Sublethal and Transgenerational Toxicities of Chlorfenapyr on Biological Traits and Enzyme Activities of Paracoccus marginatus (Hemiptera: Pseudococcidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Toxicity of Chlorfenapyr on P. marginatus
2.3. Transgenerational and Sublethal Effects of Chlorfenapyr on P. marginatus
2.4. Enzyme Activity Assay
2.5. Data Analysis
3. Results
3.1. Toxicity of Chlorfenapyr on P. marginatus
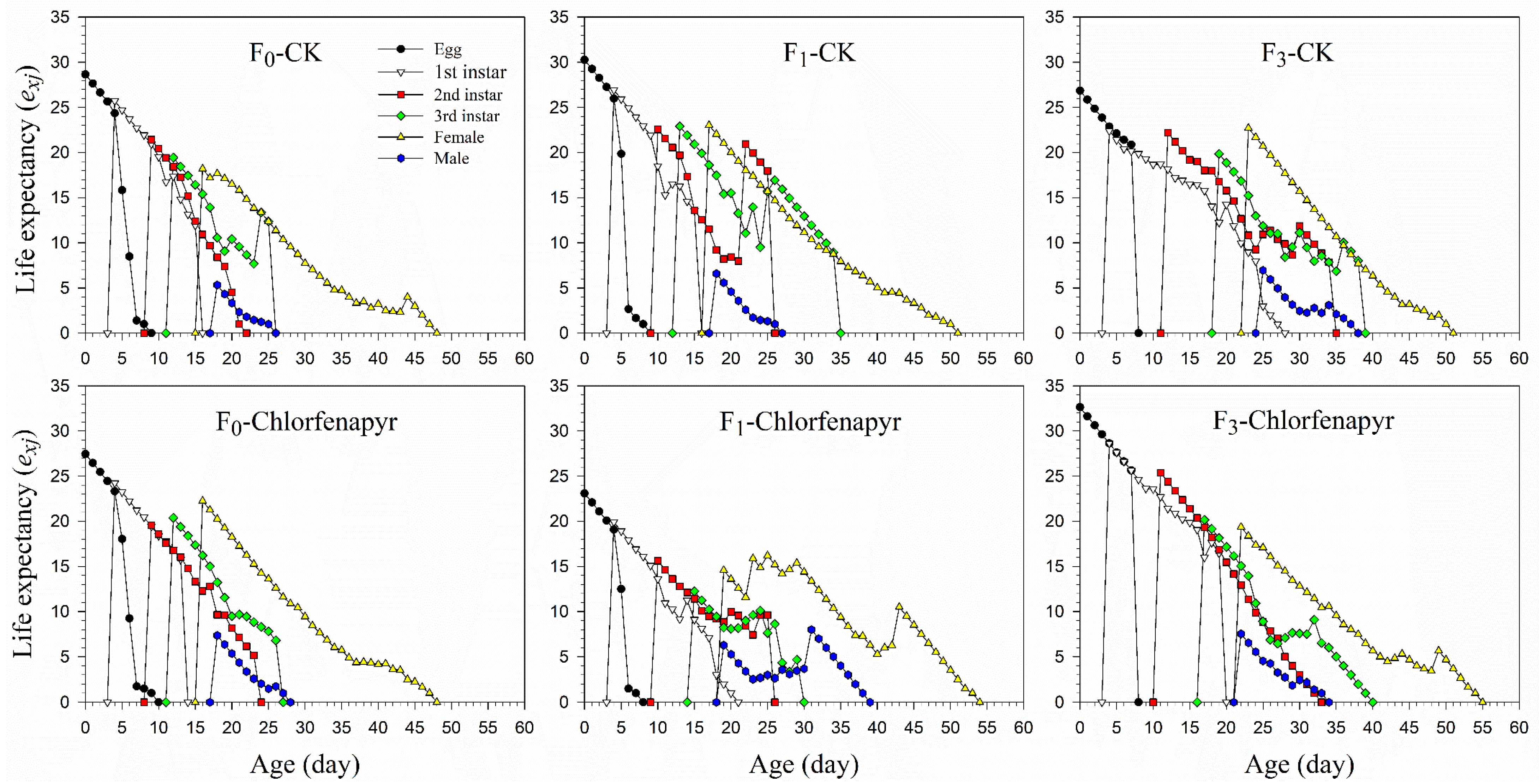
3.2. Life History Traits
3.3. Population Parameters
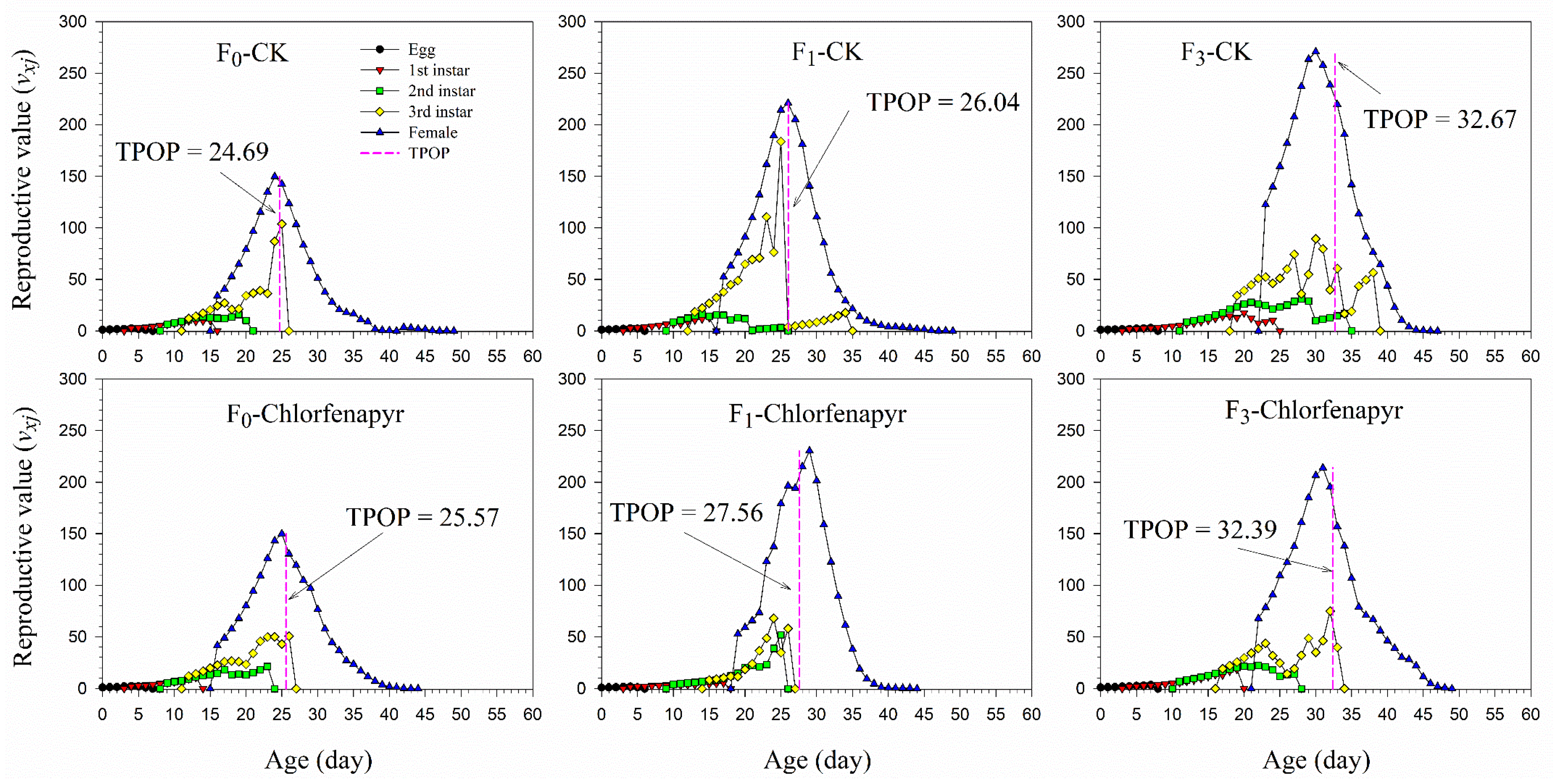
3.4. Survival and Fecundity
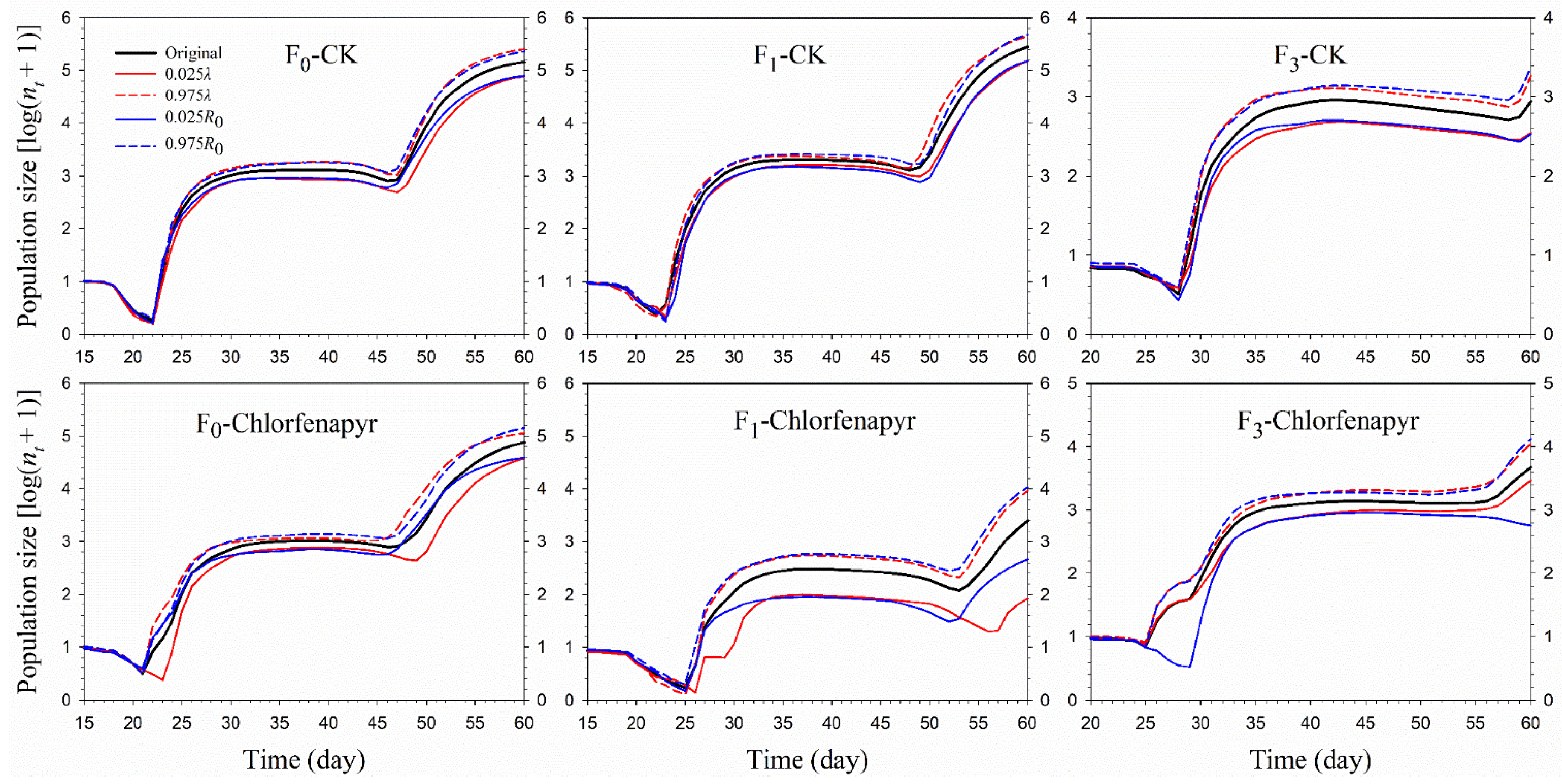
3.5. Population Prediction
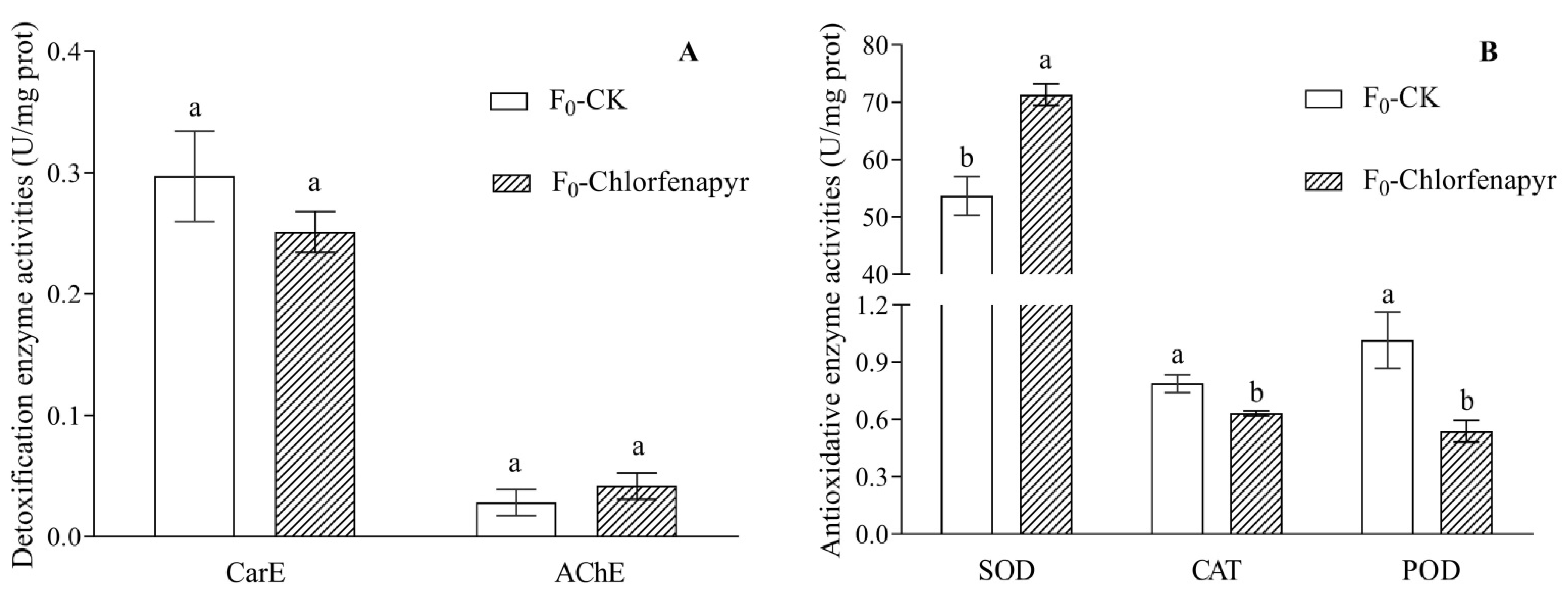
3.6. Detoxification Enzyme and Antioxidative Enzyme Activities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finch, E.A.; Beale, T.; Chellappan, M.; Goergen, G.; Gadratagi, B.G.; Khan, M.A.M.; Rehman, A.; Rwomushana, I.; Sarma, A.K.; Wyckhuys, K.A. The potential global distribution of the papaya mealybug, Paracoccus marginatus, a polyphagous pest. Pest Manag. Sci. 2021, 77, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Miller, G.L.; Watson, G.W. Invasive species of mealybugs (Hemiptera: Pseudococcidae). Proc. Entomol. Soc. Wash. 2002, 104, 825–836. [Google Scholar]
- Zhang, J.T.; Wu, S.A. A new invasive mealybug, Paracoccus marginatus (Hemiptera: Coccoidea: Pseudococcidae), in mainland China. Environ. Entomol. 2015, 37, 441–447. [Google Scholar]
- Miller, D.R.; Miller, G.L. Redescription of Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Coccoidea: Pseudococcidae), including descriptions of the immature stages and adult male. Proc. Entomol. Soc. Wash. 2002, 104, 1–23. [Google Scholar]
- Krishnan, J.U.; George, M.; Ajesh, G.; Jithine, J.; Lekshmi, N.; Deepasree, M. A review on Paracoccus marginatus Williams, papaya mealybug (Hemiptera: Pseudococcidae). J. Entomol. Zool. Study 2016, 4, 528–533. [Google Scholar]
- Galanihe, L.D.; Jayasundera, M.U.P.; Vithana, A.; Asselaarachchi, N.; Watson, G.W. Occurrence, distribution and control of papaya mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae), an invasive alien pest in Sri Lanka. Trop. Agric. Res. Ext. 2011, 13, 81–86. [Google Scholar] [CrossRef]
- Seni, A.; Sahoo, A.K. Efficacy of certain insecticides on papaya mealybug, Paracoccus marginatus Williams & Granara de Willink (Hemiptera: Pseudococcidae). J. Entomol. Zool. Study 2015, 3, 14–17.8. [Google Scholar]
- Li, J.Y.; Shi, M.Z.; Wang, Q.Y.; Luo, Y.Y.; Zheng, L.Z.; Fu, J.W. Screening and sensitivity of pesticides for controlling new invasive pest Paracoccus marginatus on papaya plants. Fujian J. Agric. Sci. 2020, 35, 74–79. [Google Scholar]
- Black, B.C.; Hollingworth, R.M.; Ahammadsahib, K.I.; Kukel, C.D.; Donovan, S. Insecticidal action and mitochondrial uncoupling activity of AC-303,630 and related halogenated pyrroles. Pestic. Biochem. Physiol. 1994, 50, 115–128. [Google Scholar] [CrossRef]
- Gao, Y.; Kim, M.J.; Kim, J.H.; Jeong, I.H.; Clark, J.M.; Lee, S.H. Transcriptomic identification and characterization of genes responding to sublethal doses of three different insecticides in the western flower thrips, Frankliniella occidentalis. Pestic. Biochem. Physiol. 2020, 167, 104596. [Google Scholar] [CrossRef]
- Darabian, K.; Yarahmadi, F. Field efficacy of azadirachtin, chlorfenapyr, and Bacillus thuringensis against Spodoptera exigua (Lepidoptera: Noctuidae) on sugar beet crop. J. Entomol. Res. Soc. 2017, 19, 45–52. [Google Scholar]
- Wang, X.L.; Wang, J.; Cao, X.W.; Wang, F.L.; Yang, Y.H.; Wu, S.W.; Wu, Y.D. Long-term monitoring and characterization of resistance to chlorfenapyr in Plutella xylostella (Lepidoptera: Plutellidae) from China. Pest Manag. Sci. 2019, 75, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Lin, R.H.; You, Y.; Lin, T.; Zeng, Z.H.; Yu, C.H. Comparative sensitivity of Neoseiulus cucumeris and its prey Tetranychus cinnabarinus, after exposed to nineteen pesticides. Ecotoxicol. Environ. Safe 2021, 217, 112234. [Google Scholar] [CrossRef] [PubMed]
- British Crop Production Council Pesticide Manual (The e-Pesticide Manual). In British Crop Production Council, Version 6.0; British Crop Production Council: Cambridge, UK, 2012.
- Havasi, M.; Kheradmand, K.; Mosallanejad, H.; Fathipour, Y. Sublethal effects of diflovidazin on life table parameters of two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae). Int. J. Acarol. 2018, 44, 115–120. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Wang, Q.H.; Ding, J.F.; Wang, Y.; Zhang, Z.Q.; Liu, F.; Mu, W. Sublethal effects of chlorfenapyr on the life table parameters, nutritional physiology and enzymatic properties of Bradysia odoriphaga (Diptera: Sciaridae). Pestic. Biochem. Physiol. 2018, 148, 93–102. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Güncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Mahmoodi, L.; Mehrkhou, F.; Guz, N.; Forouzan, M.; Atlihan, R. Sublethal effects of three insecticides on fitness parameters and population projection of Brevicoryne brassicae (Hemiptera: Aphididae). J. Econ. Entomol. 2020, 113, 2713–2722. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.G.; Zhang, Y.Z.; Yang, R.; Zhang, S.R.; Xu, X.; Zhu, M.J.; Gong, C.W.; Hasnain, A.; Shen, L.T. The population growth, development and metabolic enzymes of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae) under the sublethal dose of triflumezopyrim. Chemosphere 2020, 247, 125865. [Google Scholar] [CrossRef]
- Shan, Y.X.; Zhu, Y.; Li, J.J.; Wang, N.M.; Yu, Q.T.; Xue, C.B. Acute lethal and sublethal effects of four insecticides on the lacewing (Chrysoperla sinica Tjeder). Chemosphere 2020, 250, 126321. [Google Scholar] [CrossRef]
- Su, J.Y.; Lai, T.C.; Li, J. Susceptibility of field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in China to chlorantraniliprole and the activities of detoxification enzymes. Crop. Prot. 2012, 42, 217–222. [Google Scholar] [CrossRef]
- Zou, C.S.; Lv, C.H.; Wang, Y.J.; Cao, C.W.; Zhang, G.C. Larvicidal activity and insecticidal mechanism of Chelidonium majus on Lymantria dispar. Pestic. Biochem. Physiol. 2017, 142, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Zhang, B.W.; Yang, J.; Zou, C.S.; Li, T.; Zhang, G.C.; Chen, G.S. Detoxification, antioxidant, and digestive enzyme activities and gene expression analysis of Lymantria dispar larvae under carvacrol. J. Asia-Pac. Entomol. 2021, 24, 208–216. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, S.; Hetzel, F.; Frampton, C. Lacewings (Neuroptera: Hemerobiidae and Chrysopidae) and integrated pest management: Enzyme activity as biomarker of sublethal insecticide exposure. J. Econ. Entomol. 1997, 90, 102–108. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, X.; Wang, X.; Ullah, F.; Gao, X.W.; Song, D.L. Characterization and functional analysis of two acetylcholinesterase genes in Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae). Pestic. Biochem. Physiol. 2021, 174, 104807. [Google Scholar] [CrossRef]
- Ullah, F.; Gul, H.; Desneux, N.; Said, F.; Gao, X.W.; Song, D.L. Fitness costs in chlorfenapyr-resistant populations of the chive maggot, Bradysia odoriphaga. Ecotoxicology 2020, 29, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2022; Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 1 August 2022).
- Wei, M.F.; Chi, H.; Guo, Y.F.; Li, X.W.; Zhao, L.L.; Ma, R.Y. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2022; Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 8 August 2022).
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Brevik, K.; Lindström, L.; McKay, S.D.; Chen, Y.H. Transgenerational effects of insecticides—Implications for rapid pest evolution in agroecosystems. Curr. Opin. Insect Sci. 2018, 26, 34–40. [Google Scholar] [CrossRef]
- Tang, Q.L.; Ma, K.S.; Chi, H.; Hou, Y.M.; Gao, X.W. Transgenerational hormetic effects of sublethal dose of flupyradifurone on the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). PLoS ONE 2019, 14, e0208058. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Liu, J.; Chi, B.J.; Chen, P.; Liu, Y.J. Sublethal and transgenerational effects of six insecticides on Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Asia-Pac. Entomol. 2021, 24, 14–23. [Google Scholar] [CrossRef]
- Nozad-Bonab, Z.; Hejazi, M.J.; Iranipour, S.; Arzanlou, M.; Biondi, A. Lethal and sublethal effects of synthetic and bio-insecticides on Trichogramma brassicae parasitizing Tuta absoluta. PLoS ONE 2021, 16, e0243334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.R.; Wang, X.u.; Gu, F.C.; Gong, C.W.; Chen, L.; Zhang, Y.M.; Hasnain, A.; Shen, L.T.; Jiang, C.X. Sublethal effects of triflumezopyrim on biological traits and detoxification enzyme activities in the small brown planthopper Laodelphax striatellus (Hemiptera: Delphacidae). Front. Physiol. 2020, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Ullah, F.; Hafeez, M.; Tariq, K.; Desneux, N.; Gao, X.W.; Song, D.L. Sublethal concentrations of clothianidin affect fecundity and key demographic parameters of the chive maggot, Bradysia odoriphaga. Ecotoxicology 2021, 30, 1150–1160. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.J.; Xie, W.; Wu, Q.J.; Wang, S.L. Sublethal effects of spinetoram on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Pestic. Biochem. Physiol. 2016, 132, 102–107. [Google Scholar] [CrossRef]
- Dong, J.F.; Wang, K.; Li, Y.; Wang, S.L. Lethal and sublethal effects of cyantraniliprole on Helicoverpa assulta (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2017, 136, 58–63. [Google Scholar] [CrossRef]
- Guedes, R.; Smagghe, G.; Stark, J.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 3.1–3.20. [Google Scholar] [CrossRef]
- Filipović, A.; Mrdaković, M.; Ilijin, L.; Vlahović, M.; Todorović, D.; Grčić, A.; Perić-Mataruga, V. Effect of fluoranthene on antioxidative defense in different tissues of Lymantria dispar and Euproctis chrysorrhoea larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 224, 108565. [Google Scholar] [CrossRef]



| Stage | Generation | Control | LC30 | ||
|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | ||
| Egg (d) | F0 | - | - | - | - |
| F1 | 97 | 4.78 ± 0.06 bB | 95 | 5.07 ± 0.03 aB | |
| F3 | 100 | 6.23 ± 0.12 aA | 100 | 6.04 ± 0.12 aA | |
| 1st instar (d) | F0 | - | - | - | - |
| F1 | 90 | 5.88 ± 0.12 bB | 82 | 6.11 ± 0.15 aB | |
| F3 | 62 | 10.08 ± 0.31 aA | 84 | 8.40 ± 0.16 bA | |
| 2nd instar (d) | F0 | 85 | 3.93 ± 0.12 bC | 74 | 4.74 ± 0.17 aC |
| F1 | 73 | 4.45 ± 0.14 bB | 67 | 5.60 ± 0.25 aB | |
| F3 | 49 | 6.57 ± 0.37 aA | 82 | 6.35 ± 0.27 aA | |
| 3rd instar (d) | F0 | 81 | 4.28 ± 0.08 aB | 68 | 4.41 ± 0.09 aC |
| F1 | 68 | 4.97 ± 0.16 aA | 61 | 4.79 ± 0.10 bB | |
| F3 | 45 | 5.04 ± 0.28 aA | 68 | 5.21 ± 0.17 aA | |
| Adult longevity (d) | F0 | 81 | 12.89 ± 0.93 aB | 68 | 12.78 ± 0.99 aA |
| F1 | 68 | 16.87 ± 1.09 aA | 61 | 6.62 ± 0.84 bB | |
| F3 | 45 | 10.96 ± 1.11 aB | 68 | 11.94 ± 1.04 aA | |
| APOP (d) | F0 | 49 | 6.14 ± 0.36 aA | 35 | 6.11 ± 0.25 aA |
| F1 | 51 | 5.96 ± 0.25 aA | 9 | 5.11 ± 0.42 aB | |
| F3 | 21 | 5.48 ± 0.48 bA | 36 | 7.11 ± 0.62 aA | |
| TPOP (d) | F0 | 49 | 24.69 ± 0.38 aC | 35 | 25.57 ± 0.40 aC |
| F1 | 51 | 26.04 ± 0.41 aB | 9 | 27.56 ± 0.84 aB | |
| F3 | 21 | 32.67 ± 0.78 aA | 36 | 32.39 ± 0.72 aA | |
| Oviposition days (Od) (d) | F0 | 49 | 7.37 ± 0.41 aB | 35 | 8.37 ± 0.59 aA |
| F1 | 51 | 9.31 ± 0.61 aA | 9 | 9.11 ± 1.54 aA | |
| F3 | 21 | 8.43 ± 0.61 aA | 36 | 8.19 ± 0.69 aA | |
| Population Parameter | Generation | Control | LC30 |
|---|---|---|---|
| Intrinsic rate of increase, r (day−1) | F0 | 0.18 ± 0.01 aA | 0.16 ± 0.01 aA |
| F1 | 0.18 ± 0.01 aA | 0.11 ± 0.02 bC | |
| F3 | 0.13 ± 0.01 aB | 0.15 ± 0.01 aB | |
| Finite rate of increase, λ (day−1) | F0 | 1.19 ± 0.01 aA | 1.18 ± 0.01 aA |
| F1 | 1.20 ± 0.01 aA | 1.11 ± 0.02 bC | |
| F3 | 1.14 ± 0.01 aB | 1.16 ± 0.01 aB | |
| Net reproductive rate, R0 (offspring) | F0 | 137.10 ± 22.22 aB | 109.48 ± 18.34 aA |
| F1 | 212.85 ± 29.45 aA | 31.62 ± 13.05 bB | |
| F3 | 96.91 ± 23.10 aB | 148.14 ± 27.12 aA | |
| Mean generation time, T (day) | F0 | 27.77 ± 0.40 aC | 28.65 ± 0.48 aC |
| F1 | 29.04 ± 0.25 bB | 31.25 ± 0.52 aB | |
| F3 | 34.67 ± 0.65 aA | 34.22 ± 0.48 aA | |
| Ratio of female adults in total individuals (Nf/N) | F0 | 0.56 ± 0.05 aA | 0.40 ± 0.05 bA |
| F1 | 0.54 ± 0.05 aA | 0.14 ± 0.03 bB | |
| F3 | 0.22 ± 0.04 bB | 0.44 ± 0.05 aA | |
| Ratio of reproductive females (Nfr/Nf) | F0 | 0.88 ± 0.04 aA | 0.88 ± 0.05 aA |
| F1 | 0.94 ± 0.03 aA | 0.64 ± 0.13 bA | |
| F3 | 0.95 ± 0.05 aA | 0.82 ± 0.06 aA | |
| Fecundity (F) (eggs/female) | F0 | 244.82 ± 33.28 aB | 273.70 ± 31.78 aA |
| F1 | 394.17 ± 41.19 aA | 225.86 ± 77.39 aA | |
| F3 | 440.50 ± 66.18 aA | 336.68 ± 49.23 aA | |
| Fecundity (Fr) (eggs/reproductive female) | F0 | 279.80 ± 35.31 aB | 312.80 ± 31.05 aA |
| F1 | 417.35 ± 41.36 aA | 351.33 ± 98.12 aA | |
| F3 | 461.48 ± 65.83 aA | 411.50 ± 52.53 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-Y.; Chen, Y.-T.; Wang, Q.-Y.; Zheng, L.-Z.; Fu, J.-W.; Shi, M.-Z. Sublethal and Transgenerational Toxicities of Chlorfenapyr on Biological Traits and Enzyme Activities of Paracoccus marginatus (Hemiptera: Pseudococcidae). Insects 2022, 13, 874. https://doi.org/10.3390/insects13100874
Li J-Y, Chen Y-T, Wang Q-Y, Zheng L-Z, Fu J-W, Shi M-Z. Sublethal and Transgenerational Toxicities of Chlorfenapyr on Biological Traits and Enzyme Activities of Paracoccus marginatus (Hemiptera: Pseudococcidae). Insects. 2022; 13(10):874. https://doi.org/10.3390/insects13100874
Chicago/Turabian StyleLi, Jian-Yu, Yan-Ting Chen, Qiu-Yue Wang, Li-Zhen Zheng, Jian-Wei Fu, and Meng-Zhu Shi. 2022. "Sublethal and Transgenerational Toxicities of Chlorfenapyr on Biological Traits and Enzyme Activities of Paracoccus marginatus (Hemiptera: Pseudococcidae)" Insects 13, no. 10: 874. https://doi.org/10.3390/insects13100874
APA StyleLi, J.-Y., Chen, Y.-T., Wang, Q.-Y., Zheng, L.-Z., Fu, J.-W., & Shi, M.-Z. (2022). Sublethal and Transgenerational Toxicities of Chlorfenapyr on Biological Traits and Enzyme Activities of Paracoccus marginatus (Hemiptera: Pseudococcidae). Insects, 13(10), 874. https://doi.org/10.3390/insects13100874







