Simple Summary
Entomopathogenic nematodes (Steinernematidae) are obligate insect parasites and are used for insect pest control, particularly on amenity grassland and in horticulture. Extensive surveys have been conducted across the globe to isolate locally adapted entomopathogenic nematodes species. The biological activity and morphology of three new isolates of Steinernema feltiae from Poland are described. New S. feltiae isolates from Poland showed close genetic similarity to other isolates of this species and exhibited a high reproductive rate and invasive capacity compared to the commercial biopesticide.
Abstract
Insect trap studies were carried out to determine the presence of entomopathogenic nematodes (EPN) from the family Steinernematidae in the soils of Poland and to compare the biological activities of field nematode isolates with nematodes from commercial biopesticide. The fauna of these organisms in central Poland is poorly studied in both taxonomic and biological terms. Tilled soils representative of this region were sampled from cultivated fields. EPN were isolated from soil samples under laboratory conditions and identified using a key for species identification and molecular analysis. Basic morphometric parameters of infective juveniles and adult males of the first generation were determined. The research showed that males and infective juveniles Steinernema feltiae from Łoniów were the largest. The smallest infective juveniles were found in the isolate from Oblasy, and the smallest males in the isolate from Danków. In Poland, new field isolates showed close genetic similarity to other S. feltiae isolates. The research showed that the field isolates from Poland had greater infectivity and rate of reproduction compared with nematodes from the commercial biopesticide. The findings indicate the potential use of field S. feltiae isolates from Poland (iso1Lon, iso1Dan and iso1Obl) to develop new biopesticide products.
1. Introduction
The abundance and activity of soil fauna are largely dependent on the level of human activity within a given sampling area. Species diversity usually declines with increasing land development, resulting in a detrimental effect on biogeochemical cycles in ecosystems, disrupting the natural food webs and increasing the populations of plant pests. In crops, pest control may be particularly difficult in some areas due to, among other things, regulatory restrictions for applying plant protection chemicals. Therefore, the development of biological control methods to manage insect pests, using natural enemies, is an effective alternative strategy by which to achieve pest management, but without damage to the environment.
The widely used biocontrol factors include: bacteria, viruses, fungi, entomophagous insects and entomopathogenic nematodes (EPN) [1,2,3,4,5,6,7,8,9]. With regard to EPN, it has been reported that local populations of nematodes can be more effective at reducing pests than the isolates recovered from other environments [10,11]. It has been demonstrated that the animal hosts of the EPN are positive factors, and it has been demonstrated that EPN can infect more than 200 insect species from several orders [12].
Research into new isolates of EPN indicates that they can differ greatly in terms of invasiveness and survival, and tolerance to various environmental factors [13,14,15,16]. This variability indicates that a screening program may identify new species and isolates that might be particularly useful for the biological control of insect crop pests.
EPN are natural inhabitants of the soil environment [17,18,19,20,21]. Their development cycle is associated with those of the host insects, whose bodies are used by EPN for the multiplication and subsequent emergence of up to several generations of nematodes. In addition to the adult stage, there is an egg and four juvenile stages in the EPN lifecycle, with the third (infective) stage juvenile adapted to survive in the external environment outside the host insect. EPN are closely associated with bacteria of the genera Xenorhabdus (in Steinernema spp.) and Photorhabdus (in Heterorhabditis spp.), e.g., X. bovienii Akhurst, 1983, and P. luminescens Thomas et Poinar, 1979 [22], although it has been proven that these nematodes also form relationships with other symbiotic bacterial species [23,24]. The efficacy and biocontrol success of EPN can be enhanced through improved understanding of their biology and ecology. Many endogenous (microbiome) and environmental factors influence the survival of EPN and their transmission to the target species following their application as a biocontrol agent [23,25].
The biological activity of EPN depends on numerous environmental factors. For example, S. feltiae Filipjev, 1934, is a cold-adapted species, infecting hosts within the range 8–30 °C and reproducing at 10–25 °C [26,27,28]. Another important factor is soil moisture, which regulates nematode survival and motility [29,30,31]. Nematode infective juveniles find the most favorable conditions for invasion to be within the 25–40% soil moisture range. When the soil exceeds 50% moisture content, the biological activity of EPN declines [32]. The motility of nematode juveniles in the soil also depends on the soil structure. The greatest invasiveness was observed in sandy and sandy loamy soils, as the pore spaces in such environments provide optimal moisture and oxygenation conditions [17,32].
EPN populations are also influenced by biotic factors. The soil-dwelling infective juveniles are exposed to contacts with many organisms that share the same biotope, including bacteria, fungi and other nematodes. These organisms may reduce the EPN populations by food and territorial competency, or by their negative impact on EPN survival [33,34].
EPN are reported to be safe biological plant protection agents [35,36], and due to numerous advantages, such as tolerance of unfavorable conditions and the ability to infect several pest species, they are a good alternative to chemical plant protection products in agroecosystems where different phytophagous species may be present at the same time [37].
The broad host range of most EPN and their potential to reproduce and survive in the soil environment are the main advantages for the use of EPN for biological pest control. Research data suggest that selection of native isolates of nematodes offers more benefits than using commercial preparations that are based on one specific nematode isolate, which is not necessarily local to the agroecosystem in question [11]. Studies on the occurrence, biology and utilization of field EPN isolates have been conducted in many countries around the world [38,39,40,41,42]. Other studies have provided evidence that EPN show considerable variation in terms of biological activity, reproduction, host selection and tolerance to various environmental conditions. Thus, there is a need to gain a thorough knowledge on the natural populations of these potentially valuable organisms [43,44,45].
It is hypothesized that the activities of the isolates will be different, owing to their different origins and adaptations.
It has been demonstrated that local populations of EPN can be more effective at reducing pests than the isolates recovered from other environments [11]. Studying the biology of new isolates from Poland may be beneficial for plant protection. A better understanding of the national entomopathogenic nematode fauna in Poland and their distribution is another important aspect of the research.
The aim of the work was achieved by identifying new environmental Polish isolates of S. feltiae with the use of morphometric and molecular methods, and by comparing the biological activities of isolated nematodes with the commercial EPN biopesticide tested on larvae of the greater wax moth (Galleria mellonella Lepidoptera, Pyralidae).
2. Materials and Methods
2.1. Soil Samples’ Collection and Nematodes’ Isolation
Galleria mellonella for bioassays were obtained from laboratory cultures held at the Department of Microbiology and Parasitology, Jan Kochanowski University, Poland. Insects were cultured at 25 °C on beeswax patches in ventilated polypropylene containers. The fourth-instar of G. mellonella larvae, with an average body weight of 140 mg, was used for all analyses.
Ten organic farms on which wheat was grown were selected for the study (Appendix A, Table A1, Figure A1). For EPN isolation, soil samples were taken from sandy loam or loamy sand soils. At each farm, 50 soil samples were randomly collected to a depth of 25 cm from an area of 100 m2, using an Egner’s soil sampler.
Under laboratory conditions, soil samples were mixed to obtain homogeneity and placed into 6 sterile 250 mL vessels. Field nematodes were isolated from soil samples using the insect trap method (G. mellonella) [46]. The samples were incubated in a thermostatically controlled temperature at 20 °C (POL-EKO Aparatura, Wodzisław Śląski, Poland) (optimal for the species [27]) and checked every 48 h over a 16-day period.
Dead moth larvae, cleaned of soil particles and washed three times with distilled water, were transferred to migration sponges (modified White traps—patent No. PL 212617 B1 [47]), and fresh live larvae were introduced into the soil sample [46]. Nematodes’ migration on Petri dishes (Anumbra, Šumperk, Czech Republic) was also checked every 48 h, and newly emerging nematode larvae were collected into culture bottles and stored at 4 °C for further analysis [48].
2.2. Reproduction of the Collected EPN
Larvae of G. mellonella were infected with 50 EPN juveniles/insect on Petri dishes with filter paper, which were then stored in the incubation cabinet at 20 °C over a 5-day period. The cadavers of insects parasitized by nematodes were transferred into migration Petri dishes [47] (Anumbra, Šumperk, Czech Republic). The emerging juvenile nematodes were collected into tissue culture bottles, area 75 cm2 (Nunc EasyFlasks, Roskilde, Denmark), and stored at 4 °C. Field EPN isolates were subjected to morphometric and genetic analyses for species identification.
2.3. Identification of Nematode Species
The preliminary nematode differentiation was based on the species identification key [49]. Morphometric and molecular analyses were used to identify the EPN species. Morphometric analysis of variables was carried out on invasive juveniles and adult males of the first generation [12,50,51]. For descriptive purposes, 25 specimens per variable were used. The following were measured: total body length, maximum body width, distance from anterior end to excretory pore, distance from anterior end to nerve ring, distance from anterior end to end of pharynx, tail length, anal body width and additionally, in males, spicule length and gubernaculum length.
2.4. Gene Sequencing
Genomic DNA was extracted using a Genomic Mini kit (A&A Biotechnology, Gdańsk, Poland). The internal transcribed spacer (ITS) region was amplified by PCR using forward and reverse primers SF18SL (5′GTACACACCGCCCGTCGCTGC3′) and SF18SR (5′AAATCCTAGTTAGTTTCTTTTCCTCCGC3′) [45].
The primers were used to determine the species. The PCR conditions were: 94 °C for 3 min and then 35 cycles (94 °C for 30 s, 66 °C for 30 s, 72 °C for 30 s), followed by an extension step at 72 °C for 5 min. After the last step, temperature was lowered to 4 °C, where the products were maintained until the purification step. The purification of the PCR product was performed using the PCR/DNA Clean-Up kit (A&A Biotechnology, Gdańsk, Poland) and sequenced in the laboratory CoreLab of the Medical University of Łódź, Poland. The purified PCR products were sequenced using the appropriate primers SF18SL and SF18SR. PCR sequencing was carried out in a 10 µL reaction volume, using Big Dye® Terminator v1.1 (Applied Biosystems, South San Francisco, CA, USA). The PCR reaction was performed in a Gene Amp PCR System 9700 thermal cycler (Applied Biosystems, South San Francisco, CA, USA). Purification of the reaction products was performed using the BigDye XTerminator Purification Kit (Applied Biosystems, South San Francisco, CA, USA). Reaction conditions were set according to the manufacturer’s instructions. The reaction products were separated using a 3130xl Genetic Analyzer capillary sequencer (Applied Biosystems, South San Francisco, CA, USA). Sample analysis was performed using the DNA Baser tool for the DNA Sequence Assembler program (CoreLab, Medical University, Łódź, Poland).
2.5. Phylogenetic Analysis
The ITS gene sequence of isolates iso1Lon (from Łoniów), iso1Dan (Danków Duży) and iso1Obl (Oblasy), obtained in this study, were compared to the GenBank nucleotide sequences of other S. feltiae species using BLAST, available on the NCBI website (https://www.ncbi.nlm.nih.gov, accessed on 15 July 2021). The evolutionary relationship of the three isolates was inferred using the Neighbor-Joining (NJ) method with the phylogeny.fr (http://phylogeny.lirmm.fr/phylo_cgi/index.cgi, accessed on 15 July 2021) online software version 2021 for phylogenetic analysis [52].
2.6. Quantification of Biological Activity of EPN
The S. feltiae isolate from the commercial Owinema biopesticide (Owiplant, Owińska, Poland) was used as a control to compare the biological activities of the three field isolates (iso1Lon, iso1Dan, iso1Obl). The insect mortality (percentage of insects killed due to any cause), infectivity (percentage of insects killed by nematodes), number of nematodes invading per insect, time to kill (days), time to first emergence of infective juveniles from infection (days) and number of infective juveniles emerging/insect were determined.
Larvae of G. mellonella were infected with 50 EPN juveniles/insect on Petri dishes with filter paper (50 IJs/0.1 mL), which were then stored in the incubation cabinet at 20 °C. For each sample (three field isolates and control sample), 60 larvae insects were used.
EPN infectivity was determined by dissection of insects 3 days after their death. Thirty insects from each study group (three field S. feltiae isolates and one control isolate) were dissected in Petri dishes. Using preparation needles, the body of one dead larvae was dissected into small sections and the number of nematodes inside the insect body was counted under a light microscope (number of nematodes invading per insect).
A similar approach was taken when analyzing the number of infective juveniles migrating from one insect. Insects that had been infected with the same number of nematodes (50 IJs/insect) were transferred on migration sponges, and after observing the emergence of the first nematode juveniles, the nematodes were collected. Five harvests (one every two days) were performed over a period of nine days. The experiment was repeated twice.
2.7. Statistical Analysis
The Statistica version 13.3 software (statsoft.pl) was used for statistical analysis of data: morphometry and parameters of biological activity of nematodes. One-way analysis of variance (ANOVA) was performed for morphometric variables and the number of migrating infectious larvae. Mean and standard deviation were calculated for each morphometric variable in a given group. For biological activity parameters, Tukey’s test was used, with p < 0.05 used to differentiate homogeneous groups in multiple pairwise comparisons. Mean and standard deviation were calculated for the number of nematode juveniles migrating from one insect at successive harvests at two-day intervals and for the total number of migrating nematode juveniles from host insects from the different S. feltiae isolates.
3. Results
Steinernema feltiae was isolated from only three (Danków Duży (isolate iso1Dan), Łoniów (iso1Lon) and Oblasy (iso1Obl)) out of the ten regions sampled. S. feltiae was extracted from soils within a pH range of 5.2–5.4. The pH of the remaining soil samples was within the wider range of 5.2–5.6 (Table A1).
Molecular analysis and comparisons of the morphometric variables from the three EPN isolates with those specified in the key were performed, and in each case, the nematodes were identified as S. feltiae (Table 1 and Table 2, Figure 1). Invasive juveniles of the iso1Lon isolate were the largest of the isolates tested, whereas juveniles of the iso1Obl isolate were the smallest (Table 1).

Table 1.
Morphometric variables (mean ± SD) of infective juveniles of Steinernema feltiae from Poland. Variables (in µm) are analyzed by one-way ANOVA with correspondent F statistic, with degrees of freedom (df) and p-value.

Table 2.
Morphometric variables (mean ± SD) of first-generation adult males of Steinernema feltiae from Poland. Variables (in µm) are analyzed by one-way ANOVA with correspondent F statistic, with degrees of freedom and p-value.
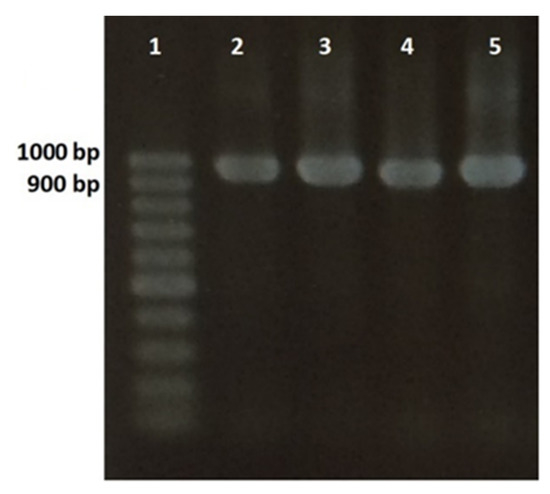
Figure 1.
Agarose gel electrophoresis of internal transcribed spacer (ITS) PCR products from four Steinernema feltiae isolates: Lane 1: 100 bp DNA Ladder; Lane 2: PCR product of control sample (S. feltiae from biopreparation); Lane 3: PCR product of iso1Lon; Lane 4: PCR product of iso1Dan; Lane 5: PCR product of iso1Obl.
The males of the first-generation isolate from Łoniów were also larger than the males from Danków and Oblasy. In this generation, the iso1Dan isolate was the smallest. It was also observed that the length of the spicules was very similar for the isolates iso1Lon and iso1Obl (Table 2).
Phylogenetic tree analysis, using the Neighbor-Joining method, based on ITS gene sequencing, revealed that the isolates from the three sites were very similar to other S. feltiae populations from Poland (Figure 2).
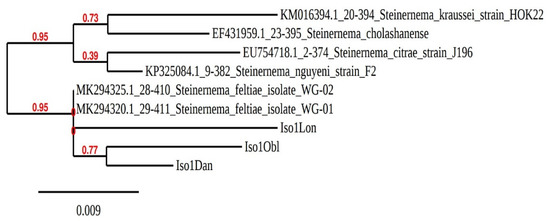
Figure 2.
Phylogenetic relationships of three Poland EPN isolates based on analysis of internal transcribed spacers (ITS), using the Neighbor-Joining method.
Insect mortality was shown to be 100% for the majority of the samples. In the sample infected with the iso1Obl isolate, the total mortality of the infected insects was lower, at 95%.
With respect to the infectivity, only 2% of test insects in the control sample and 3% of insects in the samples infected with iso1Lon or iso1Obl isolates died for other reasons (dead insects were black and no nematodes were present at necropsy) (Table 3).

Table 3.
Biological activity of Steinernema feltiae isolates (mean values).
It was found that the number of nematodes invading per insect was significantly higher in the case of the iso1Lon isolate than with the other isolates (Table 3).
By analyzing the rate of development, it was shown that the control (Owinema) isolate and the iso1Dan isolate developed faster than the iso1Lon and iso1Obl isolates. A similar relationship was noted in the case of nematode reproduction, as the nematodes from the commercial preparation and the iso1Dan isolate started migrating within ten days of host infection, whereas the first progeny of the iso1Lon and iso1Obl isolates were observed migrating a day later (Table 3).
When examining the number of migrating infective juveniles on successive days of migration, was observed that the emergence of infective juveniles from insect cadavers with field isolates reached a peak on the fourth day of emergence, whereas the nematodes from the control sample reached a peak on the third day of emergence (Figure 3).
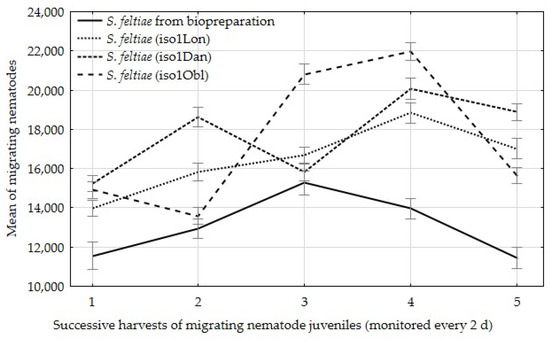
Figure 3.
Mean of nematode juveniles migrating from one insect at successive harvests at 2-day intervals, ±standard deviation (SD).
A comparison of the reproduction potentials revealed that the number of migrating infective juveniles was significantly (p < 0.05) higher, in the range of 5000, for all three environmental nematode isolates than for the control sample (Table 3, Figure 4).
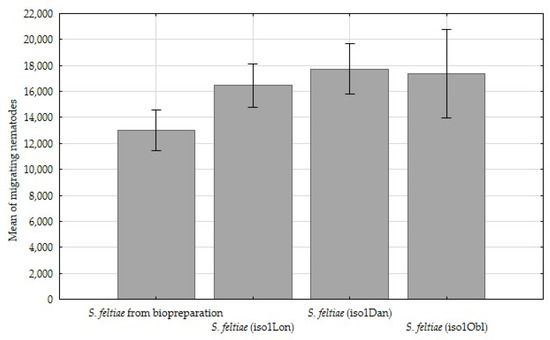
Figure 4.
Comparison of mean of migrating nematode juveniles from host insects from the different Steinernema feltiae isolates, ±standard deviation (SD).
4. Discussion
One of the topics that has attracted particular attention among entomonematologists is the study of the diversity and biological activity of EPN recovered from natural habitats [20,33,43,44,54,55,56,57,58,59,60,61,62]. About 93 species of Steinernema have been identified to date [55], of which 7 have been reported from the area of Poland [10,17,62].
The current study has shown that EPN recovered from cultivated soils in Poland were identified as S. feltiae. Morphological traits of the examined isolates matched the original description of the species, but the means of these dimensions were lower than in the original description [53]. Other studies indicate that the species has a global range, although it is most frequent under temperate climatic conditions [62,63].
Phylogenetic analysis using the Neighbor-Joining method based on ITS gene sequencing showed that new isolates (iso1Lon, iso1Dan and iso1Obl) formed a subcluster with other S. feltiae isolates.
The biological activity of the nematode isolates studied merits attention as it makes it possible to use local EPN isolates for the biological control of plant pests. The higher rate of survival and invasiveness by these field isolates, compared with the commercial biopesticidal isolate, would increase the quality and effectiveness of the biopreparation which could be prepared from one or more of these isolates.
Recent studies have provided evidence that EPN isolates originating from various sources differ in their biological activity [10,43,44,64], while other investigations found that the local populations of EPN were better adapted to native conditions and displayed a greater biological activity under such conditions than that achieved by isolates originating elsewhere [11]. Similar results were noted in this current study. The field nematode isolates were a little slower in developing than the commercial isolate, but penetrated insect bodies more readily than did the nematodes derived from commercial biopreparation.
EPN vary in their development rate [10,65] and may also be influenced by differences in their microbial association [25]. Some data from the literature suggest that EPN may kill insects within 24–48 h [61]. Other research has shown that to kill the insect, the nematodes needed more time [66]. In the current study, nematodes from the commercial biopesticide and the isolate from Danków Duży killed the insect host within two days, while the nematodes from Łoniów and Oblasy needed two to three days to achieve the same effect.
In this study, in terms of nematode biological activity (invasion extensiveness and intensity), the results were approximate to the results found by other authors from other countries [65,67]. The significantly greater invasion intensity in the field isolates than in the control isolate appears to reflect the greater invasiveness reported of field EPN isolates [44].
Other parameters of EPN bioactivity include their reproductive potential and their capacity to migrate from the host body. In the present study, it was demonstrated that nematodes isolated from the field needed more time to start migrating than did nematodes derived from the commercial biopesticide. A comparison of nematode reproductivity throughout the early days of migration showed that nematode isolates recovered from the field had a higher reproduction rate than did nematodes from the commercial biopesticide. Similar findings have been reported for these organisms in other studies [10,65,68,69].
The field S. feltiae nematodes were isolated from sandy soil, one of the dominant agricultural soil types in Poland, as well as throughout northwest Europe (France, Germany, Austria, Scandinavia, Great Britain). Therefore, the production of biopesticides from field S. feltiae isolates could have a wide economic impact for the protection of crops in Poland, as well as in other European countries.
The results obtained from this research confirm that field isolates are characterized by greater biological activity (higher invasion intensity and greater number of juvenile offspring from one host insect) than S. feltiae isolates originating from the Owinema biopreparation.
Additionally, this study supports the potential use of field entomopathogenic S. feltiae isolates for the production of effective biopreparations for crop protection.
5. Conclusions
Steinernema feltiae was reported in cultivated soils in Poland. New isolates showed close genetic and morphological similarity to other S. feltiae isolates. The field nematode isolates were characterized by a similar insecticidal effectiveness, which was confirmed by comparable infectivity. Field nematode isolates exhibited a high reproduction rate and number of nematodes invading per insect capacity compared with S. feltiae from commercial biopesticide.
Author Contributions
Conceptualization, J.M.-Ł.; methodology, J.M.-Ł. and W.K.; software, J.M.-Ł.; validation, J.M.-Ł.; formal analysis, J.M.-Ł.; investigation, J.M.-Ł.; resources, J.M.-Ł.; data curation, J.M.-Ł.; writing—original draft preparation, J.M.-Ł.; writing—review and editing, J.M.-Ł., W.K. and P.Ż.; visualization, J.M.-Ł. and P.Ż.; supervision, J.M.-Ł.; project administration, J.M.-Ł. and W.K.; funding acquisition, J.M.-Ł. and W.K. All authors have read and agreed to the published version of the manuscript.
Funding
Studies were supported by grant SUPB.RN.21.235 (2021/22) from Jan Kochanowski University, Kielce, Poland, awarded to W.K.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Dataset available upon request to the corresponding authors.
Acknowledgments
The authors would very much like to thank Barbara Wodecka (Department of Economics and Finance, Jan Kochanowski University in Kielce) for help with statistical analyzes.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Characteristics of the Areas of S. feltiae Isolation
The isolation of local populations of S. feltiae was carried out in the summer of 2019 on arable soils on which wheat had been grown. Soils were of the type representative of the arable soils in Poland (acidic sandy loam and loamy sand soils) and were typical of a temperate climate (Table A1, Figure A1).

Table A1.
Characteristics of soil samples tested for entomopathogenic nematodes.
Table A1.
Characteristics of soil samples tested for entomopathogenic nematodes.
| Locality | Latitude (N) | Longitude (E) | Soil Type | pH |
|---|---|---|---|---|
| Bałtów | 51°01′02.6″ | 21°32′10.7″ | loamy sand | 5.2 |
| Busko-Zdrój | 50°28′06.5″ | 20°41′47.3″ | sandy loam | 5.2 |
| Danków Duży | 50°52′26.5″ | 19°55′29.6″ | sandy loam | 5.2 |
| Końskie | 51°11′38.9″ | 20°23′17.3″ | loamy sand | 5.4 |
| Łoniów | 50°33′31.1″ | 21°31′37.2″ | sandy loam | 5.2 |
| Miechów | 50°21′04.5″ | 20°03′07.4″ | loamy sand | 5.6 |
| Morawica | 50°44′48.4″ | 20°36′52.5″ | loamy sand | 5.4 |
| Oblasy | 50°50′20.1″ | 19°46′31.5″ | sandy loam | 5.4 |
| Stalowa Wola | 50°36′15.2″ | 22°02′32.3″ | loamy sand | 5.2 |
| Szczekociny | 50°37′14.5″ | 19°48′01.1″ | loamy sand | 5.6 |
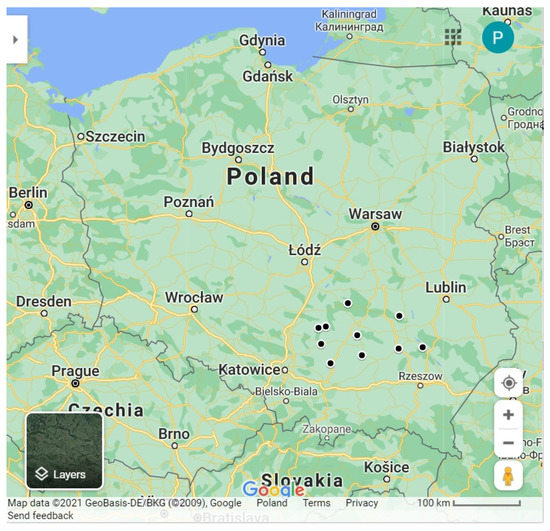
Figure A1.
Location of sampling sites—dark circles (https://www.google.com/maps, accessed on 15 July 2021).
References
- Abd-Alla, A.M.M.; Meki, I.K.; Demirbas-Uzel, G. Insect Viruses as Biocontrol Agents: Challenges and Opportunities. In Cottage Industry of Biocontrol Agents and Their Applications; El-Wakeil, N., Saleh, M., Abu-hashim, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 277–295. [Google Scholar]
- Ehler, L.E. Some Contemporary Issues in Biological Control of Insects and Their Relevance to the Use of Entomopathogenic Nematodes. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–20. ISBN 9781351071741. [Google Scholar]
- Gonzalez, F.; Tkaczuk, C.; Dinu, M.M.; Fiedler, Ż.; Vidal, S.; Zchori-Fein, E.; Messelink, G.J. New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. J. Pest Sci. 2016, 89, 295–311. [Google Scholar] [CrossRef]
- Lahiri, S.; Orr, D. Biological Control in Tomato Production Systems: Theory and Practice. In Sustainable Management of Arthropod Pests of Tomato; Wakil, W., Brust, G.E., Perring, T.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 253–267. ISBN 9780128135082. [Google Scholar]
- Dillman, A.R.; Sternberg, P.W. Entomopathogenic Nematodes. Curr. Biol. 2012, 22, 430–431. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.; Ratcliffe, N.A. Entomopathogenic Fungi: New Insights into Host-Pathogen Interactions. In Genetics and Molecular Biology of Entomopathogenic Fungi; Lovett, B., St Leger, R.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 94, p. 364. ISBN 9780128046944. [Google Scholar]
- Brivio, M.F.; Mastore, M. Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War. Insects 2018, 9, 117. [Google Scholar] [CrossRef]
- Melo, A.L.D.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2014, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Burke, G.R. Polydnaviruses: Evolution and Function. Curr. Issues Mol. Biol. 2020, 34, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Matuska-Łyżwa, J. Ecological and morphological characteristics of Steinernema kraussei (Rhabditida: Steinernematidae): Comparison of nematodes isolated from the natural environments and originated from the commercial pesticide. Ecol. Quest. 2014, 19, 51–55. [Google Scholar] [CrossRef][Green Version]
- Mráček, Z.; Bečvář, S.; Kindlmann, P.; Webster, J.M. Infectivity and specificity of Canadian and Czech isolates of Steinernema kraussei (Steiner, 1923) to some insect pests at low temperatures in the laboratory. Nematologica 1998, 44, 437–448. [Google Scholar] [CrossRef]
- Nguyen, K.B.; Hunt, D.J.; Mráček, Z. Steinernematidae: Species description. In Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts; Nguyen, K.B., Hunt, D., Eds.; Brill: Leiden, The Netherland, 2007; pp. 270–282. [Google Scholar]
- Kagimu, N.; Ferreira, T.; Malan, A.P. The Attributes of Survival in the Formulation of Entomopathogenic Nematodes Utilised as Insect Biocontrol Agents. Afr. Entomol. 2017, 25, 275–291. [Google Scholar] [CrossRef]
- Makirita, W.E.; Zhang, F.; Mbega, E.R.; He, N.; Li, X.; Chacha, M.; Liu, T. Influence of Metal Oxides Nanoparticles on Pathogenicity of Steinernema carpocapsae Nematodes Against Lepidopteran Galleria mellonella. J. Nanosci. Nanotechnol. 2019, 20, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Santhi, V.S.; Salame, L.; Muklada, H.; Azaizeh, H.; Haj-Zaroubi, M.; Awwad, S.; Landau, S.Y.; Glazer, I. Toxicity of phenolic compounds to entomopathogenic nematodes: A case study with Heterorhabditis bacteriophora exposed to lentisk (Pistacia lentiscus) extracts and their chemical components. J. Invertebr. Pathol. 2019, 160, 43–53. [Google Scholar] [CrossRef]
- Widiyaningrum, P.; Fauziyah, L.; Indriyanti, D.R. Biological activity of local entomopathogenic nematodes from two different origins based on various temperatures. Pak. J. Biol. Sci. 2018, 21, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska, M.; Berdzik, M.; Myśków, B. The first molecular characterisation of Steinernema silvaticum recorded in Poland and ITS differentiation from Steinernema kraussei using ribosomal DNA (rDNA) sequences. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2015, 316, 41–48. [Google Scholar]
- Grewal, P.S.; De Nardo, E.A.B.; Aguillera, M.M. Entomopathogenic nematodes: Potential for exploration and use in south America. Neotrop. Entomol. 2001, 30, 191–205. [Google Scholar]
- Kaspi, R.; Ross, A.; Hodson, A.K.; Stevens, G.N.; Kaya, H.K.; Lewis, E.E. Foraging efficacy of the entomopathogenic nematode Steinernema riobrave in different soil types from California citrus groves. Appl. Soil Ecol. 2010, 45, 243–253. [Google Scholar] [CrossRef]
- Kaya, H.K.; Aguillera, M.M.; Alumai, A.; Choo, H.Y.; de la Torre, M.; Fodor, A.; Ganguly, S.; Hazir, S.; Lakatos, T.; Pye, A.; et al. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biol. Control 2006, 38, 134–155. [Google Scholar] [CrossRef]
- San-Blas, E.; Luzardo, M.; Larreal, J.; Portillo, E.; Bastidas, B. Biological control of the fungus gnats Bradysia difformis (Diptera, Mycetophilidae) in mushrooms with Heterorhabditis amazonensis in tropical conditions. Sci. Hortic. 2017, 216, 120–125. [Google Scholar] [CrossRef]
- Burnell, A.M.; Stock, S.P. Heterorhabditis, Steinernema and their bacterial symbionts—Lethal pathogens of insects. Nematology 2000, 2, 31–42. [Google Scholar] [CrossRef]
- Lechowicz, L.; Chrapek, M.; Czerwonka, G.; Korzeniowska-Kowal, A.; Tobiasz, A.; Urbaniak, M.; Matuska-Lyzwa, J.; Kaca, W. Detection of ureolytic activity of bacterial strains isolated from entomopathogenic nematodes using infrared spectroscopy. J. Basic Microbiol. 2016, 56, 922–928. [Google Scholar] [CrossRef]
- Somvanshi, V.S.; Lang, E.; Stäubler, B.; Spröer, C.; Schumann, P.; Ganguly, S.; Saxena, A.K.; Stackebrandt, E. Providencia vermicola sp. nov., isolated from infective juveniles of the entomopathogenic nematode Steinernema thermophilum. Int. J. Syst. Evol. Microbiol. 2006, 56, 629–633. [Google Scholar] [CrossRef]
- Ogier, J.C.; Pagès, S.; Frayssinet, M.; Gaudriault, S. Entomopathogenic nematode-associated microbiota: From monoxenic paradigm to pathobiome. Microbiome 2020, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Grewal, P.S.; Selvan, S.; Gaugler, R. Thermal adaptation of entomopathogenic nematodes: Niche breadth for infection, establishment, and reproduction. J. Therm. Biol. 1994, 19, 245–253. [Google Scholar] [CrossRef]
- Trdan, S.; Valič, N.; Urek, G.; Milevoj, L. Concentration of suspension and temperature as factors of pathogenicity of entomopathogenic nematodes for the control of granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Acta Agric. Slov. 2005, 1, 117–124. [Google Scholar]
- Mastore, M.; Quadroni, S.; Toscano, A.; Mottadelli, N.; Brivio, M.F. Susceptibility to entomopathogens and modulation of basal immunity in two insect models at different temperatures. J. Therm. Biol. 2019, 79, 15–23. [Google Scholar] [CrossRef]
- Fodor, A.; Vecseri, G.; Farkas, T. Caenorhabditis elegans as a model for the study of entomopathogenic nematodes. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 247–270. ISBN 9781351071741. [Google Scholar]
- McGraw, B.A.; Schlossberg, M.J. Fine-scale spatial analysis of soil moisture and entomopathogenic nematode distribution following release in wetting agent-treated turf. Appl. Soil Ecol. 2017, 114, 52–61. [Google Scholar] [CrossRef]
- Radová, Š.; Trnková, Z. Effect of soil temperature and moisture on the pathogenicity of two species of entomopathogenic nematodes (Rhabditida: Steinernematidae). J. Agrobiol. 2010, 27, 1–7. [Google Scholar] [CrossRef]
- Grant, J.A.; Villani, M.G. Soil moisture effects on entomopathogenic nematodes. Environ. Entomol. 2003, 32, 80–87. [Google Scholar] [CrossRef]
- Jaffuel, G.; Blanco-Pérez, R.; Büchi, L.; Mäder, P.; Fließbach, A.; Charles, R.; Degen, T.; Turlings, T.C.J.; Campos-Herrera, R. Effects of cover crops on the overwintering success of entomopathogenic nematodes and their antagonists. Appl. Soil Ecol. 2017, 114, 62–73. [Google Scholar] [CrossRef]
- Wu, S.Y.; El-Borai, F.E.; Graham, J.H.; Duncan, L.W. Geospatial relationships between native entomopathogenic nematodes and Fusarium solani in a Florida citrus orchard. Appl. Soil Ecol. 2019, 140, 108–114. [Google Scholar] [CrossRef]
- Boemare, N.; Laumond, C.; Mauleon, H. The entomopathogenic nematode-bacterium complex: Biology, life cycle and vertebrate safety. Biocontrol Sci. Technol. 1996, 6, 333–346. [Google Scholar] [CrossRef]
- Ehlers, R.U. Current and future use of nematodes in biocontrol: Practice and commercial aspects with regard to regulatory policy issues. Biocontrol Sci. Technol. 1996, 6, 303–316. [Google Scholar] [CrossRef]
- Gaugler, R. Ecological considerations in the biological control of soil-inhabiting insects with entomopathogenic nematodes. Agric. Ecosyst. Environ. 1988, 24, 351–360. [Google Scholar] [CrossRef]
- Agazadeh, M.; Mohammadi, D.; Kary, N.E. Distribution of entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae in potato fields in North-West Iran (Nematoda: Rhabditida). Munis Entomol. Zool. 2010, 5, 758–763. [Google Scholar]
- Iraki, N.; Salah, N.; Sansour, M.A.; Segal, D.; Glazer, I.; Johnigk, S.A.; Hussein, M.A.; Ehlers, R.U. Isolation and characterization of two entomopathogenic nematode strains, Heterorhabditis indica (Nematoda, Rhabditida), from the West Bank, Palestinian territories. J. Appl. Entomol. 2000, 124, 375–380. [Google Scholar] [CrossRef]
- Lakatos, T.; Toth, T. Biological control of European cockchafer larvae [Melolontha melolontha L.]—Preliminary results. J. Fruit Ornam. Plant Res. 2006, 14, 73–78. [Google Scholar]
- Toth, T. Collection of entomopathogenic nematodes for the biological control of insect pests. J. Fruit Ornam. Plant Res. 2006, 14, 225–230. [Google Scholar]
- Matuska-Łyzwa, J. Effectiveness of selected strains of entomopathogenic nematodes in peat substrate in container breeding. Ecol. Quest. 2013, 18, 63–67. [Google Scholar] [CrossRef][Green Version]
- Matuska-Łyżwa, J. Invasiveness and the morphometry of Steinernema feltiae from different agrocoenoses of Poland. Ecol. Chem. Eng. A 2013, 20, 565–572. [Google Scholar]
- Stock, S.P. Molecular approaches and the taxonomy of insect-parasitic and pathogenic nematodes. In Insect Pathogens: Molecular Approaches and Techniques; Stock, S.P., Vandenberg, J., Glazer, I., Boemare, N., Eds.; CABI International: Oxfordshire, UK, 2009; pp. 71–100. ISBN 9781845934781. [Google Scholar]
- Matuska-Łyżwa, J.; Kaca, W.; Żarnowiec, P. Biological activity of wild isolates of entomopathogenic nematodes to horse-chestnut leaf miner (Cameraria ohridella). Pol. J. Environ. Stud. 2015, 24, 1181–1184. [Google Scholar] [CrossRef]
- Fan, X.; Hominick, W.M. Efficiency of the Galleria (wax moth) baiting technique for recovering infective stages of entomopathogenic rhabditids (Steinernematidae and Heterorhabditidae) from Sand and soil. Rev. Nématol. 1991, 14, 381–387. [Google Scholar]
- Matuska-Łyżwa, J. Method of Multiplication of Entomopathogenic Nematodes for the Plant Protection Research Purposes. WUP, Patent PL 212617 B1, 31 October 2012. [Google Scholar]
- Koppenhöfer, A.M. Nematodes. In Field Manual of Techniques in Invertebrate Pathology; Lacey, L., Kaya, H.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 249–264. [Google Scholar]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Field Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Ed.; Academic Press: Cambridge, MA, USA, 1997; pp. 281–324. [Google Scholar]
- Filipjev, I.N. On the classification of the Tylenchinae. Proc. Helminthol. Soc. Wash. 1936, 3, 80–82. [Google Scholar]
- Nguyen, K.B. Methodology, morphology and identification. In Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts; Nguyen, K.B., Hunt, D., Eds.; Brill: Leiden, The Netherland, 2007; pp. 59–119. [Google Scholar]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.B.; Mráček, Z.; Webster, J.M. Morphological and molecular characterization of a new isolate of Steinernema feltiae (Filipjev, 1934) from Vancouver, Canada, with morphometrical comparison with the topotype population from Russia. Zootaxa 2006, 1132, 51–61. [Google Scholar] [CrossRef]
- Helmberger, M.S.; Shields, E.J.; Wickings, K.G. Ecology of belowground biological control: Entomopathogenic nematode interactions with soil biota. Appl. Soil Ecol. 2017, 121, 201–213. [Google Scholar] [CrossRef]
- Keshari, A.K.; Hari, B.K.C.; Bhat, A.H.; Shah, M.M. Prospects and Present Status and of Entomopathogenic Nematodes (Steinernematidae and Heterorhabditidae) in Nepal. J. Appl. Adv. Res. 2019, 4, 31–35. [Google Scholar] [CrossRef]
- Tarasco, E.; Clausi, M.; Rappazzo, G.; Panzavolta, T.; Curto, G.; Sorino, R.; Oreste, M.; Longo, A.; Leone, D.; Tiberi, R.; et al. Biodiversity of entomopathogenic nematodes in Italy Biodiversity of entomopathogenic nematodes in Italy. J. Helminthol. 2015, 89, 359–366. [Google Scholar] [CrossRef]
- Malan, A.P.; Knoetze, R.; Tiedt, L.R. Steinernema jeffreyense n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. J. Helminthol. 2016, 90, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Nikdel, M.; Niknam, G.; Kary, N.E. First report of an entomopathogenic nematode, Steinernema kraussei (Rhabditida, Steinernematidae) from Iran. J. Entomol. Soc. Iran 2011, 30, 39–51. [Google Scholar]
- Campos-Herrera, R.; Escuer, M.; Robertson, L.; Gutiérrez, C. Morphological and ecological characterization of Steinernema feltiae (Rhabditida: Steinernematidae) rioja strain isolated from Bibio hortulanus (Diptera: Bibionidae) in Spain. J. Nematol. 2006, 38, 68–75. [Google Scholar] [PubMed]
- Çimen, H.; Půža, V.; Nermuť, J.; Hatting, J.; Ramakuwela, T.; Faktorová, L.; Hazir, S. Steinernema beitlechemi n. sp., a new entomopathogenic nematode (Nematoda: Steinernematidae) from South Africa. Nematology 2016, 18, 439–453. [Google Scholar] [CrossRef]
- De Brida, A.L.; Rosa, J.M.O.; De Oliveira, C.M.G.; De Castro E Castro, B.M.; Serrão, J.E.; Zanuncio, J.C.; Leite, L.G.; Wilcken, S.R.S. Entomopathogenic nematodes in agricultural areas in Brazil. Sci. Rep. 2017, 7, 45254. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska, M.; Skwiercz, A. The Influence of Selected Abiotic Factors on the Occurrence of Entomopathogenic Nematodes (Steinernematidae, Heterorhabditidae) in Soil. Pol. J. Soil Sci. 2018, 51, 11–21. [Google Scholar] [CrossRef]
- Hominick, W.M.; Reid, A.P.; Bohan, D.A.; Briscoe, B.R. Entomopathogenic nematodes: Biodiversity, geographical distribution and the convention on biological diversity. Biocontrol Sci. Technol. 1996, 6, 317–332. [Google Scholar] [CrossRef]
- El Khoury, Y.; Oreste, M.; Noujeim, E.; Nemer, N.M.; Tarasco, E. Effect of temperature on the pathogenicity of mediterranean native entomopathogenic nematodes (steinernematidae and heterorhabditidae) from natural ecosystems effect of temperature on the pathogenicity of mediterranean native entomopathogenic nematodes. Redia 2018, 101, 123–127. [Google Scholar] [CrossRef]
- Matuska-Łyżwa, J. Invasiveness and reproduction of the Steinernema feltiae from selected agrocoenose located in Wielun. Ecol. Chem. Eng. A 2015, 22, 327–334. [Google Scholar] [CrossRef]
- Lortkipanidze, M.; Gorgadze, O.; Kokhia, M.; Melashvili, N.; Kuchava, M. Effectiveness of entomopathogenic nematodes (Steinernema carpocapsae) against the Melolontha hippocastani (Coleoptera: Scarabaeidae). Bull. Georgian Natl. Acad. Sci. 2011, 5, 155–157. [Google Scholar]
- Noosidum, A.; Hodson, A.K.; Lewis, E.E.; Chandrapatva, A. Characterization of new entomopathogenic nematodes from thailand: Foraging behavior and virulence to the greater wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae). J. Nematol. 2010, 42, 281–291. [Google Scholar]
- McMullen, J.G.; Patricia Stock, S. In vivo and in vitro rearing of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae). J. Vis. Exp. 2014, 52096. [Google Scholar] [CrossRef]
- Rahoo, A.M.; Mukhtar, T.; Gowen, S.R.; Rahoo, R.K.; Abro, S.I. Reproductive potential and Host Searching Ability of Entomopathogenic nematode, Steinernema feltiae. Pak. J. Zool. 2017, 49, 229–234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).