.; Alfaro-Fernández, A.; Martínez, C.; Tanaka, F.A.O.; Kitajima, E.W.; Roselló, M.; Sanjuán, S.; Ferrándiz, J.C.; López, M.M.; et al. Association of Candidatus Liberibacter Solanacearum with a Vegetative Disorder of Celery in Spain and Development of a Real-Time Pcr Method for Its Detection. Phytopathology 2014, 104, 804–811. [Google Scholar] [CrossRef]
- Eurostat The EU Potato Sector—Statistics on Production, Prices and Trade—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=The_EU_potato_sector_-_statistics_on_production,_prices_and_trade (accessed on 16 June 2022).
- MAPA Producciones Agrícolas: Patata. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/patata/ (accessed on 17 June 2022).
- Soliman, T.; Mourits, M.C.M.; Oude Lansink, A.G.J.M.; van der Werf, W. Economic Justification for Quarantine Status—The Case Study of Candidatus Liberibacter Solanacearum in the European Union. Plant Pathol. 2013, 62, 1106–1113. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Rastas, M.; Wang, J.; Hannukkala, A.; Pirhonen, M.; Nissinen, A.I. Carrot Pathogen Candidatus Liberibacter Solanacearum Haplotype C Detected in Symptomless Potato Plants in Finland. Potato Res. 2018, 61, 31–50. [Google Scholar] [CrossRef]
- Palomo, J.; Bertolini, E.; Martín-Robles, M.; Teresani, G.; López, M.M.; Cambra, M. Detección En Patata En España de Un Haplotipo de Candidatus Liberibacter Solanacearum No Descrito En Solanáceas. In Proceedings of the XVII Congress of Spanish Phytopathological Society, Lleida, Spain, 7–10 October 2014. [Google Scholar]
- Ruiz-Padilla, A.; Redondo, C.; Asensio, A.; Garita-Cambronero, J.; Martínez, C.; Pérez-Padilla, V.; Marquínez, R.; Collar, J.; García-Méndez, E.; Alfaro-Fernández, A.; et al. Assessment of Multilocus Sequence Analysis (MLSA) for Identification of Candidatus Liberibacter Solanacearum from Different Host Plants in Spain. Microorganisms 2020, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Sengoda, V.; Cooper, W.R.; Swisher, K.D.; Henne, D.C.; Munyaneza, J.E. Latent Period and Transmission of Candidatus Liberibacter Solanacearum by the Potato Psyllid Bactericera cockerelli (Hemiptera: Triozidae). PLoS ONE 2014, 9, e93475. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.R.; Sengoda, V.; Munyaneza, J.E. Localization of Candidatus Liberibacter Solanacearum (Rhizobiales: Rhizobiaceae) in Bactericera Cockerelli (Hemiptera: Triozidae). Ann. Entomol. Soc. Am. 2014, 107, 204–210. [Google Scholar] [CrossRef]
- Nelson, W.R.; Sengoda, V.; Alfaro-Fernandez, A.O.; Font, M.I.; Crosslin, J.M.; Munyaneza, J.E. A New Haplotype of Candidatus Liberibacter Solanacearum Identified in the Mediterranean Region. Eur. J. Plant Pathol. 2013, 135, 633–639. [Google Scholar] [CrossRef]
- Munyaneza, J.; Fisher, T.W.; Sengoda, V.; Garczynski, S.F.; Nissinen, A.; Lemmetty, A. Association of Candidatus Liberibacter Solanacearum with the Psyllid, Trioza Apicalis (Hemiptera: Triozidae) in Europe. J. Econ. Entomol. 2010, 103, 1060–1070. [Google Scholar] [CrossRef]
- Teresani, G.; Hernández, E.; Bertolini, E.; Siverio, F.; Marroquín, C.; Molina, J.; de Mendoza, A.H.; Cambra, M. Search for Potential Vectors of Candidatus Liberibacter Solanacearum: Population Dynamics in Host Crops. Span. J. Agric. Res. 2015, 13, e1002. [Google Scholar] [CrossRef]
- Ben Othmen, S.; Morán, F.E.; Navarro, I.; Barbé, S.; Martínez, C.; Marco-Noales, E.; Chermiti, B.; López, M.M. Candidatus Liberibacter Solanacearum Haplotypes D and E in Carrot Plants and Seeds in Tunisia. J. Plant Pathol. 2018, 100, 197–207. [Google Scholar] [CrossRef]
- Antolínez, C.A.; Moreno, A.; Ontiveros, I.; Pla, S.; Plaza, M.; Sanjuan, S.; Palomo, J.L.; Sjölund, M.J.; Sumner-Kalkun, J.C.; Arnsdorf, Y.M.; et al. Seasonal Abundance of Psyllid Species on Carrots and Potato Crops in Spain. Insects 2019, 10, 287. [Google Scholar] [CrossRef]
- Ben Othmen, S.; Abbes, K.; El Imem, M.; Ouvrard, D.; Rapisarda, C.; Chermiti, B. Bactericera Trigonica and B. Nigricornis (Hemiptera: Psylloidea) in Tunisia as Potential Vectors of Candidatus Liberibacter Solanacearum on Apiaceae. Orient. Insects 2019, 53, 497–509. [Google Scholar] [CrossRef]
- Monger, W.A.; Jeffries, C.J. First Report of Candidatus Liberibacter Solanacearum in Parsley (Petroselinum Crispum) Seed. New Dis. Rep. 2016, 34, 31. [Google Scholar] [CrossRef]
- Haapalainen, M.; Wang, J.; Latvala, S.; Lehtonen, M.T.; Pirhonen, M.; Nissinen, A.I. Genetic Variation of Candidatus Liberibacter Solanacearum Haplotype C and Identification of a Novel Haplotype from Trioza Urticae and Stinging Nettle. Phytopathology 2018, 108, 925–934. [Google Scholar] [CrossRef]
- Swisher Grimm, K.D.; Garczynski, S.F. Identification of a New Haplotype of Candidatus Liberibacter Solanacearum in Solanum tuberosum. Plant Dis. 2019, 103, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Sun, P.; Meduri, V.R.S.; Hansen, A.K. New Ca. Liberibacter Psyllaurous Haplotype Resurrected from a 49-Year-Old Specimen of Solanum umbelliferum: A Native Host of the Psyllid Vector. Sci. Rep. 2019, 9, 9530. [Google Scholar] [CrossRef] [PubMed]
- Haapalainen, M.; Latvala, S.; Wickström, A.; Wang, J.; Pirhonen, M.; Nissinen, A.I. A Novel Haplotype of Candidatus Liberibacter Solanacearum Found in Apiaceae and Polygonaceae Family Plants. Eur. J. Plant Pathol. 2020, 156, 413–423. [Google Scholar] [CrossRef]
- Antolínez, C.A.; Fereres, A.; Moreno, A. Risk Assessment of Candidatus Liberibacter Solanacearum Transmission by the Psyllids Bactericera trigonica and B. tremblayi from Apiaceae Crops to Potato. Sci. Rep. 2017, 7, 45534. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Miranda, M.P.; Fereres, A. Psyllids as Major Vectors of Plant Pathogens. Entomol. Gen. 2021, 41, 419–438. [Google Scholar] [CrossRef]
- Ouvrard, D.; Burckhardt, D. First Record of the Onion Psyllid Bactericera tremblayi (Wagner, 1961) in France (Insecta: Hemiptera: Steirnorrhyncha: Psylloidea), New Symptoms on Leek Crops and Reassessment of the B. nigricornis-Group Distribution. EPPO Bull. 2012, 42, 585–590. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Status and Taxonomy of the Trioza (Bactericera) Nigricornis Förster Complex (Hemiptera: Triozidae). Bull. Entomol. Res. 1981, 71, 671–679. [Google Scholar] [CrossRef]
- Irwin, M.E. Sampling Aphids in Soybean Fields. In Sampling Methods in Soybean Entomology; Springer: New York, NY, USA, 1980; pp. 239–259. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid Isolation of High Molecular Weight Plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Casquet, J.; Thebaud, C.; Gillespie, R.G. Chelex without Boiling, a Rapid and Easy Technique to Obtain Stable Amplifiable DNA from Small Amounts of Ethanol-Stored Spiders. Mol. Ecol. Resour. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Levy, J.; Ravindran, A.; Gross, D.; Tamborindeguy, C.; Pierson, E. Translocation of Candidatus Liberibacter Solanacearum, the Zebra Chip Pathogen, in Potato and Tomato. Phytopathology 2011, 101, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Munyaneza, J.; Crosslin, J.M.; Buchman, J.L. Seasonal Occurrence and Abundance of the Potato Psyllid, Bactericera cockerelli, in South Central Washington. Am. J. Potato Res. 2009, 86, 513–518. [Google Scholar] [CrossRef]
- Nissinen, A.I.; Pihlava, J.M.; Latvala, S.; Jauhiainen, L. Assessment of the Efficiency of Different Control Programs to Reduce Trioza apicalis Först. (Triozidae: Hemiptera) Feeding Damage and the Spread of Candidatus Liberibacter Solanacearum on Carrots (Daucus carota ssp. sativus L.). Ann. Appl. Biol. 2020, 177, 166–177. [Google Scholar] [CrossRef]
- Goolsby, J.; Adamczyk, J.J.; Crosslin, J.M.; Troxclair, N.N.; Anciso, J.R.; Bester, G.G.; Bradshaw, J.D.; Bynum, E.D.; Carpio, L.A.; Henne, D.C.; et al. Seasonal Population Dynamics of the Potato Psyllid (Hemiptera: Triozidae) and Its Associated Pathogen Candidatus Liberibacter Solanacearum in Potatoes in the Southern Great Plains of North America. J. Econ. Entomol. 2012, 105, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, A.I.; Haapalainen, M.; Jauhiainen, L.; Lindman, M.; Pirhonen, M. Different Symptoms in Carrots Caused by Male and Female Carrot Psyllid Feeding and Infection by Candidatus Liberibacter Solanacearum. Plant Pathol. 2014, 63, 812–820. [Google Scholar] [CrossRef]
- Goolsby, J.; Bextine, B.; Munyaneza, J.; Setamou, M.; Adamczyk, J.; Bester, G. Seasonal Abundance of Sharpshooters, Leafhoppers, and Psyllids Associated with Potatoes Affected by Zebra Chip Disorder. Subtrop. Plant Sci. 2007, 59, 15–23. [Google Scholar]


| Locality | Province | Region | Date | Symptomatic Plants | CaLsol+ | Bactericera Nigricornis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Insects/Sweep | ♂ | ♀ | |||||||||
| Mean | St Dv | N | CaLsol+ | N | CaLsol+ | ||||||
| Cogeces de Íscar | Valladolid | Castile and Leon | 28 June 2016 | 13 | 0 | 0.19 | 0.19 | 9 | 0 | 10 | 0 |
| Íscar | Valladolid | Castile and Leon | 28 June 2016 | 10 | 0 | 0.31 | 0.44 | 0 | - | 14 | 0 |
| Pedrajas de San Esteban | Valladolid | Castile and Leon | 28 June 2016 | 17 | 0 | 0.04 | 0.13 | 0 | - | 4 | 0 |
| Escalona del Prado | Segovia | Castile and Leon | 30 June 2016 | 19 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Mozoncillo | Segovia | Castile and Leon | 30 June 2016 | 7 | 0 | 0.34 | 0.22 | 18 | 6 | 16 | 0 |
| Torregutiérrez | Segovia | Castile and Leon | 30 June 2016 | 10 | 0 | 0.47 | 0.18 | 14 | 0 | 33 | 0 |
| Cabizuela | Ávila | Castile and Leon | 7 July 2016 | 7 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Nava de Arévalo | Ávila | Castile and Leon | 7 July 2016 | 13 | 0 | 0.02 | 0.04 | 2 | 0 | 0 | - |
| Vinaderos | Ávila | Castile and Leon | 7 July 2016 | 11 | 0 | 0.04 | 0.07 | 0 | - | 4 | 2 |
| Quintanilla del Agua | Burgos | Castile and Leon | 21 July 2016 | 19 | 0 | 0.01 | 0.03 | 0 | - | 1 | 0 |
| Tordomar | Burgos | Castile and Leon | 21 July 2016 | 0 | 0 | 0.04 | 0.07 | 0 | - | 4 | 0 |
| Cantalpino | Salamanca | Castile and Leon | 28 July 2016 | 19 | 0 | 0.02 | 0.06 | 0 | - | 2 | 0 |
| Pedrosillo de los Aires | Salamanca | Castile and Leon | 28 July 2016 | 17 | 0 | 0.07 | 0.13 | 3 | 0 | 4 | 0 |
| Cabezón de Pisuerga | Valladolid | Castile and Leon | 9 August 2016 | 3 | 0 | 0.01 | 0.03 | 0 | 0 | 1 | 0 |
| Lomoviejo | Valladolid | Castile and Leon | 9 August 2016 | 0 | 1 | 0.05 | 0.16 | 2 | - | 3 | - |
| Velascálvaro | Valladolid | Castile and Leon | 9 August 2016 | 4 | 0 | 0.10 | 0.32 | 3 | 0 | 7 | 0 |
| Chatún | Segovia | Castile and Leon | 1 August 2017 | 0 | 0 | 0.04 | 0.05 | 3 | 0 | 2 | 0 |
| Cogeces de Íscar | Segovia | Castile and Leon | 1 August 2017 | 0 | 0 | 0.22 | 0.20 | 10 | 0 | 12 | 0 |
| Remondo | Segovia | Castile and Leon | 1 August 2017 | 6 | 6 | 0.05 | 0.05 | 1 | 0 | 4 | 0 |
| Castresana de Losa | Burgos | Castile and Leon | 3 August 2017 | 0 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Dobro | Burgos | Castile and Leon | 3 August 2017 | 0 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Fuenteurbel | Burgos | Castile and Leon | 3 August 2017 | 0 | 0 | 0.07 | 0.08 | 0 | - | 7 | 1 |
| Cubillo de Ebro | Santander | Cantabria | 17 August 2017 | 0 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Montecillo | Santander | Cantabria | 17 August 2017 | 0 | 0 | 0.22 | 0.18 | 3 | 0 | 19 | 0 |
| Renedo de Bricia | Santander | Cantabria | 17 August 2017 | 0 | 0 | 0.02 | 0.06 | 1 | 0 | 1 | 0 |
| San Martín de Elines | Santander | Cantabria | 17 August 2017 | 0 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Villamoñico | Santander | Cantabria | 17 August 2017 | 0 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Fuencaliente de Valdelucio | Burgos | Castile and Leon | 17 August 2017 | 0 | 0 | 0.19 | 0.19 | 4 | 0 | 15 | 1 |
| Becerril de Carpio | Palencia | Castile and Leon | 17 August 2017 | 0 | 0 | 0.04 | 0.07 | 3 | 1 | 1 | 0 |
| Santa Mª de Mave | Palencia | Castile and Leon | 17 August 2017 | 0 | 0 | 0.27 | 0.25 | 11 | 0 | 16 | 0 |
| Villallano | Palencia | Castile and Leon | 17 August 2017 | 0 | 0 | 0.47 | 0.13 | 18 | 0 | 29 | 0 |
| Chañe | Segovia | Castile and Leon | 12 July 2018 | 0 | 0 | 0.16 | 0.16 | 4 | 0 | 12 | 0 |
| Remondo | Segovia | Castile and Leon | 12 July 2018 | 0 | 0 | 0.27 | 0.25 | 6 | 0 | 21 | 0 |
| Cogeces de Íscar | Valladolid | Castile and Leon | 12 July 2018 | 0 | 0 | 0.03 | 0.05 | 0 | - | 3 | 0 |
| Cubillo | Santander | Cantabria | 18 July 2018 | 0 | 0 | 0.02 | 0.06 | 0 | - | 2 | - |
| Campo de Cuéllar | Segovia | Castile and Leon | 24 July 2018 | 0 | 0 | 2.26 | 0.73 | 66 | 1 | 160 | 0 |
| Ruerrero | Santander | Cantabria | 30 July 2018 | 0 | 0 | 0.10 | 0.11 | 2 | - | 8 | - |
| Espinosa | Santander | Cantabria | 7 August 2018 | 0 | 0 | 0.08 | 0.08 | 2 | - | 6 | - |
| Cubillo | Santander | Cantabria | 21 August 2018 | 0 | 0 | 0.04 | 0.10 | 0 | - | 4 | - |
| Basconcillos del Tozo | Burgos | Castile and Leon | 21 August 2018 | 0 | 0 | 0.07 | 0.11 | 2 | 0 | 5 | 0 |
| Santa Mª Mave | Palencia | Castile and Leon | 21 August 2018 | 0 | 0 | 0.18 | 0.28 | 5 | 0 | 13 | 0 |
| Villarén de Valdivia | Palencia | Castile and Leon | 21 August 2018 | 0 | 0 | 0.03 | 0.07 | 2 | 0 | 1 | 0 |
| Ruerrero | Santander | Cantabria | 6 September 2018 | 0 | 0 | 0.00 | 0.00 | 0 | - | 0 | - |
| Espinosa | Santander | Cantabria | 13 September 2018 | - | - | 0.01 | 0.03 | 0 | - | 1 | - |
| Total | 7 | 194 | 8 | 445 | 4 | ||||||
| Locality | Province | Region (1) | Year | Sampling Tool (2) | Bactericera Nigricornis | Bactericera Trigonica | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ♂ | CaLsol+ | ♀ | CaLsol+ | Total | ♂ | CaLsol+ | ♀ | CaLsol+ | |||||
| Güimar | Tenerife | CI | 2016 | MT | 0 | 0 | - | 0 | - | 152 | 139 | 136 | 5 | 5 |
| Isamar | Tenerife | CI | 2016 | MT | 0 | 0 | - | 0 | - | 17 | 13 | 8 | 4 | 0 |
| Tegueste | Tenerife | CI | 2016 | MT | 0 | 0 | - | 0 | - | 64 | 48 | 45 | 16 | 16 |
| Aldearrubia | Salamanca | CyL | 2016 | SN | 33 | 11 | 1 | 22 | 0 | 0 | 0 | - | 0 | - |
| Gomezserracín | Segovia | CyL | 2016 | SN | 39 | 11 | 1 | 28 | 2 | 71 | 51 | 34 | 20 | 15 |
| Zamadueñas | Valladolid | CyL | 2016 | SN | 88 | 26 | 0 | 62 | 0 | 15 | 8 | 4 | 7 | 3 |
| Iturrieta | Araba | Euskadi | 2016 | MT | 15 | 10 | 2 | 5 | 0 | 0 | 0 | - | 0 | - |
| Zamadueñas | Valladolid | CyL | 2017 | IT | 23 | 14 | 6 | 9 | 2 | 0 | 0 | - | 0 | - |
| Zamadueñas | Valladolid | CyL | 2017 | SN | 64 | 17 | 0 | 45 | 0 | 2 | 0 | - | 2 | 1 |
| Iturrieta | Araba | Euskadi | 2017 | MT | 31 | 26 | 2 | 5 | 0 | 0 | 0 | - | 0 | - |
| Zamadueñas | Valladolid | CyL | 2018 | IT | 0 | 0 | - | 0 | - | 0 | 0 | - | 0 | - |
| Zamadueñas | Valladolid | CyL | 2018 | SN | 226 | 91 | 0 | 135 | 2 | 1 | 0 | - | 1 | 0 |
| Iturrieta | Araba | Euskadi | 2018 | MT | 96 | 84 | 2 | 12 | 0 | 0 | 0 | - | 0 | - |
| Total | 615 | 290 | 14 | 323 | 6 | 322 | 259 | 227 | 55 | 40 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asensio-S.-Manzanera, M.C.; Santiago-Calvo, Y.; Palomo-Gómez, J.L.; Marquínez-Ramírez, R.; Bastin, S.; García-Méndez, E.M.; Hernández-Suárez, E.; Siverio-de-la-Rosa, F. Survey of Candidatus Liberibacter Solanacearum and Its Associated Vectors in Potato Crop in Spain. Insects 2022, 13, 964. https://doi.org/10.3390/insects13100964
Asensio-S.-Manzanera MC, Santiago-Calvo Y, Palomo-Gómez JL, Marquínez-Ramírez R, Bastin S, García-Méndez EM, Hernández-Suárez E, Siverio-de-la-Rosa F. Survey of Candidatus Liberibacter Solanacearum and Its Associated Vectors in Potato Crop in Spain. Insects. 2022; 13(10):964. https://doi.org/10.3390/insects13100964
Chicago/Turabian StyleAsensio-S.-Manzanera, M. Carmen, Yolanda Santiago-Calvo, José Luis Palomo-Gómez, Raquel Marquínez-Ramírez, Saskia Bastin, Eva María García-Méndez, Estrella Hernández-Suárez, and Felipe Siverio-de-la-Rosa. 2022. "Survey of Candidatus Liberibacter Solanacearum and Its Associated Vectors in Potato Crop in Spain" Insects 13, no. 10: 964. https://doi.org/10.3390/insects13100964
APA StyleAsensio-S.-Manzanera, M. C., Santiago-Calvo, Y., Palomo-Gómez, J. L., Marquínez-Ramírez, R., Bastin, S., García-Méndez, E. M., Hernández-Suárez, E., & Siverio-de-la-Rosa, F. (2022). Survey of Candidatus Liberibacter Solanacearum and Its Associated Vectors in Potato Crop in Spain. Insects, 13(10), 964. https://doi.org/10.3390/insects13100964







