Implementing Mass Rearing of Trissolcus japonicus (Hymenoptera: Scelionidae) on Cold-Stored Host Eggs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Origin and Rearing
2.2. Standard Protocol Used across All Experiments
2.3. Effect of Storage of H. halys Egg Masses on Parasitization by T. japonicus
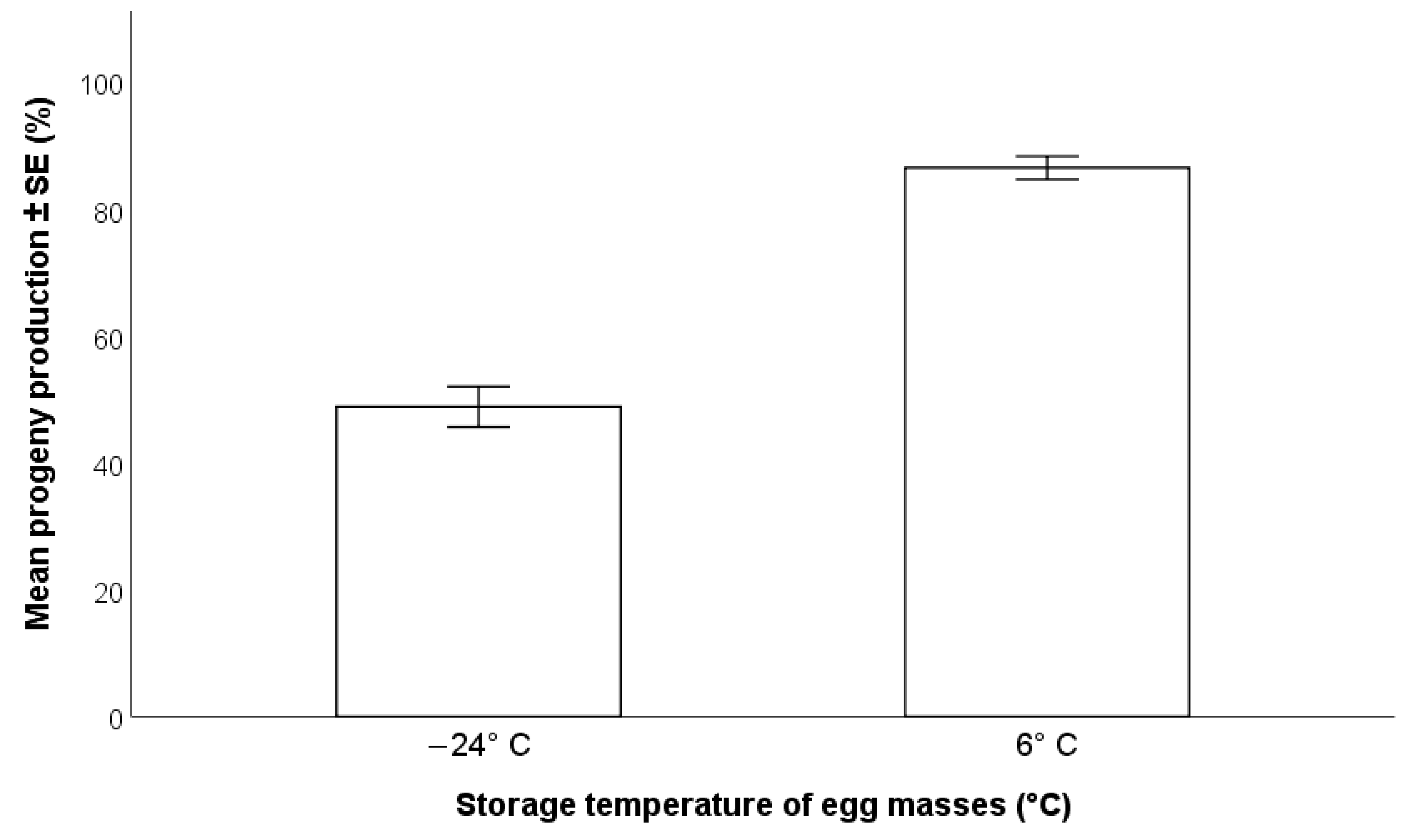
2.3.1. Effects of Pre-Exposure Storage Temperature of the H. halys Egg Masses on Parasitoid Progeny Production
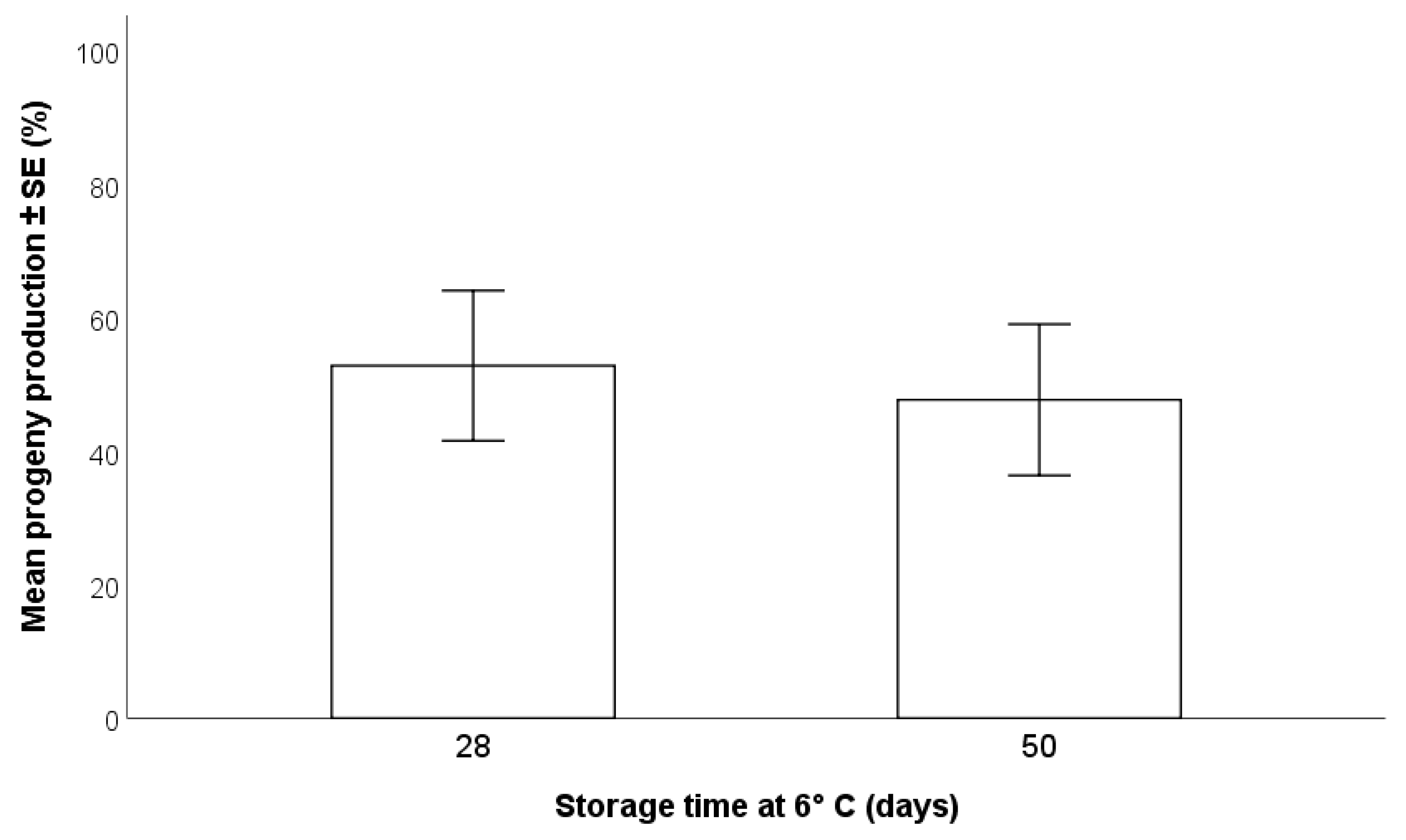
2.3.2. Duration of Storage of H. halys Egg Masses at 6 °C
2.4. Exposure of Egg Masses to Parasitoids
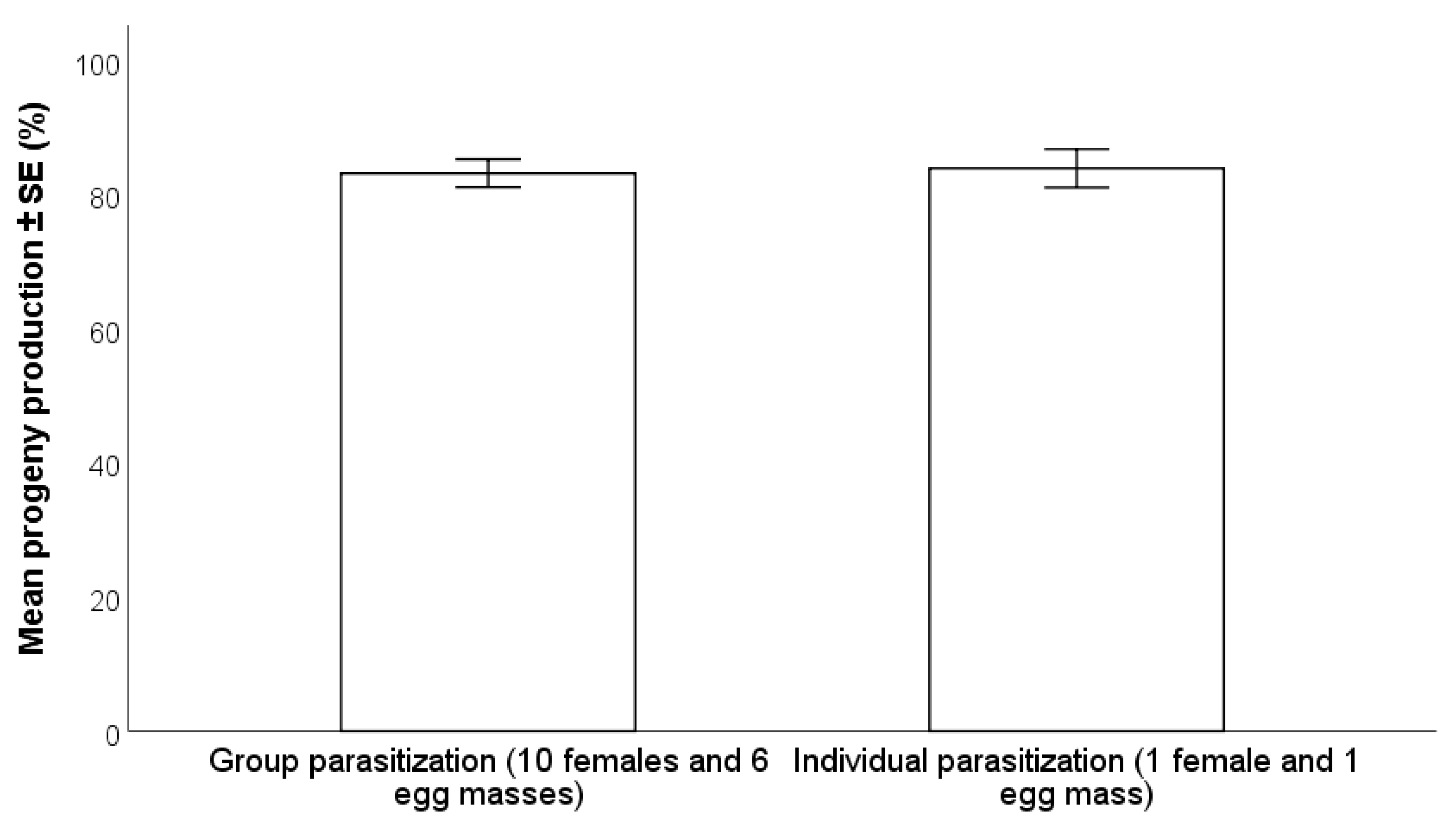
2.4.1. Individual vs. Group Parasitization
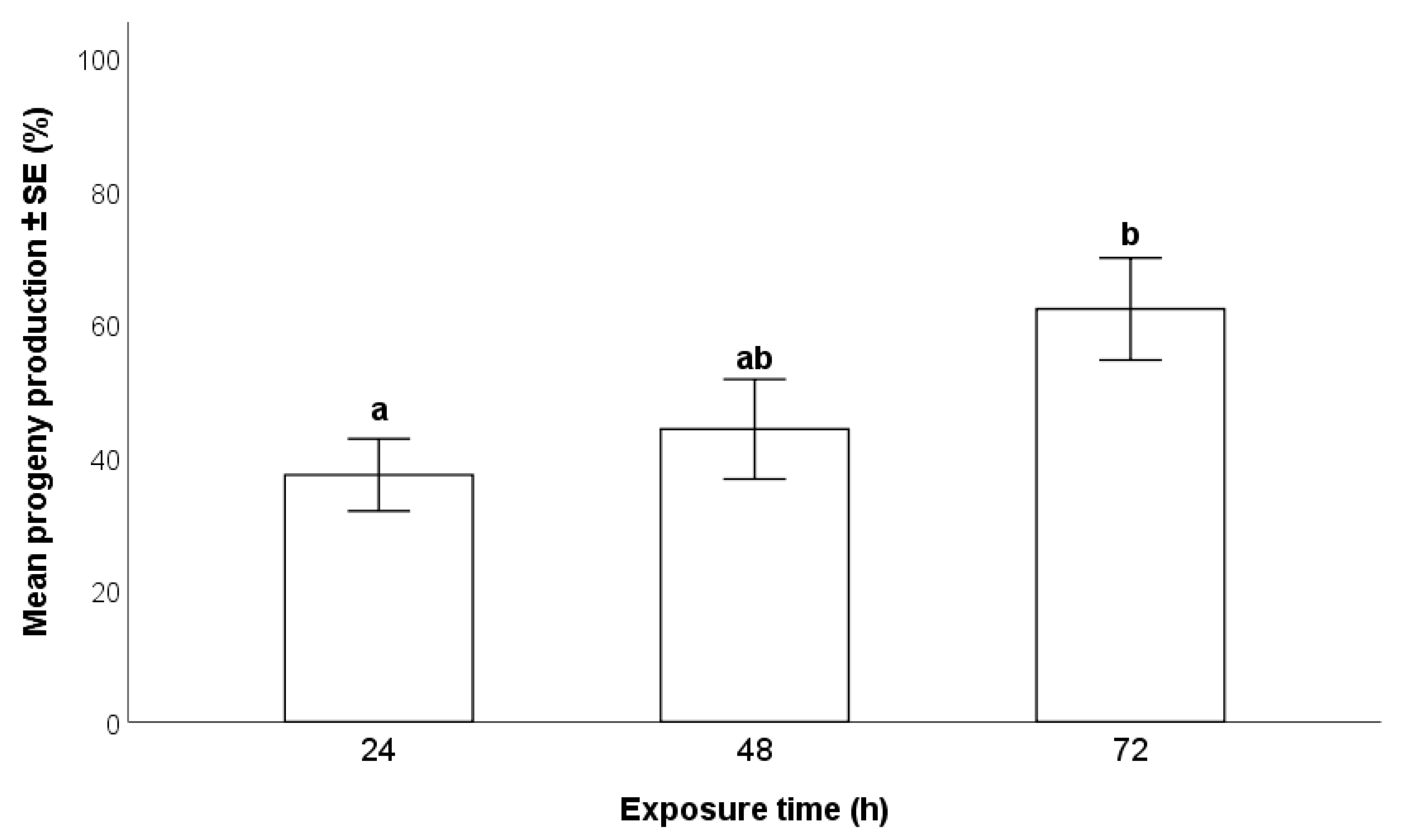
2.4.2. Duration of Exposure of Egg Masses to T. japonicus Females
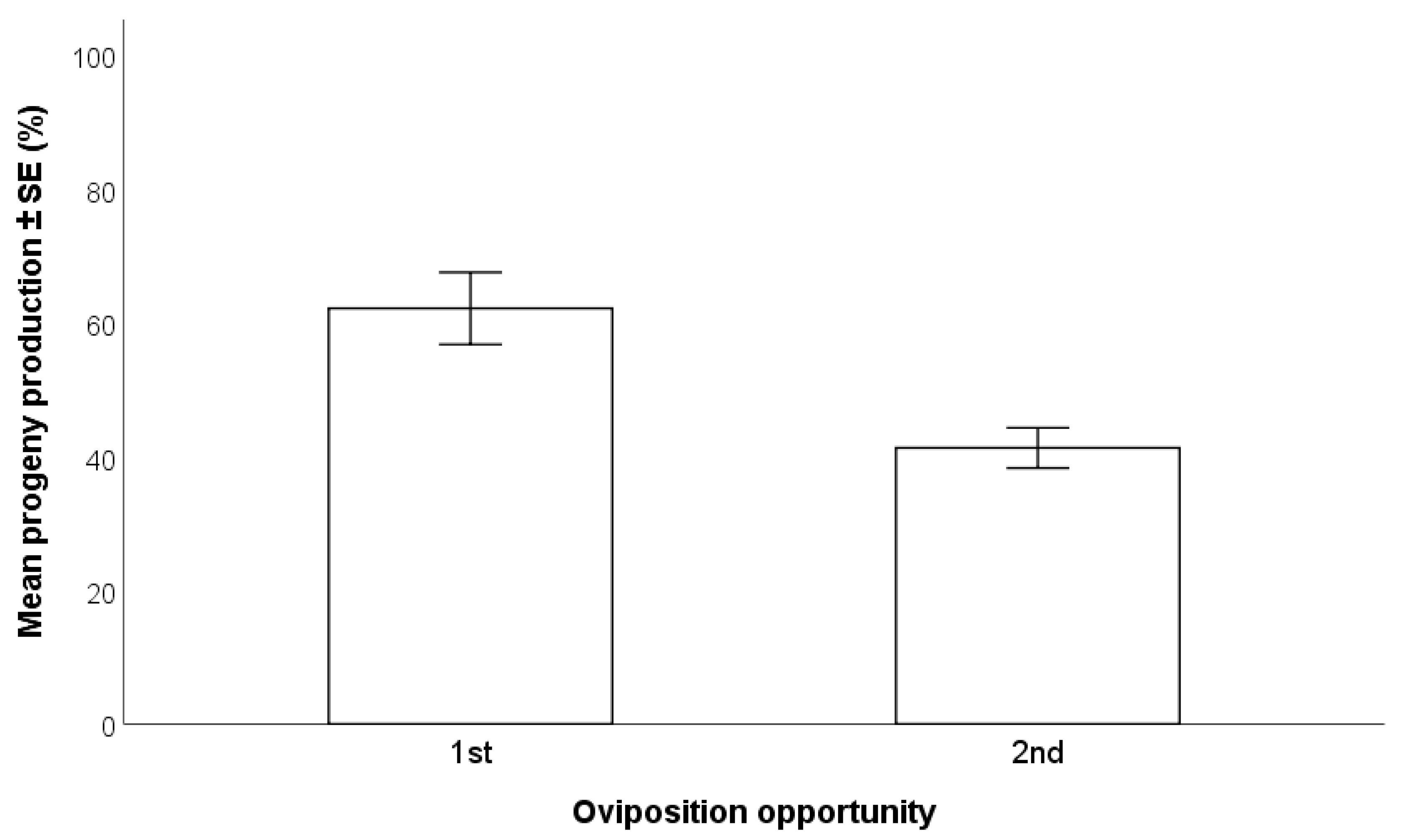
2.4.3. First vs. Second Oviposition by T. japonicus Females
2.5. Effect of Length of Storage of Adult Parasitoids at 16 °C on Their Survival and Production of Progeny
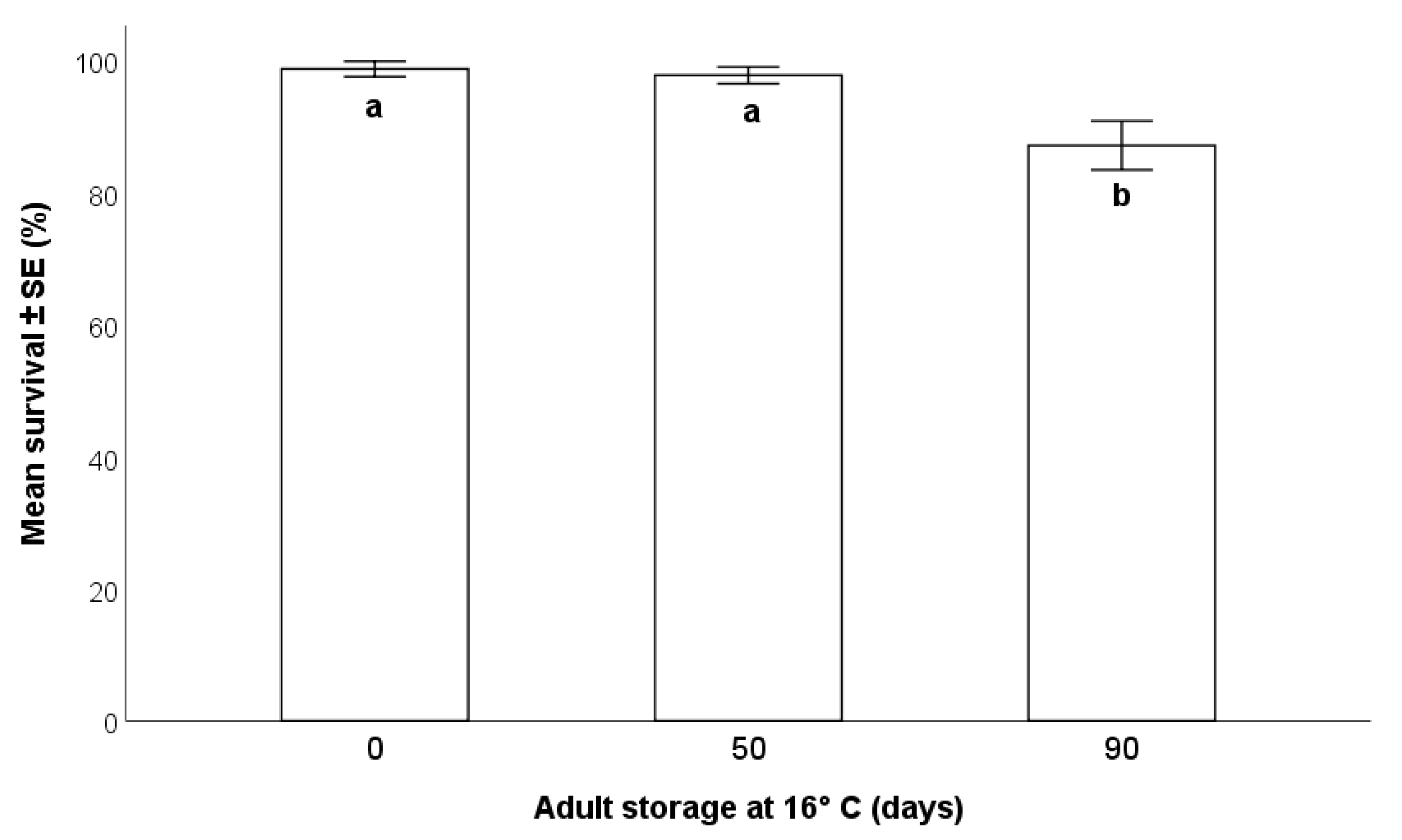
2.5.1. Effects on Survival
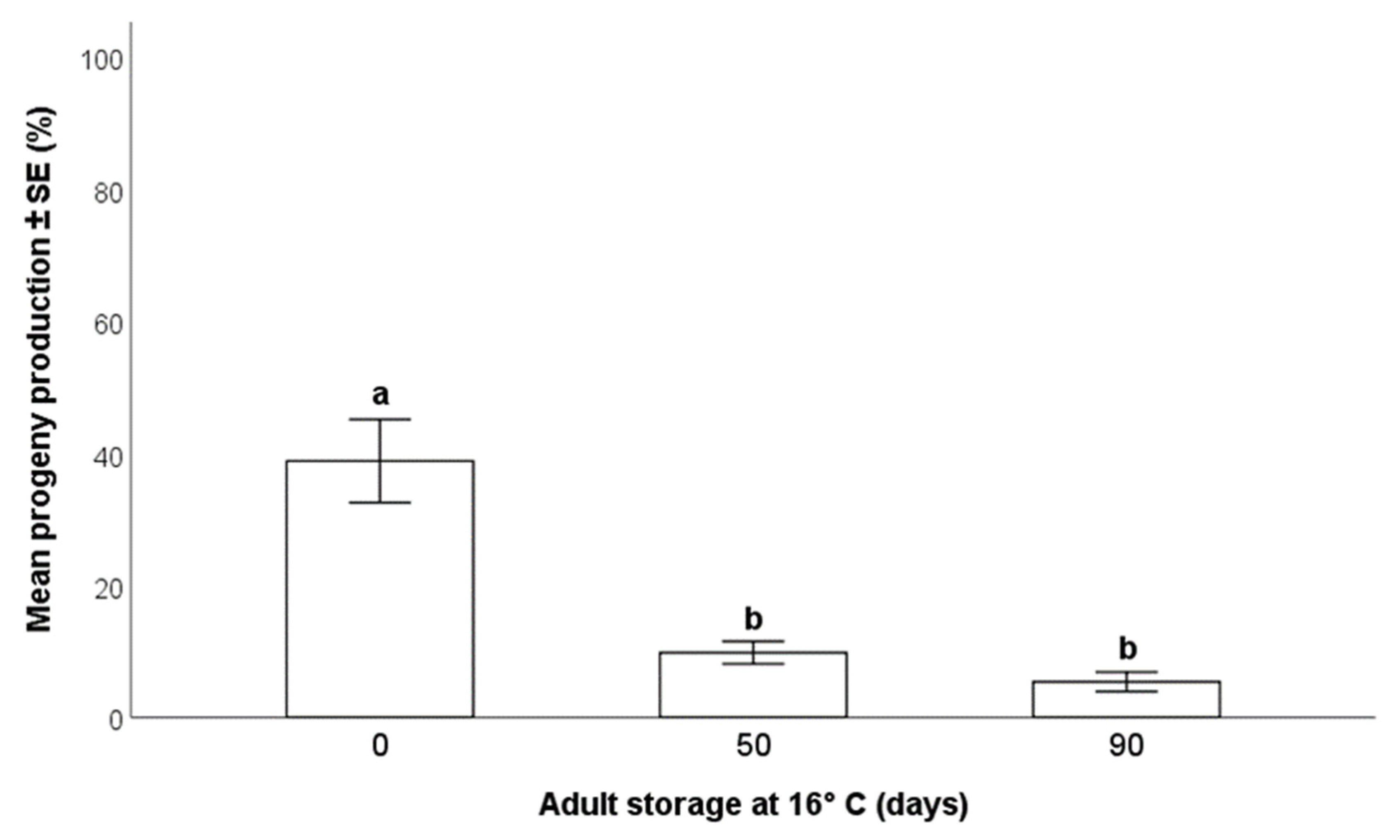
2.5.2. Effects on Progeny Production
2.6. Data Analysis
3. Results
3.1. Effect of Storage of H. halys Egg Masses on Parasitization by T. japonicus
3.1.1. Effects of Pre-Exposure Storage Temperature of the H. halys Egg Masses on Parasitoid Progeny Production
3.1.2. Duration of Storage of H. halys Egg Masses Storage at 6 °C
3.2. Exposure of Egg Masses to Parasitoids
3.2.1. Individual vs. Group Parasitization
3.2.2. Duration of Exposure of Egg Masses to T. japonicus Females
3.2.3. First vs. Second Oviposition by T. japonicus Females
3.3. Effect of Length of Storage of Adult Parasitoids at 16 °C on Their Survival and Production of Progeny
3.3.1. Effects on Parasitoid Survival
3.3.2. Effects on Progeny Production
4. Discussion
5. Conclusions
- (i)
- In terms of parasitoid production, the most effective method of storage for H. halys egg masses is refrigeration at 6 °C for a maximum of two months; beyond this time, a progressive decline in egg suitability has been shown [22]. Freezing may be considered for long term maintenance of H. halys egg masses, but negative impacts on T. japonicus production of progeny were detected.
- (ii)
- A 72-h exposure time of H. halys egg masses to parasitoids resulted in higher progeny production compared to 24 and 48 h, especially with wasps at their first oviposition opportunity. There were no differences between individual and group parasitization.
- (iii)
- The storage of the adult parasitoids at 16 °C allowed for their survival up to 90 days from emergence, but this was accompanied by a progressive decrease in the production of progeny. Consequently, the long-term conservation of adults at low temperatures may be suitable for maintenance of the rearing during less-demanding periods, but not for maximal production of parasitoids to be released in biological control programs.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Èntomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [Green Version]
- Stoeckli, S.; Felber, R.; Haye, T. Current distribution and voltinism of the brown marmorated stink bug, Halyomorpha halys, in Switzerland and its response to climate change using a high-resolution CLIMEX model. Int. J. Biometeorol. 2020, 64, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Bariselli, M.; Bugiani, R.; Maistrello, L. Distribution and damage caused by Halyomorpha halys in Italy. EPPO Bull. 2016, 46, 332–334. [Google Scholar] [CrossRef]
- Francati, S.; Masetti, A.; Martinelli, R.; Mirandola, D.; Anteghini, G.; Busi, R.; Dalmonte, F.; Spinelli, F.; Burgio, G.; Dindo, M.L. Halyomorpha halys (Hemiptera: Pentatomidae) on Kiwifruit in Northern Italy: Phenology, Infestation, and Natural Enemies Assessment. J. Econ. Èntomol. 2021, 114, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Leskey, T.C.; Hamilton, G.C.; Nielsen, A.L.; Polk, D.F.; Rodriguez-Saona, C.; Bergh, J.C.; Herbert, D.A.; Kuhar, T.P.; Pfeiffer, D.; Dively, G.P.; et al. Pest Status of the Brown Marmorated Stink Bug, Halyomorpha Halys in the USA. Outlooks Pest Manag. 2012, 23, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Masetti, A.; Depalo, L.; Pasqualini, E. Impact of Triflumuron on Halyomorpha halys (Hemiptera: Pentatomidae): Laboratory and Field Studies. J. Econ. Èntomol. 2021, 114, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, T.P.; Kamminga, K. Review of the chemical control research on Halyomorpha halys in the USA. J. Pest Sci. 2017, 90, 1021–1031. [Google Scholar] [CrossRef]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, Ecology, and Management of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Haye, T.; Moraglio, S.T.; Stahl, J.; Visentin, S.; Gregorio, T.; Tavella, L. Fundamental host range of Trissolcus japonicus in Europe. J. Pest Sci. 2020, 93, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Malek, R.; Kaser, J.M.; Anfora, G.; Ciolli, M.; Khrimian, A.; Weber, D.C.; Hoelmer, K.A. Trissolcus japonicus foraging behavior: Implications for host preference and classical biological control. Biol. Control. 2021, 161, 104700. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Yao, Y.-X.; Qiu, L.-F.; Li, Z.-X. A New Species of Trissolcus (Hymenoptera: Scelionidae) Parasitizing Eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with Comments on Its Biology. Ann. Èntomol. Soc. Am. 2009, 102, 39–47. [Google Scholar] [CrossRef]
- Talamas, E.J.; Herlihy, M.V.; Dieckhoff, C.; Hoelmer, K.A.; Buffington, M.; Bon, M.-C.; Weber, D.C. Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 2015, 43, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Qui, L.F.; Yang, Z.; Tao, W. Biology and population dynamics of Trissolcus halyomorphae. Sci. Silvae Sin. 2007, 43, 62–65. [Google Scholar] [CrossRef]
- Sabbatini-Peverieri, G.; Dieckhoff, C.; Giovannini, L.; Marianelli, L.; Roversi, P.F.; Hoelmer, K. Rearing Trissolcus japonicus and Trissolcus mitsukurii for Biological Control of Halyomorpha halys. Insects 2020, 11, 787. [Google Scholar] [CrossRef]
- Medal, J.; Smith, T.; Fox, A.; Cruz, A.S.; Poplin, A.; Hodges, A. Rearing the Brown Marmorated Stink Bug Halyomorpha halys(Heteroptera: Pentatomidae). Fla. Èntomol. 2012, 95, 800–802. [Google Scholar] [CrossRef]
- McIntosh, H.; Lowenstein, D.M.; Wiman, N.G.; Wong, J.S.; Lee, J.C. Parasitism of frozen Halyomorpha halys eggs by Trissolcus japonicus: Applications for rearing and experimentation. Biocontrol Sci. Technol. 2019, 29, 478–493. [Google Scholar] [CrossRef]
- Peverieri, G.S.; Talamas, E.; Bon, M.C.; Marianelli, L.; Bernardinelli, I.; Malossini, G.; Benvenuto, L.; Roversi, P.F.; Hoelmer, K. Two Asian egg parasitoids of Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2018, 67, 37–53. [Google Scholar] [CrossRef]
- Moraglio, S.T.; Tortorici, F.; Pansa, M.G.; Castelli, G.; Pontini, M.; Scovero, S.; Visentin, S.; Tavella, L. A 3-year survey on parasitism of Halyomorpha halys by egg parasitoids in northern Italy. J. Pest Sci. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Decreto 42967 del 9 giugno 2020. Immissione in natura della specie non autoctona Trissolcus japonicus quale Agente di Controllo Biologico del fitofago Halyomorpha halys ai sensi del Decreto del Presidente della Repubblica 8 settembre 1997, n. 357, art. 12. 2020.
- Conti, E.; Avila, G.; Barratt, B.; Cingolani, F.; Colazza, S.; Guarino, S.; Hoelmer, K.; Laumann, R.A.; Maistrello, L.; Martel, G.; et al. Biological control of invasive stink bugs: Review of global state and future prospects. Entomol. Exp. Appl. 2021, 169, 28–51. [Google Scholar] [CrossRef]
- Leppla, N.C.; Morales-Ramos, J.A.; Shapiro-Ilan, D.I.; Rojas, G.M. Introduction. In Mass Production of Beneficial Organisms-Invertebrates and Entomopathogens; Morales-Ramos, J., Ro-jas, G., Shapiro-Ilan, D., Eds.; Elsevier-Academic Press: New York, NY, USA, 2014; pp. 7–9. ISBN 9780-0-12-391453-8. [Google Scholar]
- Wong, W.H.L.; A Walz, M.; Oscienny, A.B.; Sherwood, J.L.; Abram, P.K. An Effective Cold Storage Method for Stockpiling Halyomorpha halys (Hemiptera: Pentatomidae) Eggs for Field Surveys and Laboratory Rearing of Trissolcus japonicus (Hymenoptera: Scelionidae). J. Econ. Èntomol. 2021, 114, 571–581. [Google Scholar] [CrossRef]
- Cira, T.; Santacruz, E.N.; Koch, R.L. Optimization of Trissolcus japonicus cold storage methods for biological control of Halyomorpha halys. Biol. Control 2021, 156, 104534. [Google Scholar] [CrossRef]
- Nielsen, A.; Hamilton, G.; Matadha, D. Development rate estimation and life table analysis for Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). Environ. Entomol. Environ. Entomol. 2008, 37, 348–355. [Google Scholar] [CrossRef]
- Haye, T.; Abdallah, S.J.; Gariepy, T.D.; Wyniger, D. Phenology, life table analysis and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J. Pest Sci. 2014, 87, 407–418. [Google Scholar] [CrossRef]
- Arakawa, R.; Miura, M.; Fujita, M. Effects of host species on the body size, fecundity, and longevity of Trissolcus mitsukurii (Hymenoptera: Scelionidae), a solitary egg parasitoid of stink bugs. Appl. Èntomol. Zool. 2004, 39, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Colinet, H.; Boivin, G. Insect parasitoids cold storage: A comprehensive review of factors of variability and consequences. Biol. Control 2011, 58, 83–95. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Lim, U.T. Evaluation of cold-stored eggs of Dolycoris baccarum (Hemiptera: Pentatomidae) for parasitization by Trissolcus nigripedius (Hymenoptera: Scelionidae). Biol. Control 2007, 43, 287–293. [Google Scholar] [CrossRef]
- Ludwick, D.C.; Leake, L.B.; Morrison, W.R.; Lara, J.R.; Hoddle, M.S.; Talamas, E.J.; Leskey, T.C. Influence of Holding Conditions and Storage Duration of Halyomorpha halys (Hemiptera: Pentatomidae) Eggs on Adventive and Quarantine Populations of Trissolcus japonicus (Hymenoptera: Scelionidae) Behavior and Parasitism Success. Environ. Èntomol. 2021, 50, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.K.; Haye, T.; Gariepy, T.D.; McNeil, J.N. Possible coexistence of native and exotic parasitoids and their impact on control of Halyomorpha halys. J. Pest Sci. 2017, 90, 1119–1125. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bittau, B.; Dindo, M.L.; Burgio, G.; Sabbatini-Peverieri, G.; Hoelmer, K.A.; Roversi, P.F.; Masetti, A. Implementing Mass Rearing of Trissolcus japonicus (Hymenoptera: Scelionidae) on Cold-Stored Host Eggs. Insects 2021, 12, 840. https://doi.org/10.3390/insects12090840
Bittau B, Dindo ML, Burgio G, Sabbatini-Peverieri G, Hoelmer KA, Roversi PF, Masetti A. Implementing Mass Rearing of Trissolcus japonicus (Hymenoptera: Scelionidae) on Cold-Stored Host Eggs. Insects. 2021; 12(9):840. https://doi.org/10.3390/insects12090840
Chicago/Turabian StyleBittau, Barbara, Maria Luisa Dindo, Giovanni Burgio, Giuseppino Sabbatini-Peverieri, Kim Alan Hoelmer, Pio Federico Roversi, and Antonio Masetti. 2021. "Implementing Mass Rearing of Trissolcus japonicus (Hymenoptera: Scelionidae) on Cold-Stored Host Eggs" Insects 12, no. 9: 840. https://doi.org/10.3390/insects12090840
APA StyleBittau, B., Dindo, M. L., Burgio, G., Sabbatini-Peverieri, G., Hoelmer, K. A., Roversi, P. F., & Masetti, A. (2021). Implementing Mass Rearing of Trissolcus japonicus (Hymenoptera: Scelionidae) on Cold-Stored Host Eggs. Insects, 12(9), 840. https://doi.org/10.3390/insects12090840










