Supercritical Carbon Dioxide Impregnation of Gold Nanoparticles Demonstrates a New Route for the Fabrication of Hybrid Silk Materials
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
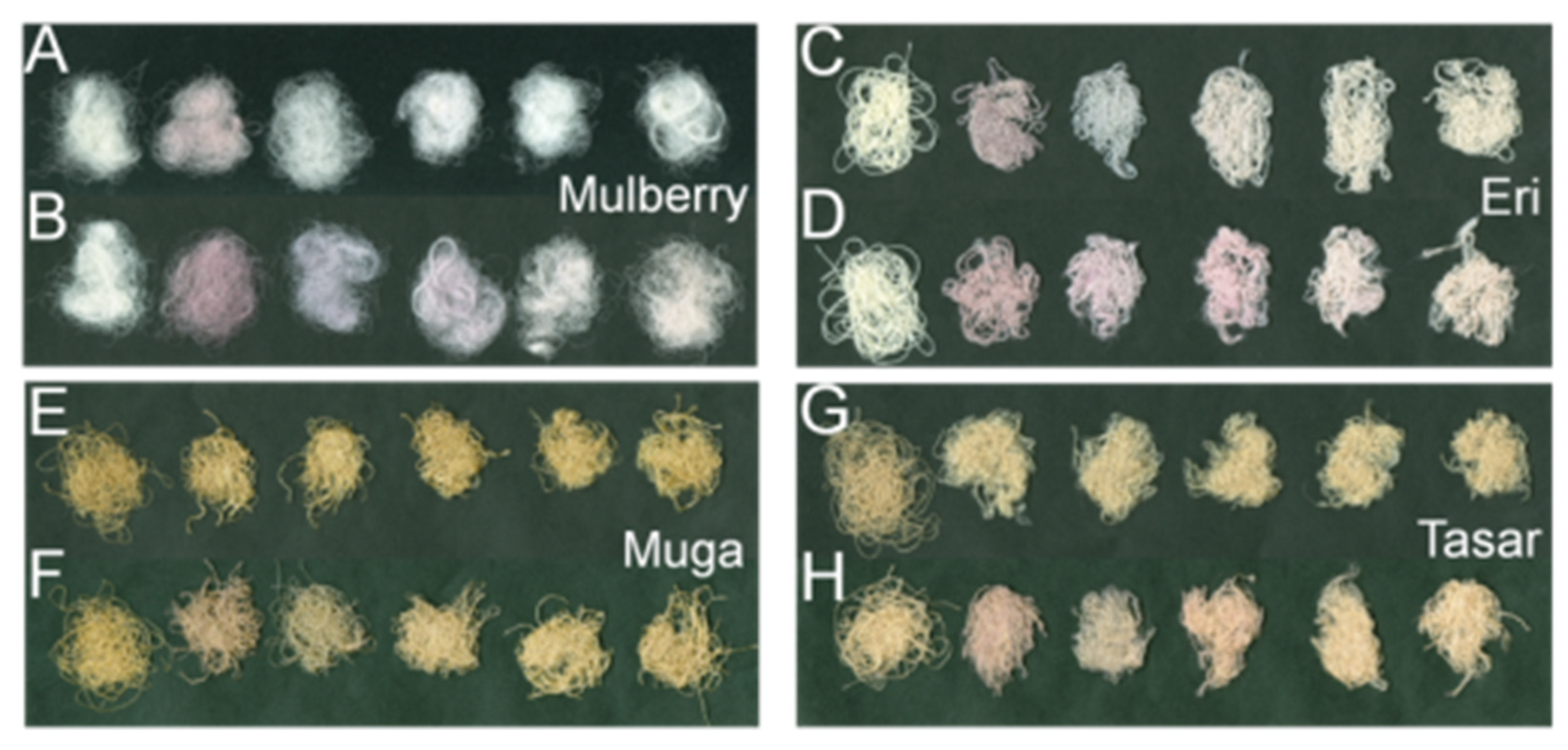
2.1. Preparation of Silks Fibers to Be Impregnated with Gold NPs
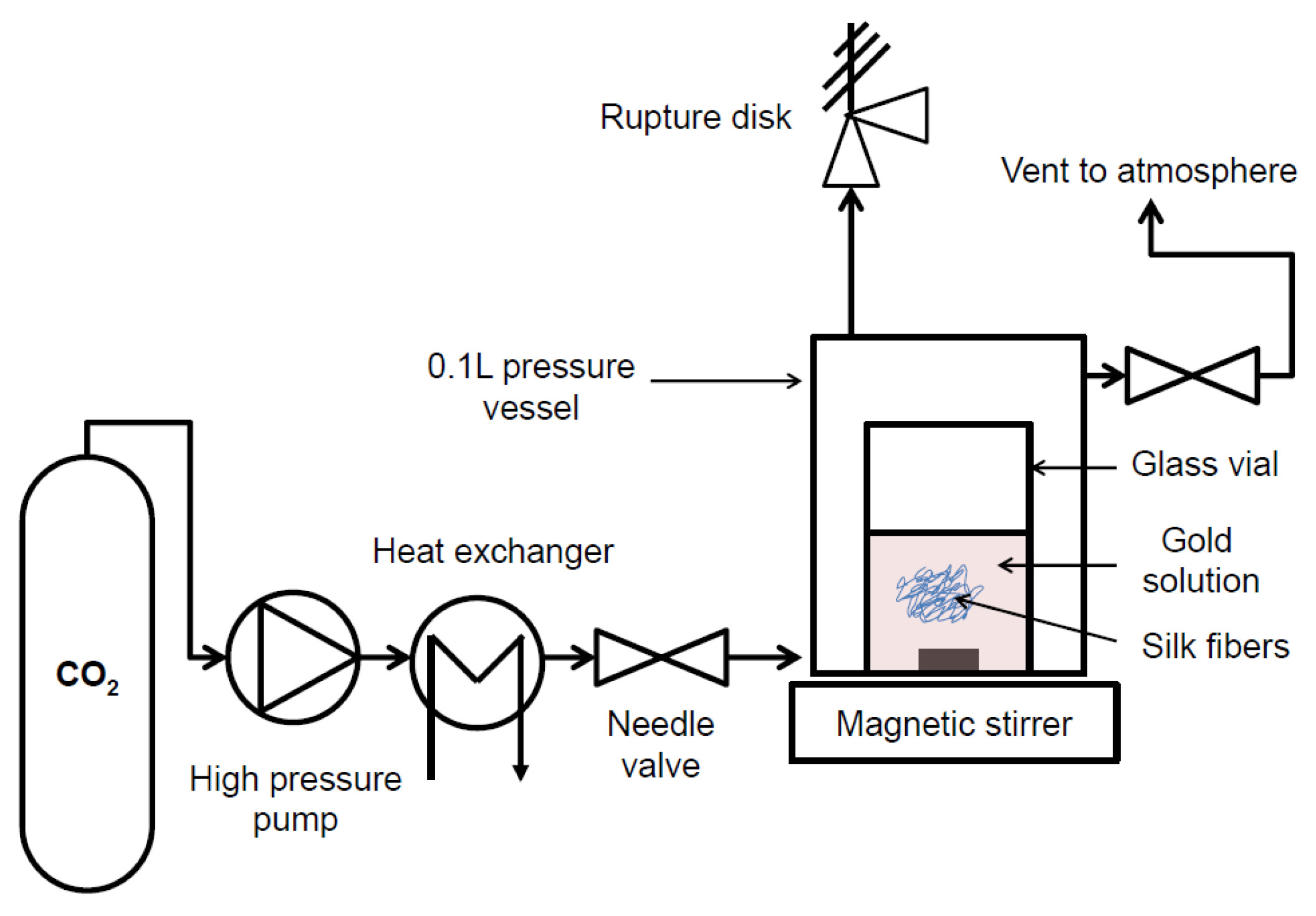
2.2. Impregnation of Silk Fibers under Supercritical and Atmospheric Conditions
2.3. Fiber Post-Processing
2.4. Characterization
2.4.1. UV-Visible Absorption Spectroscopy
2.4.2. Fourier Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR)
2.4.3. Scanning Electron Microscopy-Degummed Bombyx Mori
2.4.4. Photographs
2.5. Factorial Design for Degummed Bombyx Mori Study
3. Results
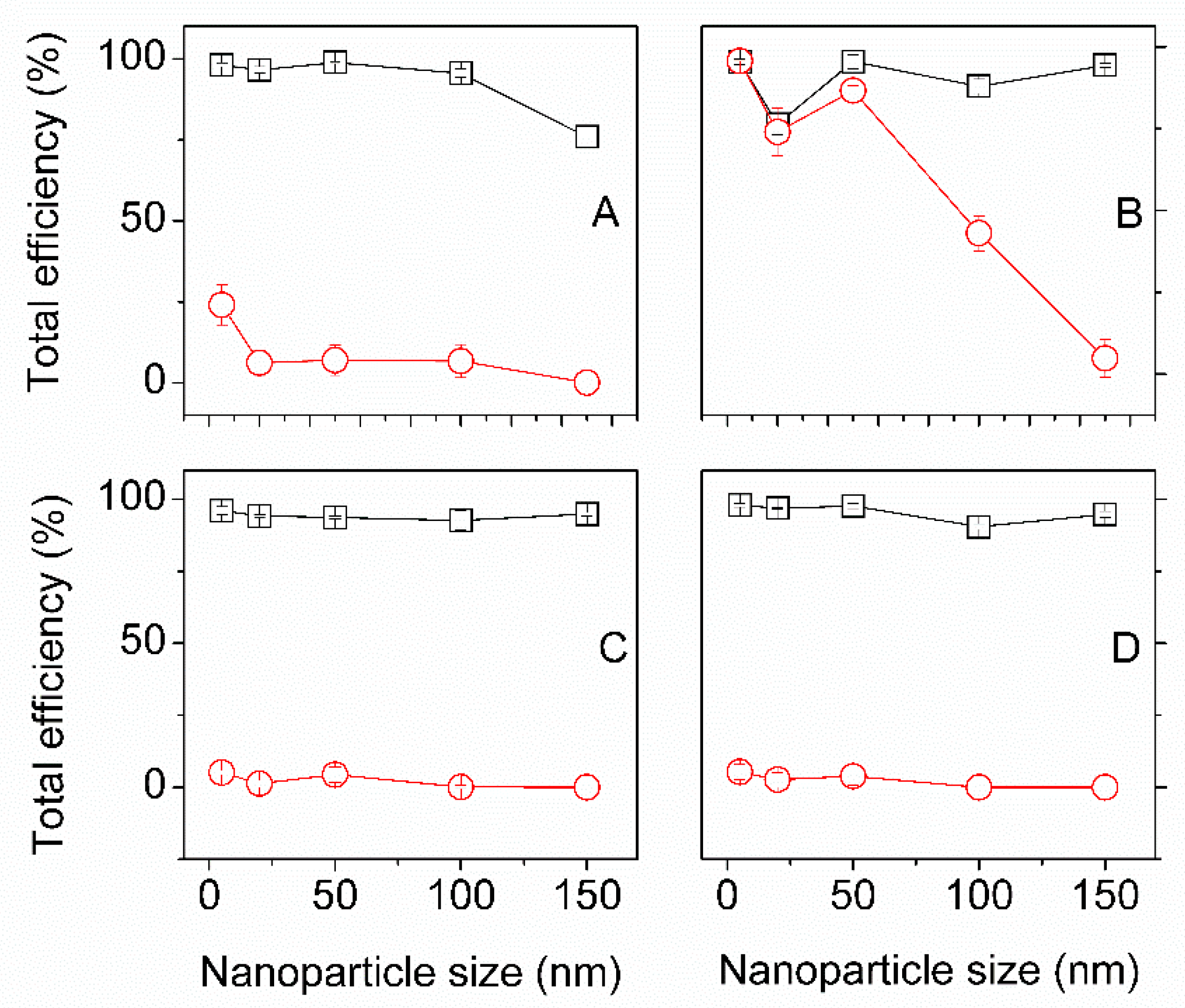
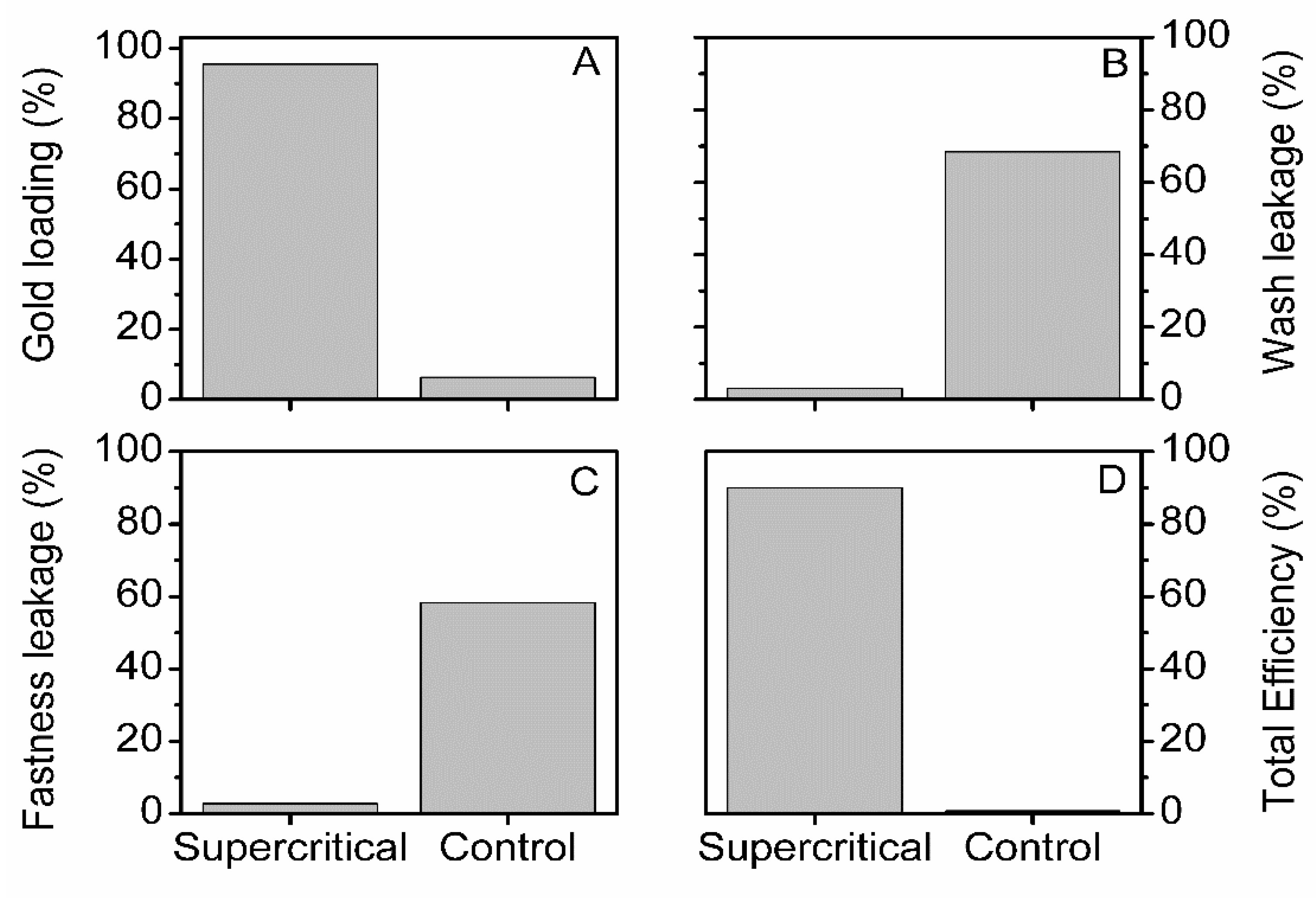
3.1. The Efficiency of the Impregnation Process and Fibers Color Change
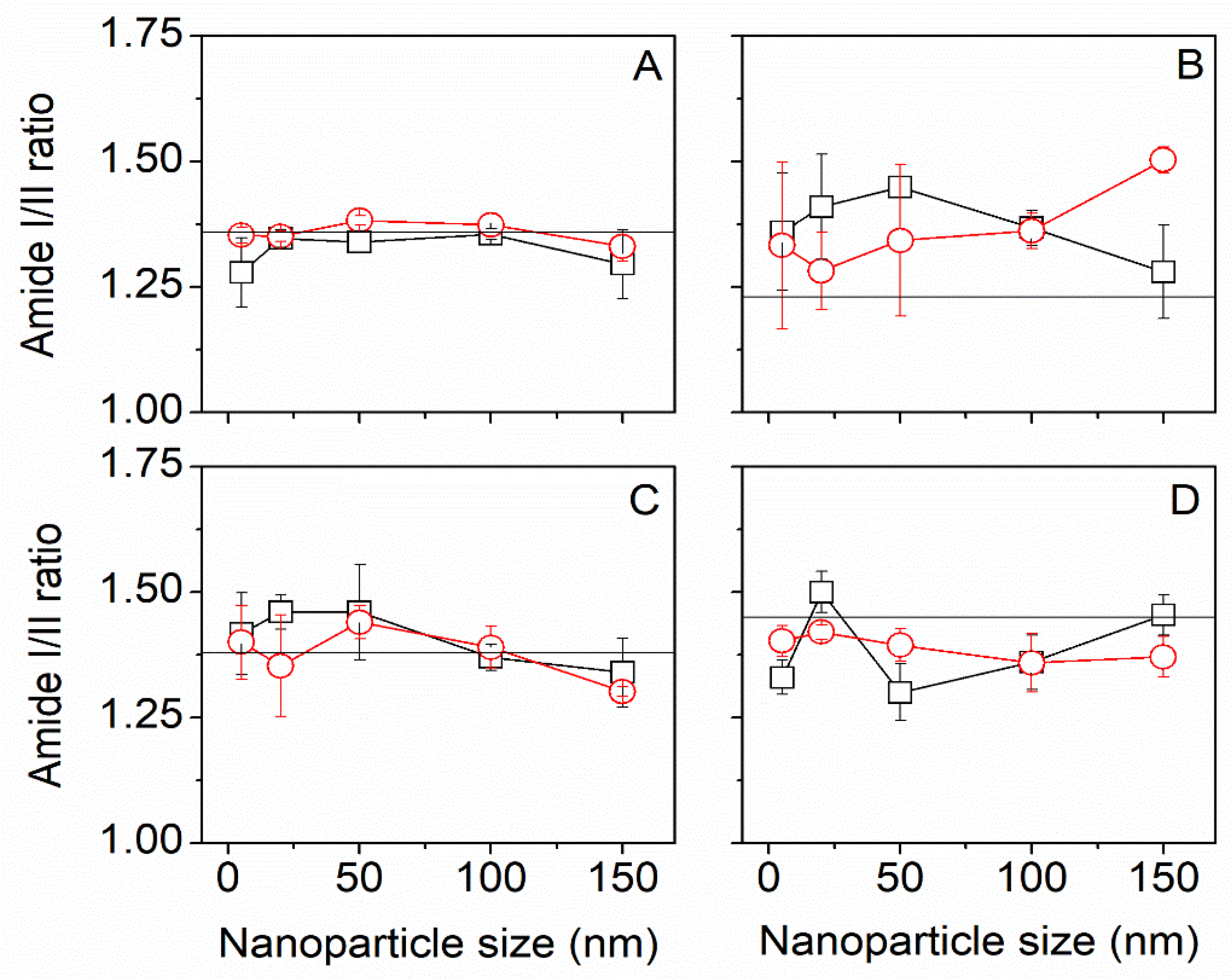
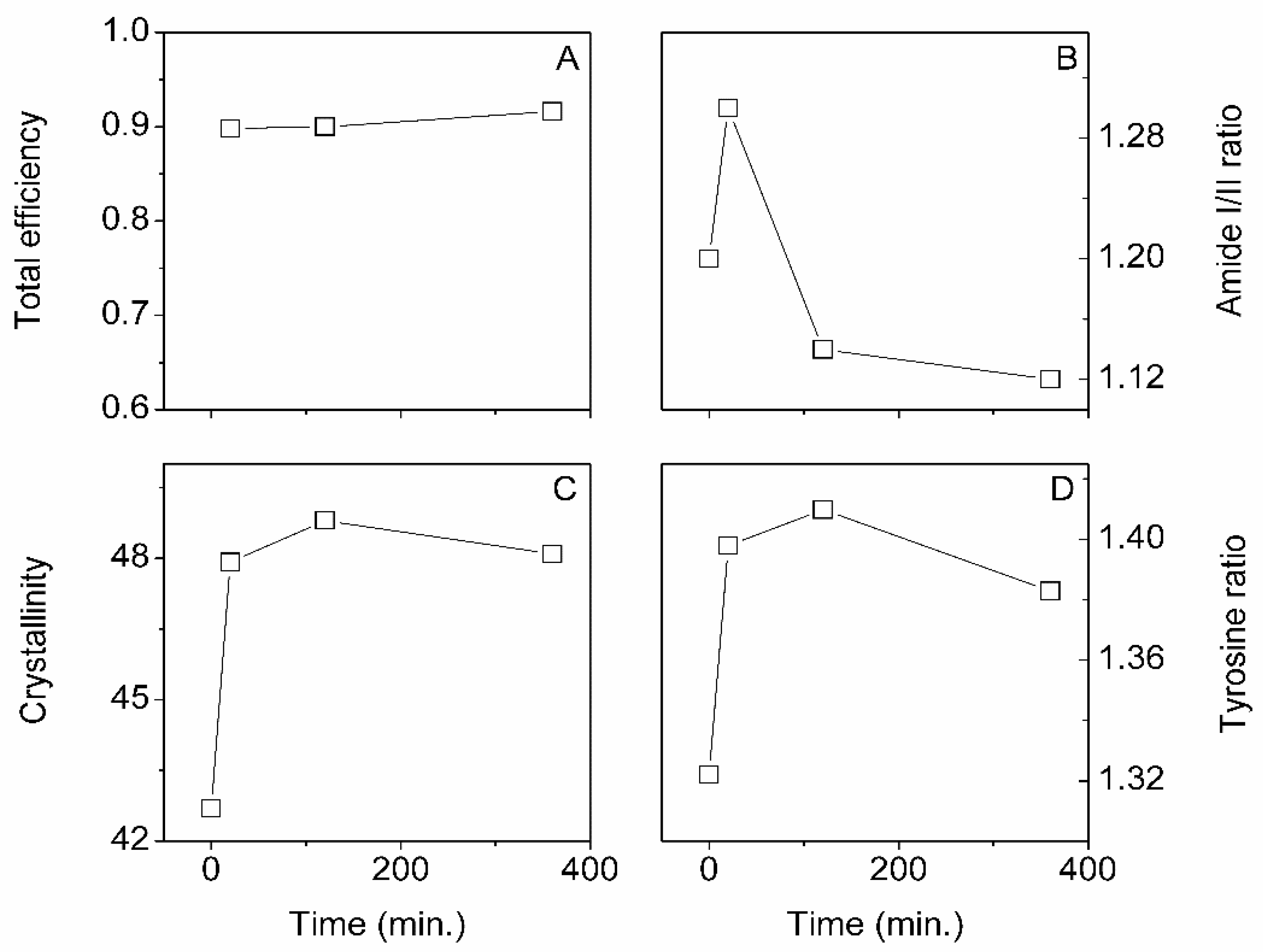
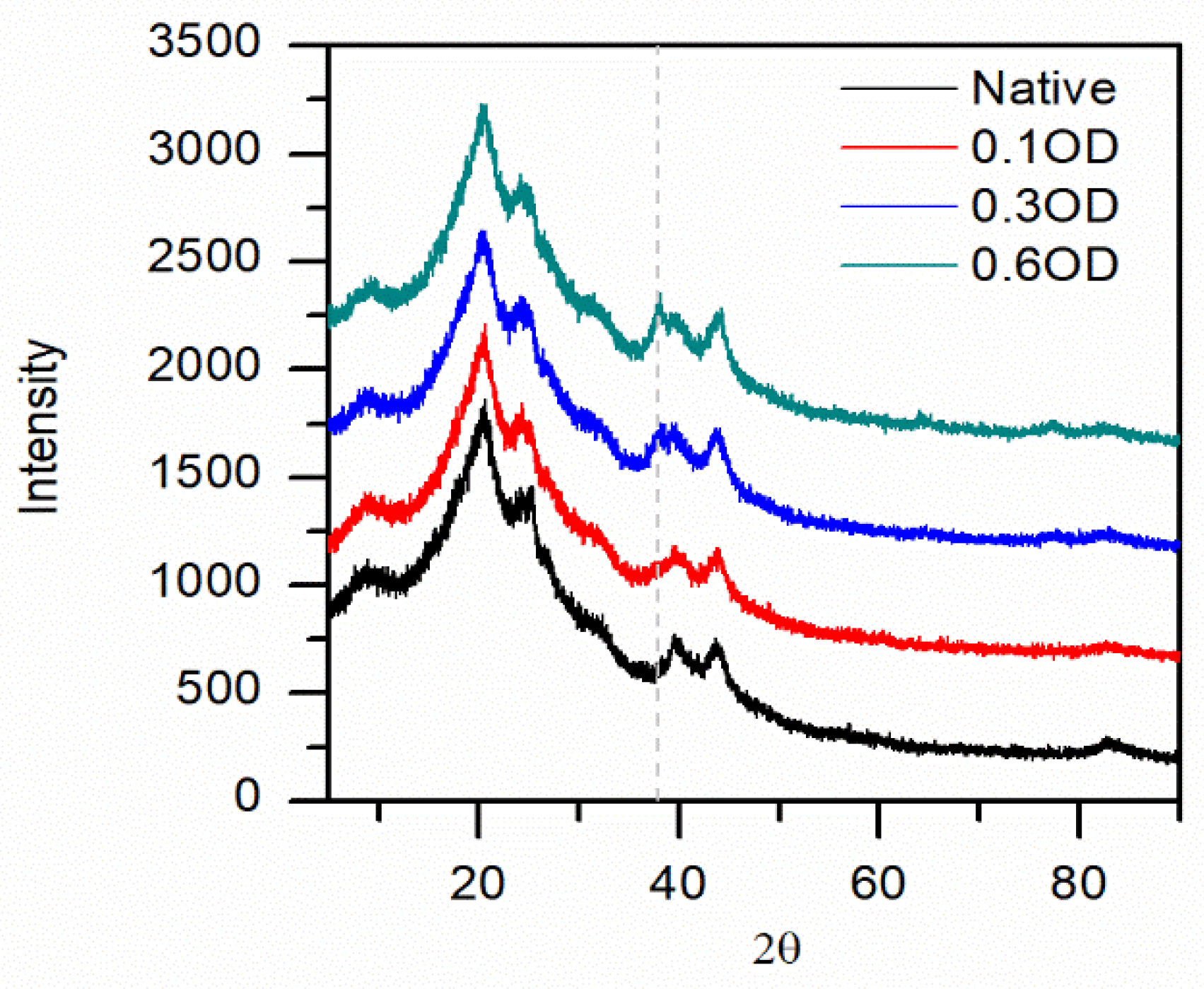
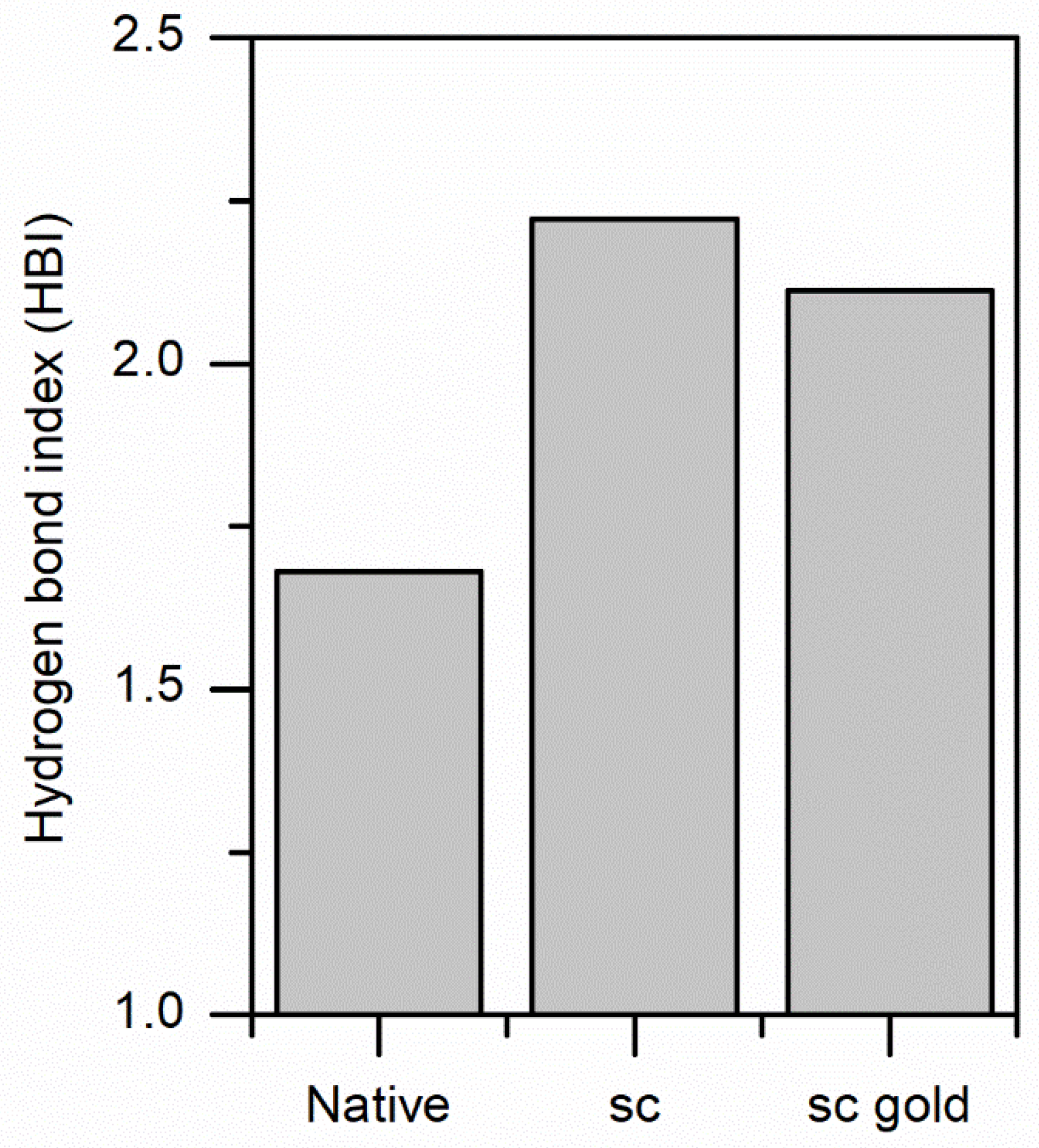
3.2. ScCO2 Impregnation Induced Structural Changes
3.3. Effect of Degumming on Bombyx Mori and 5 nm Gold NPs
3.3.1. Facile Gold Impregnation in Supercritical Carbon Dioxide at 40 °C and 200 Bars
3.3.2. Effect of Initial Gold NPs Concentration and Time of Impregnation
3.4. Factorial Design—Relationships between Process Parameters and Multiple Responses
4. Discussion
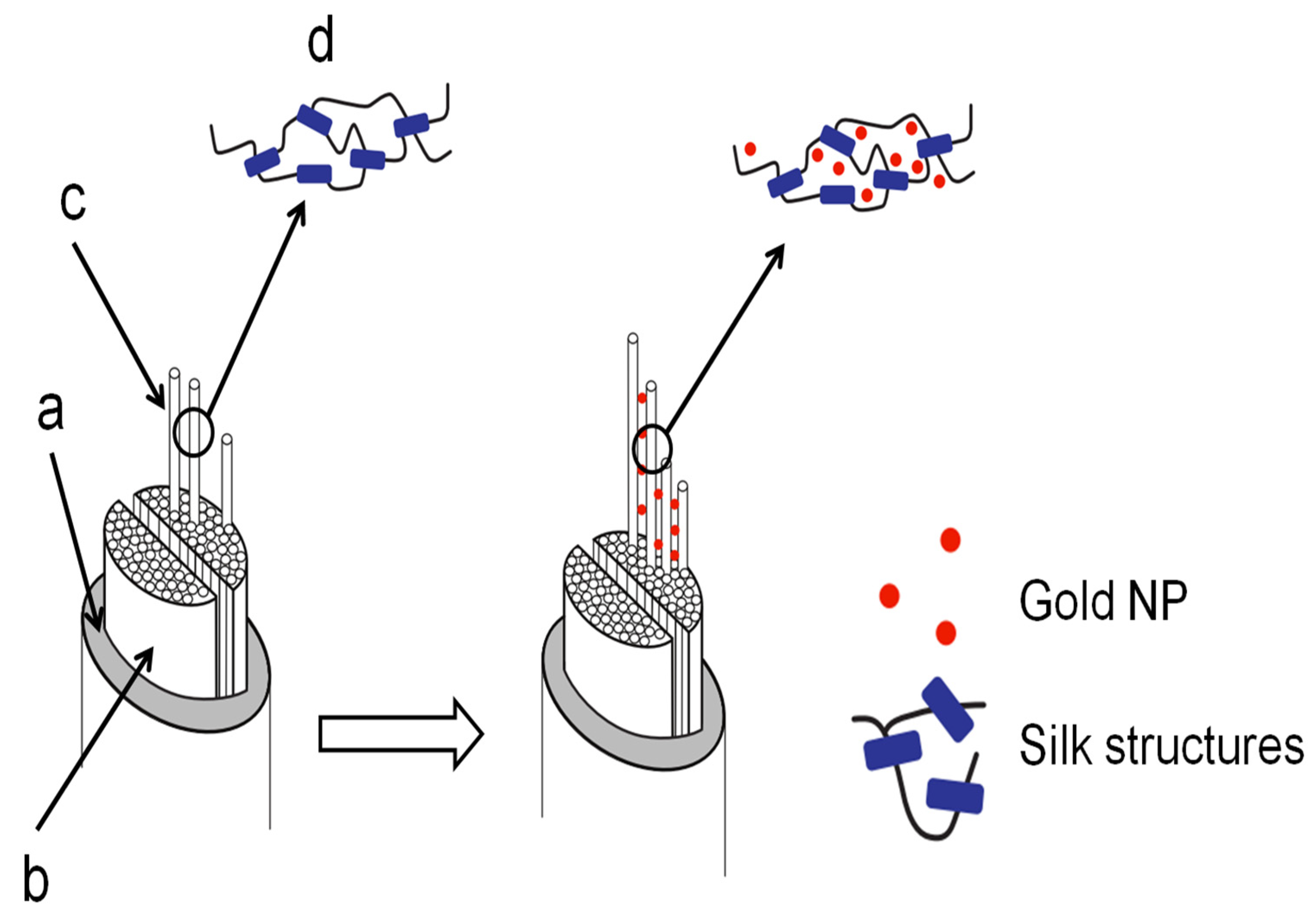
Mechanism of Impregnation and Gold Nanoparticles Location
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Shishtawy, R.M.; Asiri, A.M.; Abdelwahed, N.A.M.; Al-Otaibi, M.M. In situ production of silver nanoparticle on cotton fabric and its antimicrobial evaluation. Cellulose 2011, 18, 75–82. [Google Scholar] [CrossRef]
- Alongi, J.; Malucelli, G. Cotton flame retardancy: State of the art and future perspectives. RSC Adv. 2015, 5, 24239–24263. [Google Scholar] [CrossRef]
- Onar, N.; Aksit, A.C.; Sen, Y.; Mutlu, M. Antimicrobial, UV-protective and self-cleaning properties of cotton fabrics coated by dip-coating and solvothermal coating methods. Fiber Polym. 2011, 12, 461–470. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Dong, B.H.; Hinestroza, J.P. Metal nanoparticles on natural cellulose fibers: Electrostatic assembly and in situ synthesis. ACS Appl. Mater. Interfaces 2009, 1, 797–803. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Zhao, H.; Gao, Y.; Wang, J.; Jiang, H.; Boughton, R.I. Chemical assembly of TiO2 and TiO2@Ag nanoparticles on silk fiber to produce multifunctional fabrics. J. Colloid Interface Sci. 2011, 358, 307–315. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E.; Etemadifar, A. Fabricating multifunctional silver nanoparticles-coated cotton fabric. Arab. J. Chem. 2017, 10, S2355–S2362. [Google Scholar] [CrossRef]
- Tang, B.; Sun, L.; Kaur, J.; Yu, Y.; Wang, X. In-situ synthesis of gold nanoparticles for multifunctionalization of silk fabrics. Dye. Pigm. 2014, 103, 183–190. [Google Scholar] [CrossRef]
- Tang, B.; Li, J.; Hou, X.; Afrin, T.; Sun, L.; Wang, X. Colorful and antibacterial silk fiber from anisotropic silver nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 4556–4563. [Google Scholar] [CrossRef]
- Grand, J.; Ferreira, S.R.; de Waele, V.; Mintova, S.; Nenoff, T.M. Nanoparticle alloy formation by radiolysis. J. Phys. Chem. C 2018, 122, 12573–12588. [Google Scholar] [CrossRef]
- Park, J.-E.; Atobe, M.; Fuchigami, T. Sonochemical synthesis of conducting polymer–metal nanoparticles nanocomposite. Electrochim. Acta 2005, 51, 849–854. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Rimmele, E.; Wichser, A.; Erni, R.; Height, M.; Nowack, B. Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 2014, 8, 7208–7219. [Google Scholar] [CrossRef]
- Hristozov, D.; Malsch, I. Hazards and risks of engineered nanoparticles for the environment and human health. Sustainability 2009, 1, 1161–1194. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, H.; Fukushima, Y. Supercritical CO2 for making nanoscale materials. Ind. Eng. Chem. Res. 2006, 45, 3328–3331. [Google Scholar] [CrossRef]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of supercritical carbon dioxide in materials processing and synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Cansell, F.; Chevalier, B.; Demourgues, A.; Etourneau, J.; Even, C.; Pessey, V.; Petit, S.; Tressaud, A.; Weill, F. Supercritical fluid processing: A new route for materials synthesis. J. Mater. Chem. 1999, 9, 67–75. [Google Scholar] [CrossRef]
- Xu, Y.; Musumeci, V.; Aymonier, C. Chemistry in supercritical fluids for the synthesis of metal nanomaterials. React. Chem. Eng. 2019, 4, 2030–2054. [Google Scholar] [CrossRef]
- Lane, M.K.M.; Zimmerman, J.B. Controlling metal oxide nanoparticle size and shape with supercritical fluid synthesis. Green Chem. 2019, 21, 3769–3781. [Google Scholar] [CrossRef]
- Chih, Y.-W.; Cheng, W.-T. Supercritical carbon dioxide-assisted synthesis of silver nanoparticles in polyol process. Mater. Sci. Eng. B 2007, 145, 67–75. [Google Scholar] [CrossRef]
- Parandhaman, T.; Dey, M.D.; Das, S.K. Biofabrication of supported metal nanoparticles: Exploring the bioinspiration strategy to mitigate the environmental challenges. Green Chem. 2019, 21, 5469–5500. [Google Scholar] [CrossRef]
- Hua, M.; Hua, X. polymer nanoparticles prepared by supercritical carbon dioxide for in vivo anti-cancer drug delivery. Nano-Micro Lett. 2014, 6, 20–23. [Google Scholar] [CrossRef]
- Wang, Y.; Dave, R.N.; Pfeffer, R. Polymer coating/encapsulation of nanoparticles using a supercritical anti-solvent process. J. Supercrit. Fluids 2004, 28, 85–99. [Google Scholar] [CrossRef]
- Liu, Z.-T.; Zhang, L.; Liu, Z.; Gao, Z.; Dong, W.; Xiong, H.; Peng, Y.; Tang, S. Supercritical CO2 dyeing of ramie fiber with disperse dye. Ind. Eng. Chem. Res. 2006, 45, 8932–8938. [Google Scholar] [CrossRef]
- van der Kraan, M.; Fernandez Cid, M.V.; Woerlee, G.F.; Veugelers, W.J.T.; Witkamp, G.J. Dyeing of natural and synthetic textiles in supercritical carbon dioxide with disperse reactive dyes. J. Supercrit. Fluids 2007, 40, 470–476. [Google Scholar] [CrossRef]
- Guney, O.; Akgerman, A. Synthesis of controlled-release products in supercritical medium. AIChE J. 2002, 48, 856–866. [Google Scholar] [CrossRef]
- Fernandez Cid, M.V.; van Spronsen, J.; van der Kraan, M.; Veugelers, W.J.T.; Woerlee, G.F.; Witkamp, G.J. Excellent dye fixation on cotton dyed in supercritical carbon dioxide using fluorotriazine reactive dyes. Green Chem. 2005, 7, 609–616. [Google Scholar] [CrossRef]
- Singh, M.; Bollella, P.; Gorton, L.; Dey, E.; Dicko, C. Conductive and enzyme-like silk fibers for soft sensing application. Biosens. Bioelectron. 2019, 150, 111859. [Google Scholar] [CrossRef]
- Cooper, A.I. Polymer synthesis and processing using supercritical carbon dioxide. J. Mater. Chem. 2000, 10, 207–234. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, Y.; Li, D.; Niu, J.; Wang, S.; Chen, A. Carbon-doped metal oxide nanoparticles prepared from metal nitrates in supercritical CO2-enabled polymer nanoreactors. Part. Part. Syst. Charact. 2019, 36, 1900016. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, P.; Xu, P.; Li, L.; Liu, T.; Zhao, L. Synthesis of cellulose/titanium dioxide hybrids in supercritical carbon dioxide. Green Chem. 2008, 10, 1061–1067. [Google Scholar] [CrossRef]
- Gittard, S.D.; Hojo, D.; Hyde, G.K.; Scarel, G.; Narayan, R.J.; Parsons, G.N. Antifungal textiles formed using silver deposition in supercritical carbon dioxide. J. Mater. Eng. Perform. 2009, 19, 368–373. [Google Scholar] [CrossRef]
- Ding, X.; Yu, M.; Wang, Z.; Zhang, B.; Li, L.; Li, J. A promising clean way to textile colouration: Cotton fabric covalently-bonded with carbon black, cobalt blue, cobalt green, and iron oxide red nanoparticles. Green Chem. 2019, 21, 6611–6621. [Google Scholar] [CrossRef]
- Watkins, J.J.; McCarthy, T.J. Polymer/metal nanocomposite synthesis in supercritical CO2. Chem. Mater. 1995, 7, 1991–1994. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, D.; Saquing, C.; Aindow, M.; Erkey, C. Supported Platinum Nanoparticles by Supercritical Deposition. Ind. Eng. Chem. Res. 2005, 44, 4161–4164. [Google Scholar] [CrossRef]
- Ye, X.-R.; Lin, Y.; Wang, C.; Engelhard, M.H.; Wang, Y.; Wai, C.M. Supercritical fluid synthesis and characterization of catalytic metal nanoparticles on carbon nanotubes. J. Mater. Chem. 2004, 14, 908–913. [Google Scholar] [CrossRef]
- Hasell, T.; Thurecht, K.J.; Jones, R.D.W.; Brown, P.D.; Howdle, S.M. Novel one pot synthesis of silver nanoparticle–polymer composites by supercritical CO2 polymerization in the presence of a RAFT agent. Chem. Commun. 2007, 38, 3933–3935. [Google Scholar] [CrossRef]
- Wong, B.; Yoda, S.; Howdle, S.M. The preparation of gold nanoparticle composites using supercritical carbon dioxide. J. Supercrit. Fluids 2007, 42, 282–287. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.-Q.; Yan, K.; Long, J.-J. Synthesis of a novel disperse reactive dye involving a versatile bridge group for the sustainable coloration of natural fibers in supercritical Carbon Dioxide. Adv. Sci. 2019, 6, 1801368. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Xu, Q.; Sun, D.; Shao, Z. Grafting of methyl methacrylate onto Antheraea pernyi silk fiber with the assistance of supercritical CO2. J. Appl. Polym. Sci. 2006, 100, 1299–1305. [Google Scholar] [CrossRef]
- Peng, L.; Guo, R.; Lan, J.; Jiang, S.; Wang, X.; Lin, S.; Li, C. Silver nanoparticles coating on silk fabric with pretreatment of 3-aminopropyltrimethoxysilane in supercritical carbon dioxide. J. Ind. Text. 2016, 47, 883–896. [Google Scholar] [CrossRef]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk fibroin aerogels for drug delivery applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nadiger, G.S. Crystallinity in silk fibers: Partial acid hydrolysis and related studies. J. Supercrit. Fluids 1980, 25, 921–932. [Google Scholar] [CrossRef]
- Monti, P.; Freddi, G.; Bertoluzza, A.; Kasai, N.; Tsukada, M. Raman spectroscopic studies of silk fibroin from bombyx mori. J. Raman Spectrosc. 1998, 29, 297–304. [Google Scholar] [CrossRef]
- Boulet-Audet, M.; Vollrath, F.; Holland, C. Identification and classification of silks using infrared spectroscopy. J. Exp. Biol. 2015, 218, 3138–3149. [Google Scholar] [CrossRef]
- Ishida, K.P.; Griffiths, P.R. Comparison of the amide I/II intensity ratio of solution and solid-state proteins samples by transmission, attenuated total reflectance, and diffuse reflectance spectrometry. Appl. Spectrosc. 1993, 47, 584–589. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, W.; Fang, P.F.; Yu, W.; Wang, S.J.; Xiong, J.; Xu, Y.S. The hydrogen bond and free volume property of poly(ether-urethane) irradiated by neutron. J. Polym. Sci. B Polym. Phys. 2010, 48, 381–388. [Google Scholar] [CrossRef]
- Sen, K.; Babu, K.M. Studies on Indian silk. II. Structure–property correlations. J. Appl. Polym. Sci. 2004, 92, 1098–1115. [Google Scholar] [CrossRef]
- Sen, K.; Babu, M.K.; Babu, K.M. Studies on Indian silk. III. Effect of structure on dyeing behavior. J. Appl. Polym. Sci. 2004, 92, 1116–1123. [Google Scholar] [CrossRef]
- Sen, K.; Babu, K.M. Studies on Indian silk. I. macrocharacterization and analysis of amino acid composition. Polymer 2003, 92, 1080–1097. [Google Scholar] [CrossRef]
- Guan, J.; Wang, Y.; Mortimer, B.; Holland, C.; Shao, Z.; Porter, D.; Vollrath, F. Glass transitions in native silk fibres studied by dynamic mechanical thermal analysis. Soft Matter 2016, 12, 5926–5936. [Google Scholar] [CrossRef] [PubMed]
- Mazzi, S.; Zulker, E.; Buchicchio, J.; Anderson, B.; Hu, X. Comparative thermal analysis of Eri, Mori, Muga, and Tussar silk cocoons and fibroin fibers. J. Therm. Anal. Calorim. 2014, 116, 1337–1343. [Google Scholar] [CrossRef]
- Aymonier, C.; Loppinet-Serani, A.; Reverón, H.; Garrabos, Y.; Cansell, F. Review of supercritical fluids in inorganic materials science. J. Supercrit. Fluids 2006, 38, 242–251. [Google Scholar] [CrossRef]
- Brunner, G.J. Application of Supercritical Fluids. Annu. Rev. Chem. Biomol. Enginering 2010, 1, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Kikic, I.; Vecchione, F. Supercritical impregnation of polymers. Curr. Opin. Solid State Mater. Sci. 2003, 7, 399–405. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-supported nanocomposites for environmental application: A review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Kazarian, S.G. Polymer processing with supercritical fluids. Polym. Sci. 2000, 42, 78–101. [Google Scholar]
- Weidner, E. Impregnation via supercritical CO2—What we know and what we need to know. J. Supercrit. Fluids 2018, 134, 220–227. [Google Scholar] [CrossRef]
- Park, H.; Thompson, R.B.; Lanson, N.; Tzoganakis, C.; Park, C.B.; Chen, P. Effect of temperature and pressure on surface tension of polystyrene in supercritical carbon dioxide. J. Phys. Chem. B 2007, 111, 3859–3868. [Google Scholar] [CrossRef][Green Version]
- Rengasamy, R.S. Wetting phenomena in fibrous materials. In Thermal and Moisture Transport in Fibrous Materials; Pan, N., Gibson, P., Eds.; Woodhead: Melbourne, Australia, 2006. [Google Scholar]



| Factors | Levels | Range |
|---|---|---|
| A: Temperature (°C) | 2 | 35, 50 and center point at 42.5 |
| B: Pressure (bars) | 2 | 100, 250 and center point at 175 |
| C: Time (hours) | 2 | 1, 3 and center point at 2 |
| D: Mixing (rpm) | 2 | 0 and 300 |
| Mulberry | Eri | Muga | Tasar | |
|---|---|---|---|---|
| Average density (g/cm3) a | 1.357 | 1.288 | 1.34 | 1.323 |
| Average moisture regain (%) a | 7.04 | 8.03 | 7.61 | 8.52 |
| Sericin content (%) b | 10.4–24.4 | 6.5–10.1 | 8.6–12.7 | 8.2–14.4 |
| Acid dye exhaustion (%) c | 89.82 | 58.38 | 57.02 | 59.10 |
| Disperse dye exhaustion (%) d | 23.67 | 13.55 | 12.59 | 16.20 |
| Elongation (%) | 13.5 | 20.8 | 22.3 | 26.5 |
| Tenacity (g/d) e | 3.75 | 3.7 | 4.35 | 4.5 |
| Initial modulus (g/d) | 95 | 89 | 81 | 84 |
| X-ray crystallinity (%) | 38.2 | 32.6 | 35.0 | 35.2 |
| Glass transition (°C) f | 200–220 | 220–235 | 215–235 | 235–250 |
| Basic/Acidic ratio g | 0.65 | 1.3 | 1.24 | 0.97 |
| Hydrophilic/hydrophobic ratio g | 0.28 | 0.35 | 0.38 | 0.44 |
| Bulky/non bulky side groups ratio g | 0.17 | 0.24 | 0.28 | 0.33 |
| Glycine/Alanine ratio g | 1.58 | 0.8 | 0.82 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Dey, E.S.; Bhand, S.; Dicko, C. Supercritical Carbon Dioxide Impregnation of Gold Nanoparticles Demonstrates a New Route for the Fabrication of Hybrid Silk Materials. Insects 2022, 13, 18. https://doi.org/10.3390/insects13010018
Singh M, Dey ES, Bhand S, Dicko C. Supercritical Carbon Dioxide Impregnation of Gold Nanoparticles Demonstrates a New Route for the Fabrication of Hybrid Silk Materials. Insects. 2022; 13(1):18. https://doi.org/10.3390/insects13010018
Chicago/Turabian StyleSingh, Manish, Estera S. Dey, Sunil Bhand, and Cedric Dicko. 2022. "Supercritical Carbon Dioxide Impregnation of Gold Nanoparticles Demonstrates a New Route for the Fabrication of Hybrid Silk Materials" Insects 13, no. 1: 18. https://doi.org/10.3390/insects13010018
APA StyleSingh, M., Dey, E. S., Bhand, S., & Dicko, C. (2022). Supercritical Carbon Dioxide Impregnation of Gold Nanoparticles Demonstrates a New Route for the Fabrication of Hybrid Silk Materials. Insects, 13(1), 18. https://doi.org/10.3390/insects13010018






