Simple Summary
The Asian ambrosia beetle Xylosandrus crassiusculus is a polyphagous pest that causes extensive damage to several tree crops. The present research reports the incidence of X. crassiusculus infestations on areca nuts (betel nuts) in India. Beside the new host plant record, the data provided here represent the first documented case of spermatophagy for this beetle and Xileborini in general. Further, investigations confirmed the association of fungal symbiont Ambrosiella roeperi with adult beetles of X. crassiusculus. This fungal symbiont was also recovered from the infested galleries present in the arecanut kernel. A preliminary survey showed that the infestation is so far restricted to a limited number of plantations in the Coastal part of Karnataka, India. Incidence of this symbiotic insect-fungus complex in the economic part of arecanut i.e., kernel is of serious concern. In a climate change scenario, this beetle with fungal symbionts may pose a serious threat to arecanut production in India and elsewhere.
Abstract
Xylosandrus crassiusculus (Coleoptera: Curculionidae: Scolytinae) is reported causing damage to areca palm plantations (Areca catechu L.—Arecaceae) in Karnataka (India). In particular, X. crassiusculus has been observed attacking and successfully reproducing on areca nuts; besides the new host plant record, the data provided here represent the first documented case of spermatophagy for this xyleborine beetle. All infestation symptoms of this polyphagous pest were documented and illustrated. The identity of the scolytid, besides morphologically, was confirmed by its DNA barcoding. Eggs, larvae and pupae were found within the galleries of infested kernels. All galleries of the infested kernels were characterized by the presence of whitish to greyish fungal growth. The fungus was identified as Ambrosiella roeperi, a known symbiont of Xylosandrus crassiusculus. Incidence of this symbiotic insect-fungus complex in the economic part of arecanut, i.e., the kernel, is of serious concern. In a climate change scenario, this beetle with fungal symbionts may pose a serious threat to arecanut production in India and elsewhere.
1. Introduction
The granulate ambrosia beetle, Xylosandrus crassiusculus (Motschulsky, 1866) (Curculionidae: Scolytinae: Xyleborini) is a polyphagous species native to India and the entire Oriental region [1], that is now widely introduced worldwide [2,3]. It is reported to feed on more than 200 species of trees belonging to 60 families [4]. This species is considered a major pest in fruit orchards, young forest plantations, plant nurseries, and on ornamental trees [5]. In India Xylosandrus crassiusculus infests commonly cultivated trees such as Mangifera indica, Eugenia jambolana, Dalbergia sp., Cinnamoum camphor and Shorea sp. [6]. Most recently, a massive outbreak of X. crassiusculus on Syzygium aromaticum and Myristica fragrans was reported from Kerala, India [7]. Xylosandrus crassiusculus is also emerging as the key limiting factor for grape production in India [8,9]. This species develops on stressed woody host plants and more frequently on thin-barked deciduous plants [10,11,12,13]. Adult females bore galleries into the plant xylem inoculating mutualistic fungi, which serve as a food source for the developing broods. Xylosandrus crassiusculus and the other members of the tribe Xyleborini have been found in close association with species-specific fungal symbionts [14]; these fungi provide the nourishment for larvae and adults in nutrient-poor substrates such as host plant tissues [15]. As of now, various fungal symbionts of X. crassiusculus has been identified: Ambrosiella xylebori [16,17], Ambrosiella sp. 2 [18], A. xylebori with unidentified Fusarium sp. [19] and Ambrosiella roeperi [20], the only species exclusively present in the mycangium. Penetration towards plant tissues and symbiotic fungus settlement results in chewed wood extrusion from entry holes, sap emission, foliage wilting, canopy dieback, and branch and trunk necrosis. The polyphagous nature of this species in association with its dispersal capabilities combined with the great efficiency in locating and colonizing stressed plants make X. crassiusuculus management challenging and often ineffective throughout its native and invaded range [21].
Areca palm (Areca catechu L.—Arecaceae) is an important cash crop in most Southern Asian countries [22]. The economic part of the palm is known as arecanut or ‘betel nut’ and is mainly used for masticatory purpose in many parts of Asia. India ranks first in area and production of arecanut in the world, with than 16 million people depending on arecanut industry for their livelihood [23]. This nut also plays a significant role as pharmaceutical in Ayurveda (Ancient Indian system of medicine) and in Chinese medicinal practices [24].
During the regular pest and disease surveillance programme, an unusual and widespread infestation on young areca palm plantations was observed in Karnataka, India. The attacks concentrated exclusively on immature areca nuts and showed damaging symptoms consisting in the excavation of tunnels in the nut and emission of frass noodles, similarly to those that ambrosia beetles do on wood. Given the great value that areca nut has for the local economy, great effort was invested to investigate the cause and the incidence of the infestation.
The results of this investigation are presented and discussed below.
2. Materials and Methods
2.1. Sample Collection, Symptoms Documentation and Pest Distribution
The first immature areca nuts showing damaging or decaying symptoms were collected in two different sites i.e., Nelluru-Kemraje (12°34.487′ N, 75°29.412′ E) and Markanja (12°34.502′ N, 75°29.392′ E), Karnataka (India) during first week of August 2021. All the relevant information viz., age and variety of the infested plantation, cropping pattern and surrounding plantations of the infested sites were collected during the survey. All the visible and concealed damage symptoms were systematically documented by observing infested nuts (N = 64 nuts collected from eleven infested palms randomly selected between the two studied plantations). Infested nuts were opened, and the insect specimens were recovered from kernels. Insect galleries presenting fungal mycelia were characterized thoroughly.
Subsequently, the survey was extended to some additional major areca nut growing areas of Karnataka to evaluate the possible extent of the infestation. Observations on percentage of palms infested and percentage of nuts infested were recorded in the surveyed sites. The plantations were also investigated in search of possible Xylosandrus attacks on the woody parts of the areca palm. All plantations visited were the standard size of one acre.
2.2. Pest Identification
Adult scolytine beetles were morphologically identified using characters and identification keys provided in [25,26]. Four specimens, randomly selected among the previously identified material, were subjected to molecular characterization using the mitochondrial cytochrome c oxidase I (mtCOI) gene.
PCR reactions were performed to amplify partial mtCOI gene sequences with 25 µL reaction volume containing 2.5 µL of 10X PCR buffer (Thermo Fisher Scientific, Waltham, MA, USA), 1.0 µL of 10 mM dNTPs, 1.0 µL each of forward (LCO1490 5′-GGTCAACAAATCATAAAGATATTGG-3′) and reverse (HCO2198 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) primers [27] (10 pmol/mL), 1.0 µL of DNA (100 ng/µL), 1.0 µL of Taq DNA polymerase and 18.5 µL of sterile water. Thermocycling (Bio-Rad T100) consisted of an initial denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 20 s, annealing at 50 °C for 30 s, extension at 72 °C for 30 s, and final extension at 72 °C for 5 min [22]. The amplified product was evaluated with electrophoresis (Best Lab International Inc., China) using a 1.0% agarose gel [28]. PCR purification (Geneaid, New Taipei City, Taiwan) was done and purified products were sent for Sanger sequencing (Biokart India Pvt. Ltd., Bengaluru, India). Obtained sequences were aligned using BioEdit (Biological sequence alignment editor—Tom Hall. Available online: http://www.mbio.ncsu.edu/BioEdit/bioedit.html, accessed on 20 October 2021) and blasted in the NCBI (National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/, accessed on 20 October 2021) as well as the BOLD database (BOLD Database. Available online: http://www.boldsystems.org/, accessed on 20 October 2021). Sequence similarity was analysed with the available sequences and sequences were deposited in NCBI.
The voucher specimens were deposited in National Insect Museum, ICAR-NBAIR collections under reference number NIM/NBAIR/COL/4921.
2.3. Symbiont Fungus Isolation and Characterization
Adult females (n = 12) were killed after surface sterilization and the dissected mycangia were inoculated separately on potato dextrose agar (PDA) medium [29]. Simultaneously, insect galleries (n = 30) containing fungal propagules were also surface sterilized with 1% sodium hypochlorite solution, inoculated on PDA media and incubated at 28 ± 2 °C for 5 days.
Both symbiont fungi isolated from mycangia and insect galleries were identified using cultural and microscopic descriptions given by Harrington et al. [20]. Molecular characterization of the fungal replicates (n = 8) was further confirmed using amplification of ribosomal DNA.
Total fungal DNA was extracted from the mycelial mat using the CTAB method with minor modifications [30]. PCR reactions were performed to amplify the partial rDNA gene sequences with a 25 µL reaction volume containing 2.5 µL of 10X PCR buffer (Thermo Fisher Scientific, Waltham, MA, USA), 1.0 µL of 10 mM dNTPs, 1.0 µL each of ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [31] (10 pmol/µL), 1.0 µL of DNA (100 ng/µL), 1.0 µL of Taq DNA polymerase and 18.5 µL of sterile water. The amplification profile was carried out using a Bio-Rad T100 thermocycler, which was preheated at 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min, and final extension at 72 °C for 5 min. The amplified product was visualized using 1% agarose gel, purified using Geneaid PCR Purification Kit (Geneaid, New Taipei, Taiwan) and DNA fragment was sequenced by Sanger’s sequencing (Biokart India Pvt. Ltd., Bengaluru, India).
The obtained sequences were aligned using BioEdit (Biological sequence alignment editor—Tom Hall. Available online: http://www.mbio.ncsu.edu/BioEdit/bioedit.html, accessed on 20 October 2021) and compared with the available sequences in NCBI (National Center for Biotechnology Information. Available online: http://www.ncbi.nlm.nih.gov/, accessed on 20 October 2021). The obtained sequences were deposited in the GenBank.
3. Results
3.1. Ambrosia Beetle Identification
All the scolytid beetles collected inside areca nuts were identified as Xylosandrus crassiusculus (Scolytinae: Xyleborini) (Figure 1C). The specimens ranged between 2.5–2.8 mm in size, and presented all the typical features of the species: color ferruginous with elitral declivity darker and matte, posterolateral margins of elytra carinate to interstriae seven; pronotum rounded in dorsal view, as long as wide, smooth on the basal half; mycangial tuft located on the pronotal base [24].

Figure 1.
Xylosandrus crassiusculus, specimens collected from galleries on areca nuts: (A) dorsal view, alive specimen; (B) lateral view; (C) ventro-lateral view.
Molecular identification of the beetle was confirmed by amplifying 771 bp of the mtCOI gene. The nucleotide sequence of the mtCOI gene was deposited in GenBank (Accession numbers MZ895370-MZ895373) and showed 99.29% similarity with X. crassiusculus (GenBank accession No. KX035196) submitted by Natural History Museum, London, United Kingdom.
3.2. Symbiont Fungus Identification
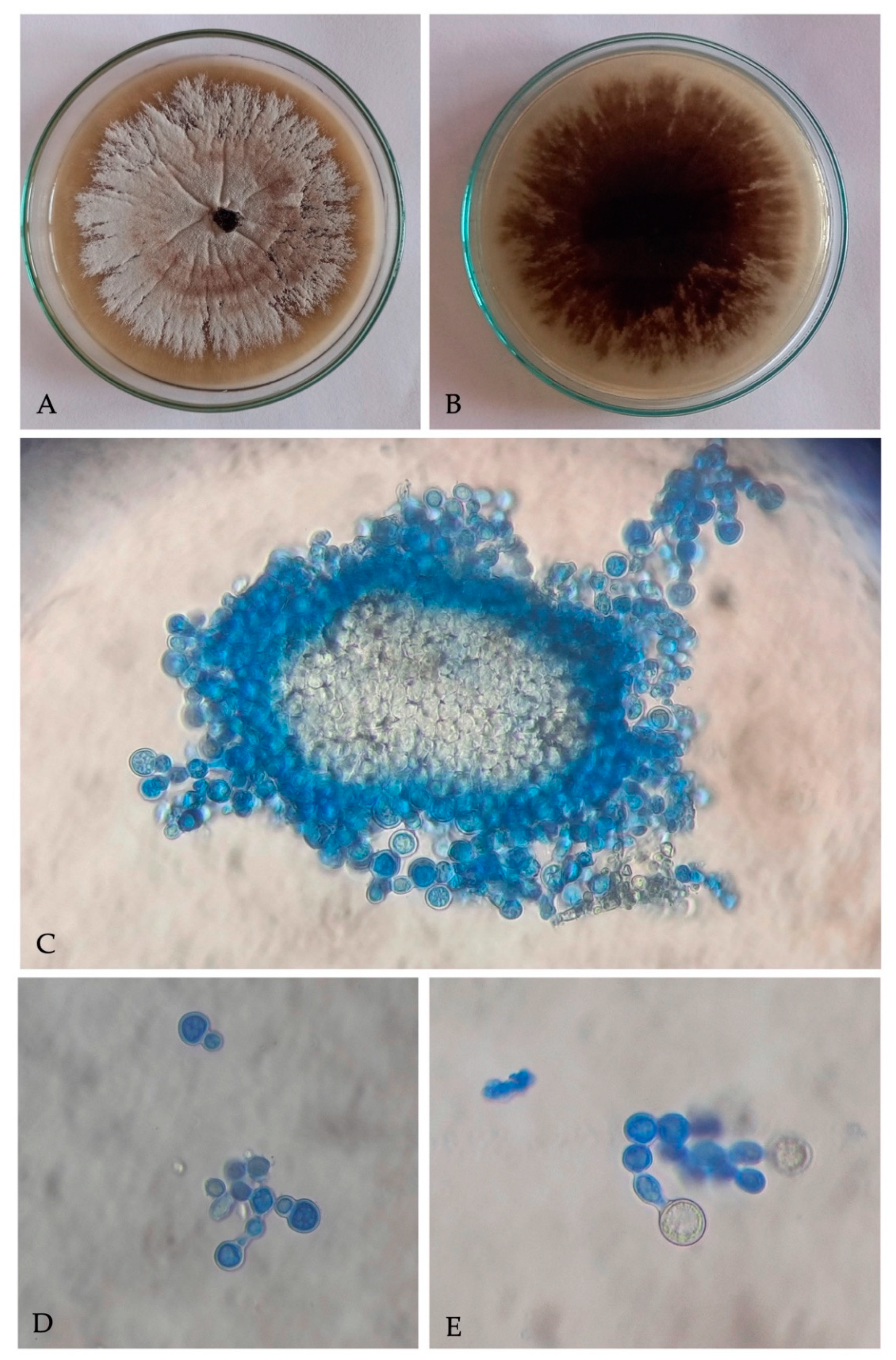
A filamentous fungus with cultural and microscopic characters in conformity with the descriptions of Ambrosiella roeperi [20] was consistently isolated from X. crassiusculus females and insect galleries. Fungal colonies on PDA, appear smoke gray at periphery, whitish to vinaceous buff at center, and 6–8 d culture emits strong sweet odor (Figure 2A). Underside of colony appears dark brown to black (Figure 2B). Microscopic characteristics of A. roeperi isolated from female mycangia and galleries observed as the presence of sporodochial mass; branched conidiophores with terminal aleurioconidia and developing aleurioconidia on swollen conidiophore cells (Figure 2C–E).
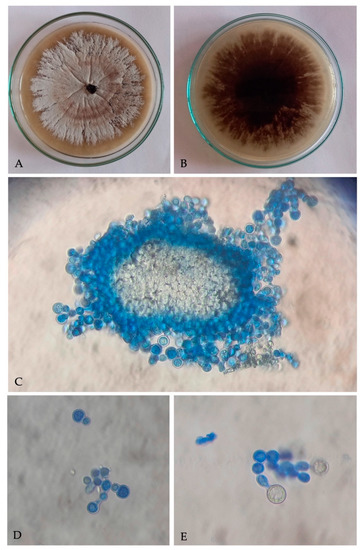
Figure 2.
Culture of Ambrosiella roeperi: (A) upper surface of culture plate; (B) under surface of culture plate; (C) sporodochial mass isolated from mycangia; (D) branched conidiophores with terminal aleurioconidia; (E) developing aleurioconidia on swollen conidiophore cells.
No other fungus was isolated from mycangia of adult females. However, all the inoculated galleries yielded a filamentous fungus matching the descriptions of A. roeperi, except for one gallery where Fusarium sp. was isolated. Isolation of a single Fusarium colony during the study could be a secondary contamination and no further efforts were taken to characterize this fungus.
Molecular identification of the fungal replicates (n = 8) was confirmed by amplifying 580 bp of the ITS gene. The nucleotide sequences of the ITS gene were submitted to GenBank (accession number MZ854170-MZ854177) and showed 100% similarity with A. roeperi (GenBank accession No. MK118927) submitted from the IFAS, University of Florida, Gainesville, FL, USA.
3.3. Infestation Symptoms, Damage and Incidence
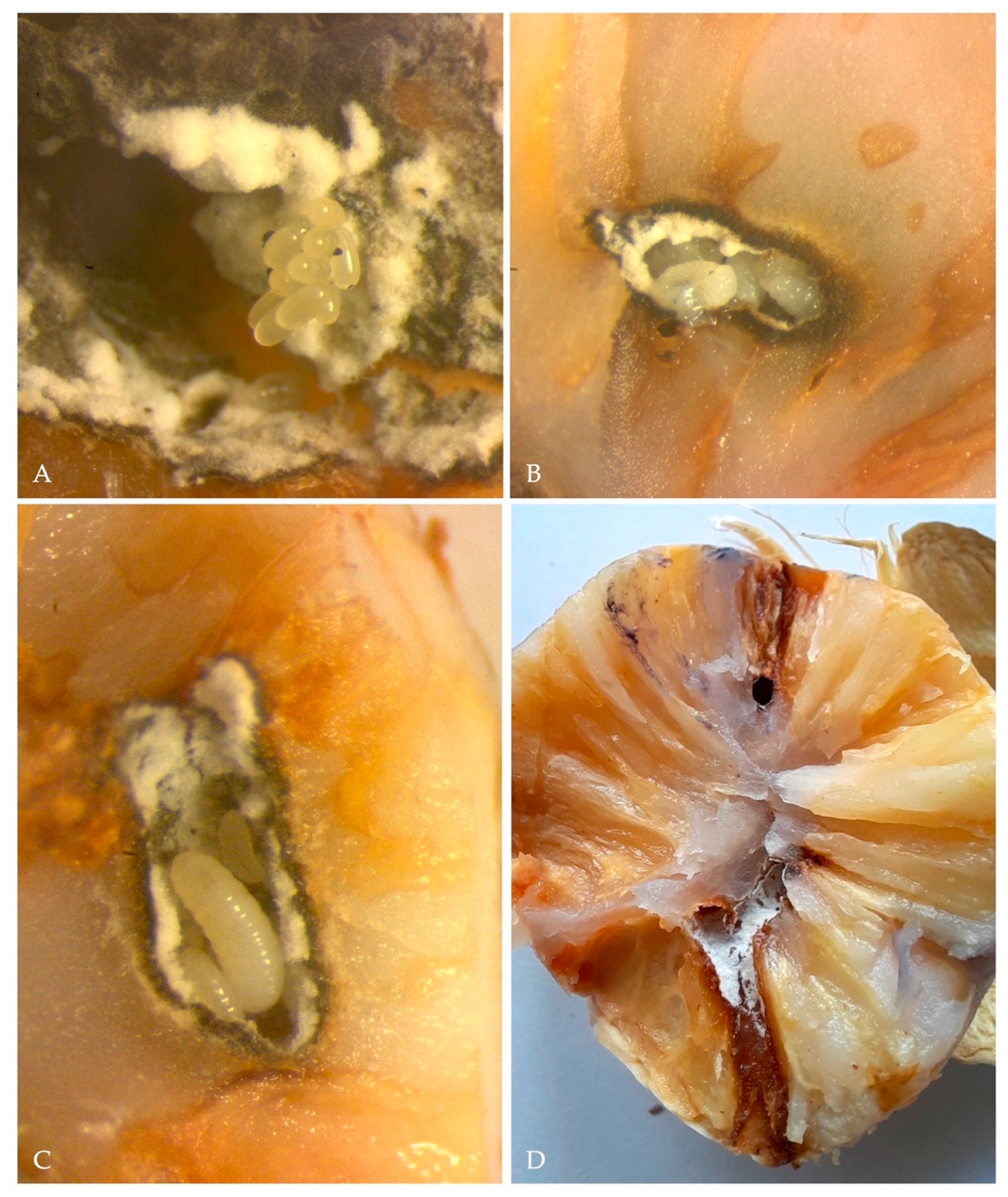
In the majority of the infested nuts (n = 60) only a single adult per fruit was observed while four nuts possessed two to three adults simultaneously. Each female bored a single gallery starting from the exocarp and penetrated through the mesocarp up to the nut kernel. This boring process resulted in the extrusion of the frass in the typical noodle-like shape, almost identical to that this species usually caused on woody trunks (Figure 3A,B). Gallery length varied from 5.0 to 16.0 mm, with an entry hole ranging from 1.06–2.39 mm (Mean ± SE = 1.46 ± 0.11 mm, n = 30) (Figure 3C,D). The gallery produced presented a series of chambers most of which hosted both eggs, larvae and pupae; furthremore, all galleries presented a black staining of the nut tisues and the profuse growth of a greyish fungal colony (Ambrosiella roeperi) (Figure 4A–C). The onset of Ambrosiella roeperi initiated a progressive decay of areca nut kernels, which caused the degeneration of the nut, compromising its maturation and consequently its marketability (Figure 4D).

Figure 3.
Areca nuts showing symptoms of the attack by Xylosandrus crassiusculus. (A,B) nut showing the typical frass noodle produced by the boring activity of the beetle; (C) nut presenting multiple entrance holes in association to a dark staining of the exocarp; (D) longitudinal section illustrating one Xylosandrus crassiusculus piercing through the nut kernel.
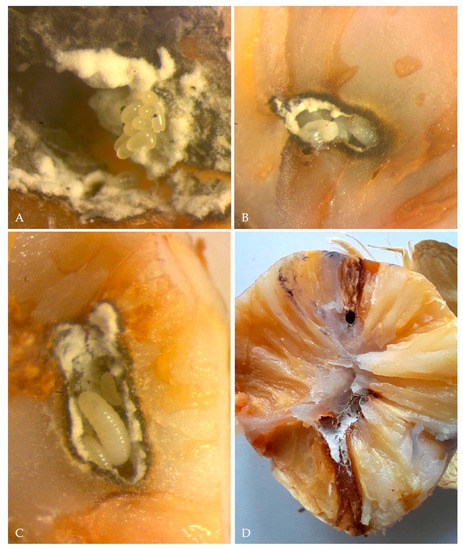
Figure 4.
(A) eggs of X. crassiusculus; (B) larvae of X. crassiusculus found in one of the galleries observed; (C) X. crassiusculus larvae at different development stages in association with A. roeperi hyphae; (D) longitudinal section illustrating X. crassiusculus galleries and kernel staining due to the onset of A. roeperi.
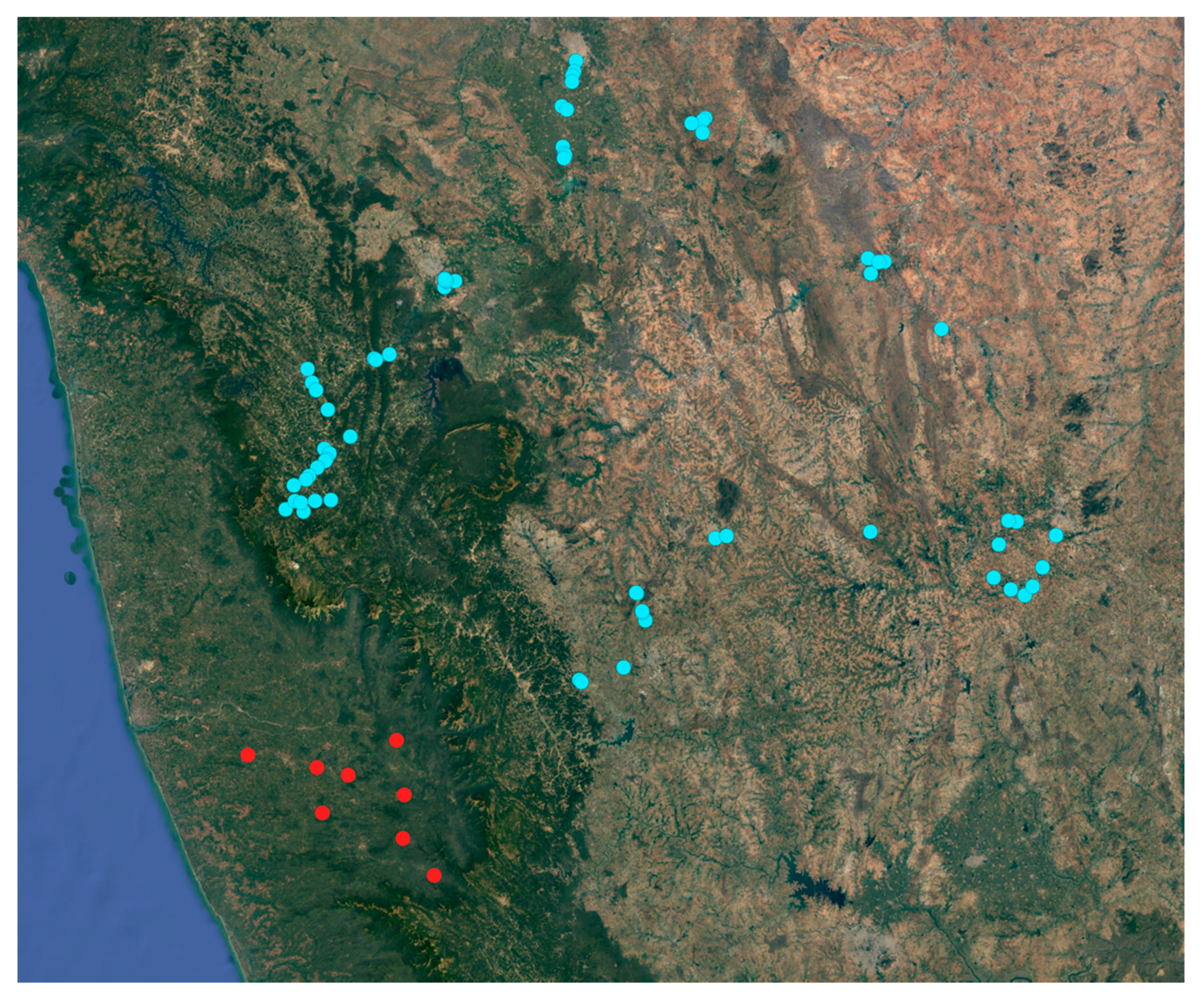
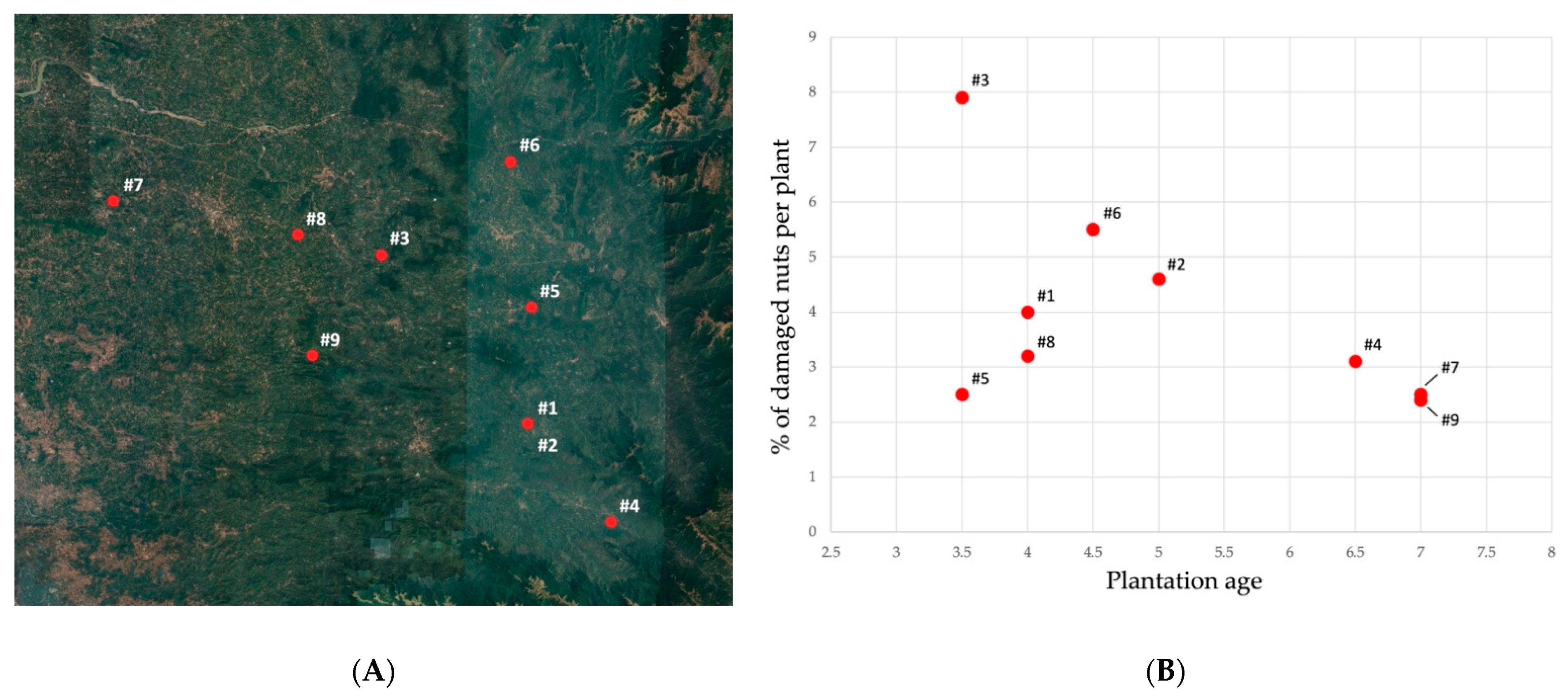
After the first detection of Xylosandrus crassiusculus in the first two localities (mentioned above), 30 additional plantations in Coastal Karnataka were surveyed (Figure 5); as a result 9 plantations out of 30 presented visible infestation symptoms (Figure 6, Table 1). These infested plantations had a plant density of 550 plants per acre and the number of infested plants was 9.3 per acre on average; in each infested palm the 4% of the total nuts presented attacks by X. crassiusculus. In terms of total areca nut production per acre, the attacks represented 0.07% on average. It was interesting to note that in the sampled plantations, Xylosandrus crassiusculus attacks only occurred on young plantations with an age between three and seven years (Figure 6). No attacks of Xylosandrus crassiusculus were recorded on woody parts of the areca palm, in all the sites surveyed.
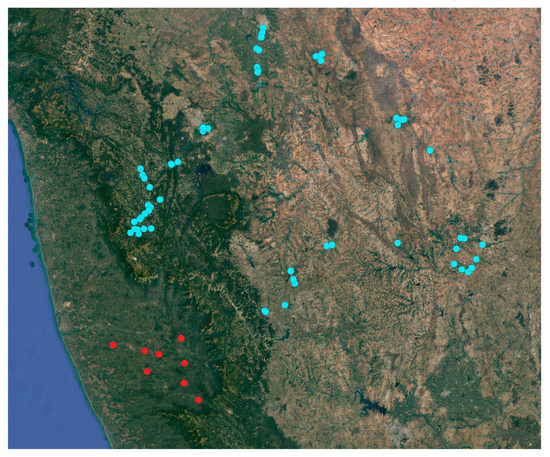
Figure 5.
Map of the areca palm plantation surveyed; sites where no infestation has been detected (blue circle), sites where damages caused by X. crassiusculus have been observed.
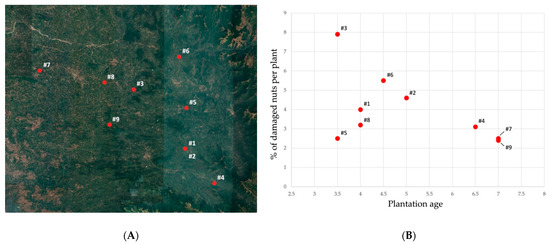
Figure 6.
(A) Map of the plantations presenting damages caused by X. crassiusculus, numbered following the order given in Table 1; (B) Plot illustrating the percentage of the damaged nuts on infested plants in association with the age of the areca palm plantation.

Table 1.
Localities where X. crassiusculus attacks were recorded during the survey in association with damage incidence.
4. Discussion
Ambrosia beetles are widespread colonizers of physiologically stressed or weakened plants [32] and X. crassiusculus preferentially colonizes apparently healthy or recently stressed trees [33]. In our study, we noticed that this species not only affected juvenile and apparently healthy areca palms, but that the attack was limited, contrary to expectations, only to the immature nuts while no other woody parts of the plant were infested.
This observation was indeed interesting because (1) Areca catechu was a new host plant and (2) for the first time spermatophagous behavior was observed in X. crassiusculus, and possibly among all Xyleborini. It was difficult to explain how this species has come to adopt a spermatophagous behavior, but the fact that the attacks were observed in multiple non-adjacent areas, and that living and prolific infestations have been found within areca nuts, would seemed to support the fact that this was not a unique event but a normal reproductive strategy.
Areca catechu is native to the Philippines and widely cultivated in the Oriental region, the geographic area in which X. crassiusculus is also native and widely distributed. Given the economic importance of areca nuts to the oriental market, it appeared very unusual that no damage caused by X. crassiusculus had been documented so far. Since the attacks were recorded only in Karnataka, where the areca palm was introduced, it is plausible that spermatophagy may be a biological feature belonging to that specific, local population of X. crassiusculus and possibly associated with a particular strain of A. roeperi.
Xylosandrus crassiusculus is a species known to respond to the ethanol emitted by stressed plants [12,34] and the fact that it attacked immature fruits might be due to ethanol content or emission by arecanut during its ripening process.
It is possible that in agroecosystems devoted to monoculture (such as the ones investigated), the historical lack of hostplants may have selected plastic scolytid-symbiont relations, capable of exploiting unusual substrates/host; alternatively, it is plausible that these monocultures generate environmental conditions that promote survival strategies that are hardly favorable in other situations. Scolytids have already been shown to start settlements on substrates of non-botanical origins simply by responding to one or more olfactory stimuli [35,36] indicating that the limiting factor in the success of a species is to identify the best substrate on which to grow the symbiotic fungus. In our specific context, the areca nut may have become the best host for A. roeperi because its general structure and constitution resembles the woody tissues where generally Ambrosiella fungi develop. In fact, the areca nut husk is predominantly composed of cellulose and varying proportions of hemicelluloses and lignin. The total hemicellulose content varies with the development and maturity, decreasing during the ripening process; contemporarily the lignin content proportionately increases with the development until maturity [37].
The causes that determined the onset of this infestation remain unknown and it will be necessary to investigate further in this regard, especially if the incidence of damage and the extent of the affected areas increases in the near future. The data collected here in preliminary form seem to indicate a relatively low damage density in this phase, with only young plantations infested. However, since areca palms are already subject to innumerable pests and diseases that compromise their productivity and survival [38,39,40,41], a further loss of product, albeit modest, could have important effects on the farming communities that depend from it. To date, very little is known about possible effects of climate change on the biology and behavior of X. crassiusculus; however, the important changes taking place could have relevant repercussions on the impact of this species both on a small and large scale [42,43].
Moreover, occurrence of Ambrosiella inside the kernels is likely to hamper the market quality and economic return of the infested product. Furthermore, Indian growers store their dry kernels for long periods (up to three years) in warehouses awaiting good market prices [44]. Under such situations, undetected infested nuts could act as a source for future infestations, facilitating the spread of this pest and the fungus in new areas.
The fact that all the nuts presenting the attacks were immature would lead to exclude that the infestation could develop on ripe areca nuts during storage. However, this possibility needs to be carefully evaluated.
5. Conclusions
The findings here presented further demonstrate the great ecological plasticity of X. crassiusculus and its symbiont A. roeperi in successfully adapting to sub-optimal conditions for their proliferation. The capability of X. crassiusculus to infest parts of the plant other than trunk and branches, particularly affecting young plants in good health, represent a potential emerging threat to arecanut production in Karnataka, especially in those areas where plantation renewal is underway. The fact that X. crassiusculus can behave as a spermatophagus species further increases the difficulty in its already complex pest management. Given the novelty and uniqueness of the find, it will be necessary to investigate the environmental and anthropogenic factors that determined the onset of the infestation and define a monitoring and decision plan that can help limit the damage through the implementation of an IPM strategy that takes into account the needs of producers, but that is at the same time sustainable for the environment.
Author Contributions
Conceptualization, S.H.T., T.P.P., A.J., M.B., V.H. and E.R.; methodology, S.H.T., T.P.P., A.B., E.R. and M.C.; writing—original draft preparation, writing—review and editing, S.H.T., T.P.P. and E.R. All authors have read and agreed to the published version of the manuscript.
Funding
S.H.T. acknowledges the support of Indian Council of Agricultural Research, New Delhi for the grant in aid through ICAR-CPCRI Institutional Project (1000765041). APC expenses were covered by World Biodiversity Association, Verona (Italy).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
Authors thank arecanut growers who allowed destructive sampling to isolate the beetles. Senior author also acknowledges the technical support given by Santhosh Paichal and Nirmal Kumar B. J. The authors thank Larry Bezark (California Department of Food and Agriculture), Sarah M. Smith (Michigan State University) and the two anonymous reviewers for providing valuable comments that improved the quality of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bright, D.E.; (Department of Agricultural Biology Colorado State University, Fort Collins, CO, USA). Personal communication, 2021.
- Dole, S.A.; Jordal, B.H.; Cognato, A.I. Polyphyly of Xylosandrus Reitter inferred from nuclear and mitochondrial genes (Coleoptera: Curculionidae: Scolytinae). Mol. Phylogenetics Evol. 2010, 54, 773–782. [Google Scholar] [CrossRef]
- Storer, C.; Payton, A.; McDaniel, S.; Jordal, B.; Hulcr, J. Cryptic genetic variation in an inbreeding and cosmopolitan pest, Xylosandrus crassiusculus, revealed using dd RAD seq. Ecol. Evol. 2017, 7, 10974–10986. [Google Scholar] [CrossRef] [Green Version]
- EPPO Database. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/alert_list (accessed on 10 September 2021).
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B.; Frank, S.D.; Addesso, K.M.; Chong, J.H.; Sampson, B.; Werle, C.; Gill, S.; et al. Biology, ecology, and management of non-native ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) in ornamental plant nurseries. J. Integr. Pest Manag. 2016, 7, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Beeson, C.F.C. The Ecology and Control of the Forest Insects of India and the Neighbouring Countries; Forest Research Institute: Dehra Dun, India, 1941; 1007p. [Google Scholar]
- Prathapan, K.D.; Hiremath, S.R. Post-flood outbreak of Xylosandrus crassiusculus and Diuncus corpulentus (Coleoptera: Curculionidae: Scolytinae: Xyleborini) on tree spices in Kerala. J. Spices Aromat. Crops 2018, 27, 161–166. [Google Scholar]
- Oregon State University. Available online: https://ir.library.oregonstate.edu/concern/conference_proceedings_or_journals/0v838255b (accessed on 21 November 2021).
- Ruzzier, E.; Prazaru, S.C.; Faccoli, M.; Duso, C. Xylosandrus germanus (Blandford, 1894) on Grapevines in Italy with a Compilation of World Scolytine Weevils Developing on Vitaceae. Insects 2021, 12, 869. [Google Scholar] [CrossRef]
- Schedl, K.E. Scolytidae und Platypodidae Afrikans. Band 2. Familie Scolytidae. Rev. De Entomol. De Mocamb. 1963, 5, 1–594. [Google Scholar]
- Ranger, C.M.; Schultz, P.B.; Frank, S.D.; Chong, J.H.; Michael, E.R. Non-native ambrosia beetles as opportunistic exploiters of living but weakened trees. PLoS ONE 2015, 10, e0131496. [Google Scholar]
- Ranger, C.M.; Reding, M.E.; Addesso, C.; Ginzel, M.; Rassati, D. Semiochemical-mediated host selection by Xylosandrus spp. ambrosia beetles (Coleoptera: Curculionidae) attacking horticultural tree crops: A review of basic and applied science. Can. Entomol. 2021, 153, 103–120. [Google Scholar] [CrossRef]
- Cavaletto, G.; Faccoli, M.; Ranger, C.M.; Rassati, D. Ambrosia beetle response to ethanol concentration and host tree species. J. Appl. Entomol. 2021, 145, 800–809. [Google Scholar] [CrossRef]
- Biedermann, P.H.W.; Vega, F.E. Ecology and evolution of insect-fungus mutualisms. Annu. Rev. Entomol. 2020, 65, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Harrington, T.C. Ecology and evolution of mycophagous bark beetles and their fungal partners. In Insect–Fungal Associations; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 257–291. [Google Scholar]
- Brader, L. Etude de la Relation Entre le Scolyte des Rameaux du Cafeier, Xyleborus Compactus Eichh. (X. morstatti Hag.), et sa Plante-Hote; Wageningen University: Wageningen, The Netherlands, 1964; Volume 64, pp. 1–109. [Google Scholar]
- Gebhardt, H.; Weiss, M.; Oberwinkler, F. Dryadomyces amasae: A nutritional fungus associated with ambrosia beetles of the genus Amasa (Coleoptera: Curculionidae, Scolytinae). Mycol. Res. 2005, 109, 687–696. [Google Scholar] [CrossRef]
- Kinuura, H. Symbiotic fungi associated with ambrosia beetles. Jpn. Agric. Res. Q. 1995, 29, 57–63. [Google Scholar]
- Hulcr, J.; Dunn, R.R. The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems. Proc. R. Soc. B Biol. Sci. 2011, 278, 2866–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, T.C.; McNew, D.; Chase, M.; Fraedrich, S.W.; Reed, S.E. Ambrosiella roeperi sp. nov. is the mycangial symbiont of the granulate ambrosia beetle, Xylosandrus crassiusculus. Mycologia 2014, 106, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzzo, A.; Biedermann, P.H.W.; Carrillo, D.; Castrillo, L.A.; Egonyu, J.P.; Gallego, D.; Haddi, K.; Hulcr, J.; Jactel, H.; Kajimura, H.; et al. Recent advances toward the sustainable management of invasive Xylosandrus ambrosia beetles. J. Pest Sci. 2021, 94, 615–637. [Google Scholar] [CrossRef]
- Thube, S.H.; Mohan, C.; Pandian, R.T.P.; Saneera, E.K.; Sannagoudra, H.M.; Hegde, V.; Chowdappa, P. First record of the invasive neotropical ambrosia beetle Euplatypus parallelus (Fabricius, 1801) (Coleoptera: Curculionidae: Platypodinae) infesting arecanut in Karnataka, India. Coleopt. Bull. 2018, 72, 713–717. [Google Scholar] [CrossRef]
- Bhat, R.; Sujatha, S.; Balasimha, D. Impact of drip fertigation on productivity of arecanut (Areca catechu L.). Agric. Water Manag. 2007, 90, 101–111. [Google Scholar] [CrossRef]
- Arjungi, K.N. Areca nut: A review. Arzneim.-Forsch. 1976, 26, 951–957. [Google Scholar]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I.; Hulcr, J.; Redford, A.J. Southeast Asian Ambrosia Beetle ID. USDA APHIS Identification Technology Program (ITP) and Michigan State University. Fort Collins, CO, USA. Available online: https://idtools.org/id/wbb/sea-ambrosia/ (accessed on 10 September 2021).
- University of Florida. Available online: https://journals.flvc.org/edis/article/view/119233 (accessed on 10 September 2021).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Francke-Grosmann, H. Ectosymbiosis in wood-inhabiting insects. In Symbiosis; Henry, S.M., Ed.; Academic Press: New York, NY, USA, 1967; Volume 11, pp. 142–206. [Google Scholar]
- Pandian, R.T.P.; Bhat, A.I.; Biju, C.N.; Sasi, S. Development of diagnostic assays for rapid and sensitive detection of Phytophthora infecting major spices and plantation crops. J. Spices Aromat. Crops 2018, 27, 119–130. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for Phylogenetic. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 1–315. [Google Scholar]
- Kühnholz, S.; Borden, J.H.; Uzunovic, A. Secondary ambrosia beetles in apparently healthy trees: Adaptations, potential causes and suggested research. Integr. Pest Manag. Rev. 2001, 6, 209–219. [Google Scholar] [CrossRef]
- Chong, J.; Reid, L.; Williamson, M. Distribution, host plants, and damage of the black twig borer, Xylosandrus compactus (Eichhoff), in South Carolina. J. Agric. Urban Entomol. 2009, 26, 199–208. [Google Scholar] [CrossRef]
- Reding, M.E.; Ranger, C.M. Attraction of invasive ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to ethanol-treated tree bolts. J. Econ. Entomol. 2020, 113, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.L. The Bark and Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae), a Taxonomic Monograph; Brigham Young University: Provo, UT, USA, 1982; Volume 6. [Google Scholar]
- Carlton, C.; Bayless, V. A case of Cnestus mutilatus (Blandford) (Curculionidae: Scolytinae: Xyleborini) females damaging plastic fuel storage containers in Louisiana, USA. Coleopt. Bull. 2011, 65, 290–291. [Google Scholar] [CrossRef]
- Rajan, A.; Kurup, J.G.; Abraham, T.E. Biosoftening of arecanut fiber for value added products. Biochem. Eng. J. 2005, 25, 237–242. [Google Scholar] [CrossRef]
- Balanagouda, P.; Sridhara, S.; Shil, S.; Hegde, V.; Naik, M.K.; Narayanswamy, H.; Balsundram, K. Assessment of the spatial distribution and risk associated with fruit rot disease in Areca catechu L. J. Fungi 2021, 7, 797. [Google Scholar] [CrossRef]
- NIPHM. Available online: https://niphm.gov.in/IPMPackages/Arecanut.pdf (accessed on 10 September 2021).
- Devasahayam, S.; Nair, C.R. Insect pest management in arecanut. Indian Farming 1982, 32, 44–46. [Google Scholar]
- Rajashekharappa, M.T. Impact of Crop Losses Due to Pests and Diseases on Cost Structure of Arecanut Cultivation—An Economic Analysis in Traditional Malnad Region of Karnataka’. Master’s Thesis, University of Agricultural Sciences, Bengaluru, India, 2001. [Google Scholar]
- Williams, G.M.; Ginzel, M.D. Spatial and climatic factors influence ambrosia beetle (Coleoptera: Curculionidae) abundance in intensively managed plantations of eastern black walnut. Environ. Entomol. 2020, 49, 49–58. [Google Scholar] [CrossRef]
- Urvois, T.; Auger-Rozenberg, M.A.; Roques, A.; Rossi, J.P.; Kerdelhue, C. Climate change impact on the potential geographical distribution of two invading Xylosandrus ambrosia beetles. Sci. Rep. 2021, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Thube, S.H.; Pandian, R.T.P.; Bhavishya, E.K.; Mohan, C.; Nagaraja, N.R. Major storage insect pests of arecanut Areca catechu L.—A Survey. J. Entomol. Zool. Stud. 2017, 5, 1471–1475. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).