UDP-Glucosyltransferases Induced by Nosema bombycis Provide Resistance to Microsporidia in Silkworm (Bombyx mori)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Cell Lines
2.2. Immune Challenge
2.3. RNA Isolation, cDNA Synthesis, and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.4. Gene Cloning
2.5. Real-Time Quantitative PCR (qPCR) Analysis
2.6. Vector Constructs
2.7. Protein Expression, Purification, and Polyclonal Antibody Production
2.8. Western Blotting
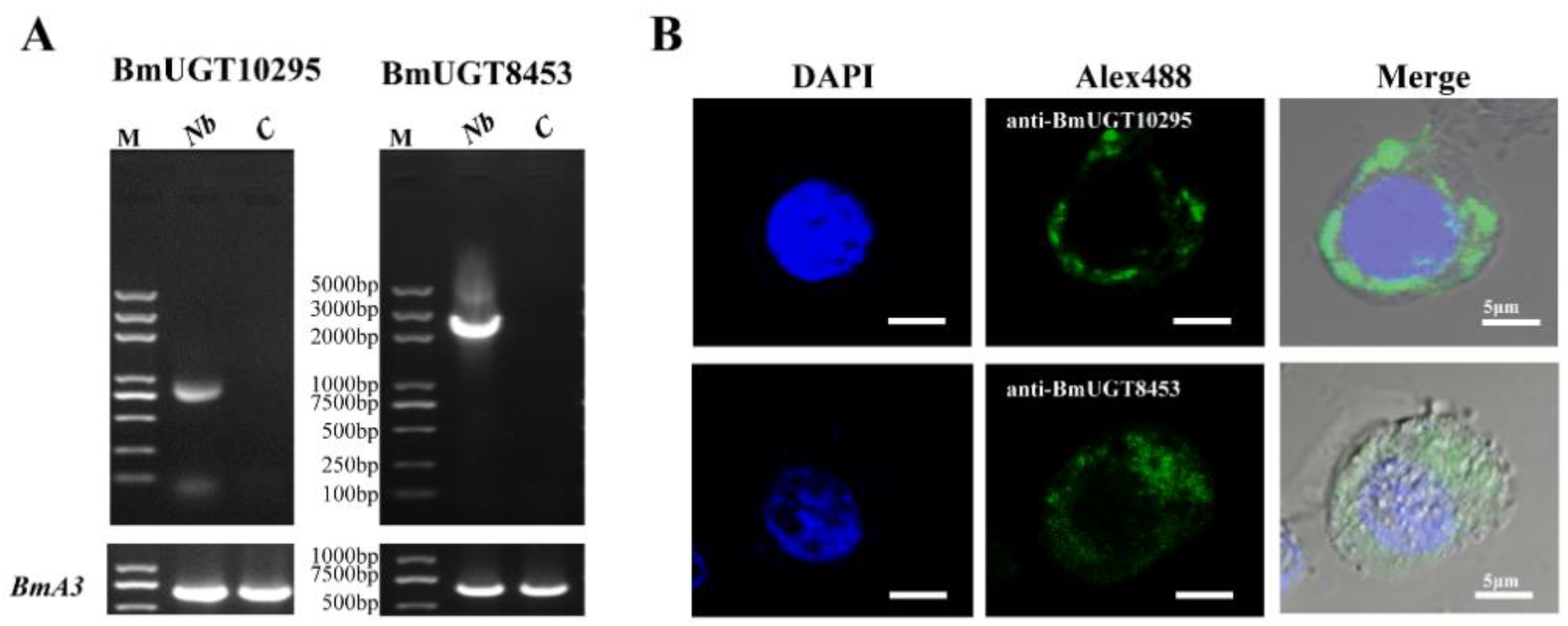
2.9. Indirect Immunofluorescence Assay
2.10. dsRNA Synthesis
2.11. Overexpression and RNAi BmUGTs
2.12. Statistical Analysis
3. Results
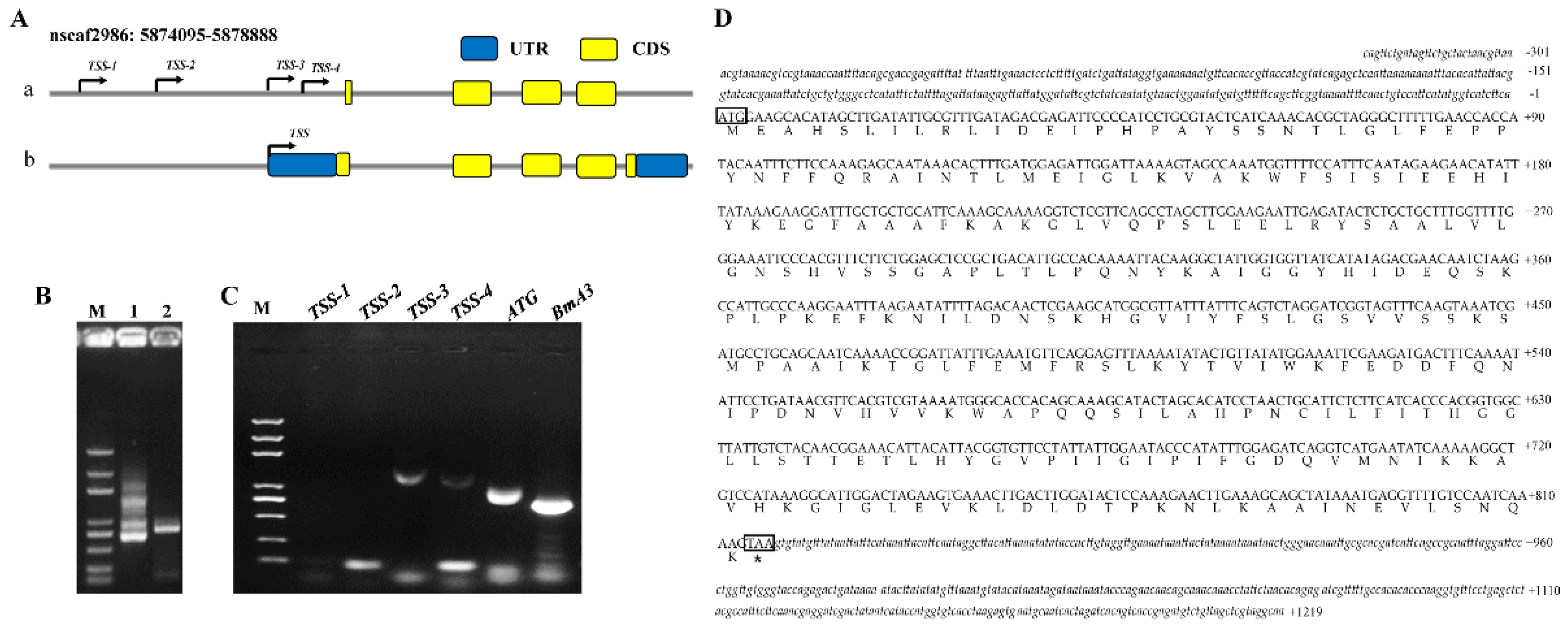
3.1. Identification of the N. bombycis-Inducible BmUGT Genes
3.1.1. Transcription of BmUGT Genes in B. mori
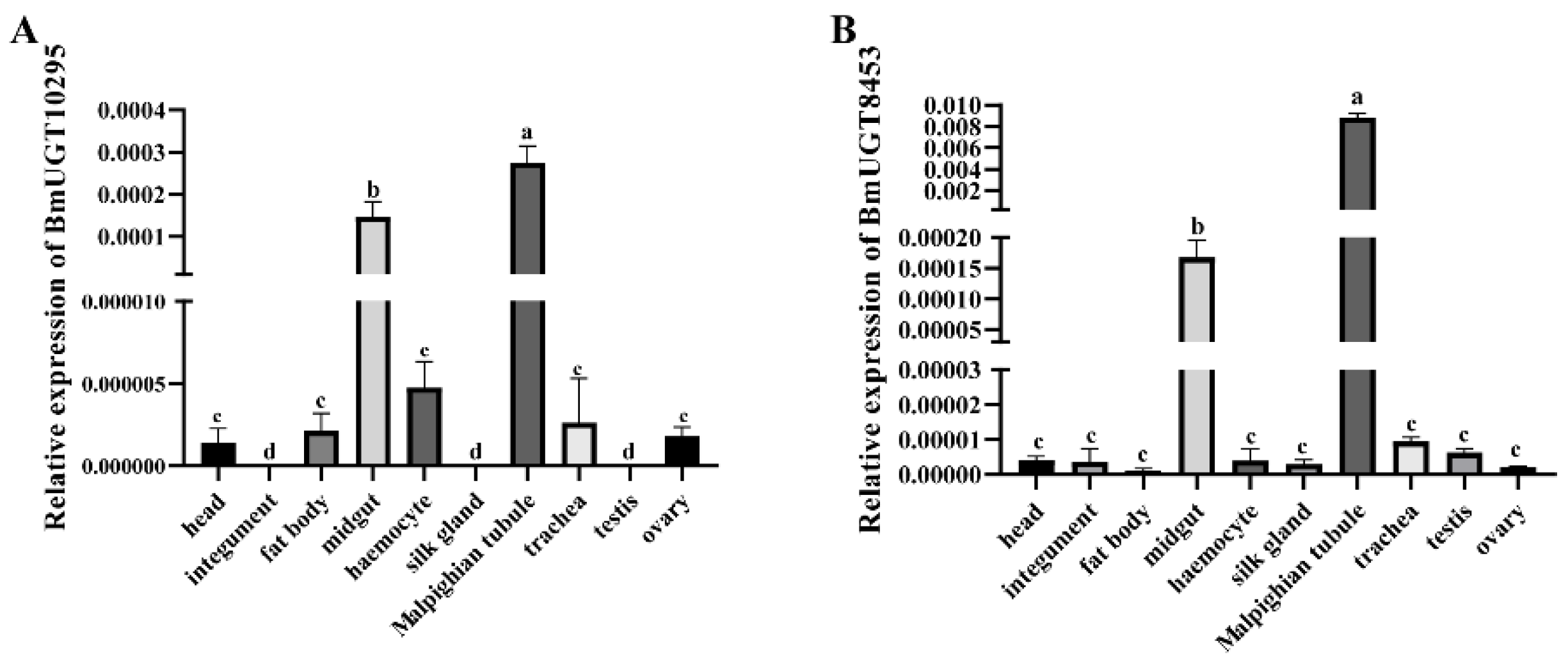
3.1.2. Transcription of BmUGT10295 and BmUGT8453 Genes in Different N. bombycis-Infected B. mori Tissues
3.2. Full ORF Clone of the BmUGT Genes
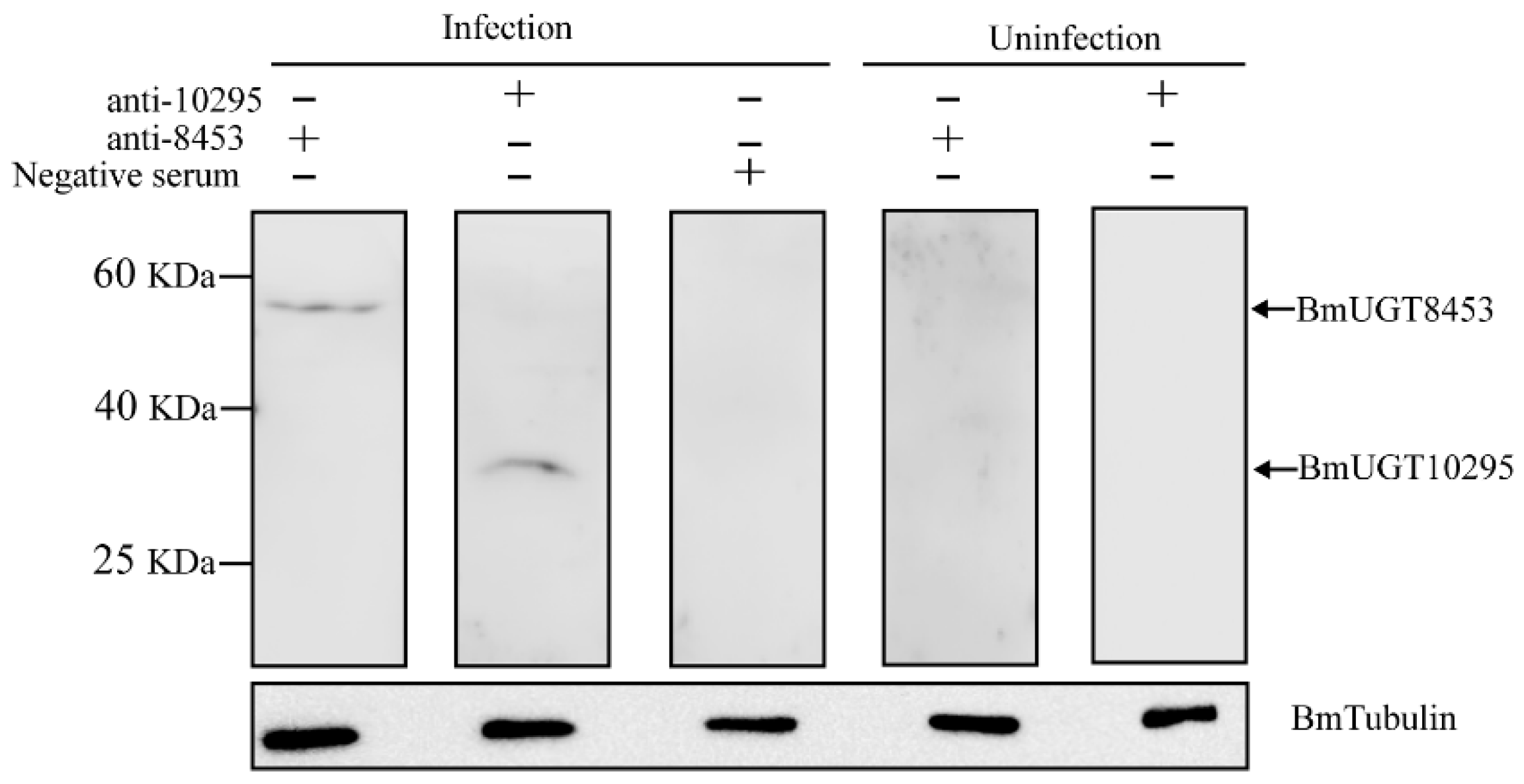
3.3. Recombinant BmUGT Purification and Immunoblot Analysis
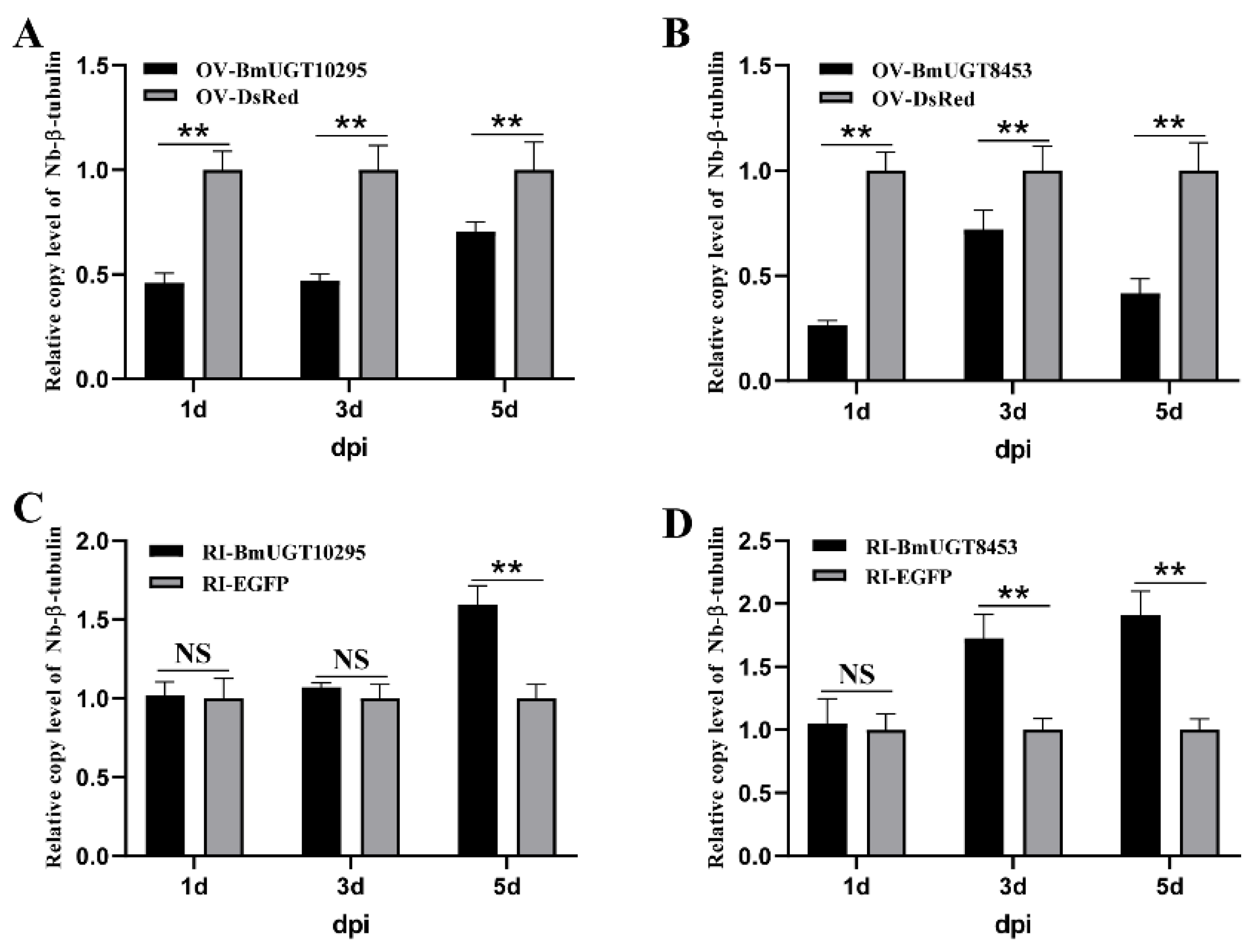
3.4. Nosema Bombycis Inhibited by BmUGT10295 and BmUGT8453
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavalier-Smith, T. A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc. 1998, 73, 203–266. [Google Scholar] [CrossRef]
- Pan, G.; Bao, J.; Ma, Z.; Song, Y.; Han, B.; Ran, M.; Li, C.; Zhou, Z. Invertebrate host responses to microsporidia infections. Dev. Comp. Immunol. 2018, 83, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Fries, I.; Feng, F.; da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n. sp.(Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Zander, E. Tierische parasiten als krankenheitserreger bei der biene. Münchener Bienenztg. 1909, 31, 196–204. [Google Scholar]
- Aranguren Caro, L.F.; Mai, H.N.; Pichardo, O.; Cruz-Flores, R.; Hanggono, B.; Dhar, A.K. Evidences supporting Enterocytozoon hepatopenaei association with white feces syndrome in farmed Penaeus vannamei in Venezuela and Indonesia. Dis. Aquat. Org. 2020, 141, 71–78. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Sudhakaran, R. Experimental horizontal transmission of Enterocytozoon hepatopenaei in post-larvae of whiteleg shrimp, Litopenaeus vannamei. J. Fish Dis. 2019, 42, 397–404. [Google Scholar] [CrossRef]
- Elkarim Laatamna, A.; Holubova, N.; Sak, B.; Kvac, M. Cryptosporidium meleagridis and C. baileyi (Apicomplexa) in domestic and wild birds in Algeria. Folia Parasitol. 2017, 64. [Google Scholar] [CrossRef]
- Shahbazi, P.; Aligolzadeh, A.; Khordadmehr, M.; Hashemzadeh Farhang, H.; Katiraee, F. Molecular study and genotyping of Cryptosporidium baileyi and Cryptosporidium parvum from free-range and commercial broiler chickens in Guilan province, Iran. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101411. [Google Scholar] [CrossRef]
- Current, W.L.; Garcia, L.S. Cryptosporidiosis. Clin. Microbiol. Rev. 1991, 4, 325–358. [Google Scholar] [CrossRef]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Not just in AIDS patients. Curr. Opin. Infect. Dis. 2011, 24, 490–495. [Google Scholar] [CrossRef]
- Muadica, A.S.; Messa, A.E., Jr.; Dashti, A.; Balasegaram, S.; Santin, M.; Manjate, F.; Chirinda, P.; Garrine, M.; Vubil, D.; Acácio, S.; et al. First identification of genotypes of Enterocytozoon bieneusi (Microsporidia) among symptomatic and asymptomatic children in Mozambique. PLoS Negl. Trop. Dis. 2020, 14, e0008419. [Google Scholar] [CrossRef]
- Ghosh, K.; Weiss, L.M. T cell response and persistence of the microsporidia. FEMS Microbiol. Rev. 2012, 36, 748–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretto, M.M.; Harrow, D.I.; Khan, I.A. Effector CD8 T cell immunity in microsporidial infection: A lone defense mechanism. Semin. Immunopathol. 2015, 37, 281–287. [Google Scholar] [CrossRef]
- Szumowski, S.C.; Troemel, E.R. Microsporidia-host interactions. Curr. Opin. Microbiol. 2015, 26, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.A.; Bashir, I.; Kamili, A.S. Microsporidiosis of silkworm, Bombyx mori L. (Lepidoptera-Bombycidae): A review. Afr. J. Agric. Res. 2009, 4, 1519–1523. [Google Scholar]
- Ni, W.; Bao, J.; Mo, B.; Liu, L.; Li, T.; Pan, G.; Chen, J.; Zhou, Z. Hemocytin facilitates host immune responses against Nosema bombycis. Dev. Comp. Immunol. 2020, 103, 103495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tang, X.; Xiao, S.; Wang, H.; Zhang, Y.; Shao, Y.; Tang, F.; Chen, S.; Bai, X. The role of Bombyx mori Bmtutl-519 protein in the infection of BmN cells by Nosema bombycis. Dev. Comp. Immunol. 2019, 92, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Xu, W.; Ma, S.; Xia, Q. STING-dependent autophagy suppresses Nosema bombycis infection in silkworms, Bombyx mori. Dev. Comp. Immunol. 2021, 115, 103862. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, Y.; Zhou, Y.; Liu, R.; Shen, Z. Quantitative proteomic analysis of ovaries from Nosema bombycis-infected silkworm (Bombyx mori). J. Invertebr. Pathol. 2020, 172, 107355. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, Z.; Li, M.; Liu, H.; Cai, S.; Man, N.; Lu, X. Nosema bombycis (Microsporidia) suppresses apoptosis in BmN cells (Bombyx mori). Acta Biochim. Biophys. Sin. 2015, 47, 696–702. [Google Scholar] [CrossRef] [Green Version]
- Bock, K.W. Vertebrate UDP-glucuronosyltransferases: Functional and evolutionary aspects. Biochem. Pharmacol. 2003, 66, 691–696. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, P.; Zeng, X.; Liu, X.; Shang, Q. Characterization of UDP-Glucuronosyltransferases and the Potential Contribution to Nicotine Tolerance in Myzus persicae. Int. J. Mol. Sci. 2019, 20, 3637. [Google Scholar] [CrossRef] [Green Version]
- Krempl, C.; Sporer, T.; Reichelt, M.; Ahn, S.J.; Heidel-Fischer, H.; Vogel, H.; Heckel, D.G.; Joußen, N. Potential detoxification of gossypol by UDP-glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 71, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wen, S.; Chen, X.; Gao, X.; Zeng, X.; Liu, X.; Tian, F.; Shang, Q. UDP-glycosyltransferases contribute to spirotetramat resistance in Aphis gossypii Glover. Pestic. Biochem. Physiol. 2020, 166, 104565. [Google Scholar] [CrossRef]
- Li, X.; Zhu, B.; Gao, X.; Liang, P. Over-expression of UDP-glycosyltransferase gene UGT2B17 is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2017, 73, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, C.; Wang, X.; Li, G.; Liu, Z.; Wang, H.; Guo, X.; Xu, B. Molecular Mechanism of the UDP-Glucuronosyltransferase 2B20-like Gene (AccUGT2B20-like) in Pesticide Resistance of Apis cerana cerana. Front. Genet. 2020, 11, 592595. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, S.H.; Ren, M.M.; Tian, X.R.; Wei, Q.; Mburu, D.K.; Su, J.Y. The expression of Spodoptera exigua P450 and UGT genes: Tissue specificity and response to insecticides. Insect Sci. 2019, 26, 199–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Xu, L.; Sun, Y.; Song, P.; Han, Z. UDP-Glycosyltransferase Genes in the Striped Rice Stem Borer, Chilo suppressalis (Walker), and Their Contribution to Chlorantraniliprole Resistance. Int. J. Mol. Sci. 2019, 20, 1064. [Google Scholar] [CrossRef] [Green Version]
- Nagare, M.; Ayachit, M.; Agnihotri, A.; Schwab, W.; Joshi, R. Glycosyltransferases: The multifaceted enzymatic regulator in insects. Insect Mol. Biol. 2021, 30, 123–137. [Google Scholar] [CrossRef]
- Rübsam, R.; Hollmann, M.; Simmerl, E.; Lammermann, U.; Schäfer, M.A.; Büning, J.; Schäfer, U. The egghead gene product influences oocyte differentiation by follicle cell-germ cell interactions in Drosophila melanogaster. Mech. Dev. 1998, 72, 131–140. [Google Scholar] [CrossRef]
- Johnson, D.M.; Mugelli, A.; Cerbai, E. Editorial: The Role of Calcium Handling in Heart Failure and Heart Failure Associated Arrhythmias. Front. Physiol. 2019, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Walski, T.; De Schutter, K.; Van Damme, E.J.M.; Smagghe, G. Diversity and functions of protein glycosylation in insects. Insect Biochem. Mol. Biol. 2017, 83, 21–34. [Google Scholar] [CrossRef]
- Wiesen, B.; Krug, E.; Fiedler, K.; Wray, V.; Proksch, P. Sequestration of host-plant-derived flavonoids by lycaenid butterflyPolyommatus icarus. J. Chem. Ecol. 1994, 20, 2523–2538. [Google Scholar] [CrossRef] [PubMed]
- Bock, K.W. The UDP-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: Animal-plant arms-race and co-evolution. Biochem. Pharmacol. 2016, 99, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Zhou, J.J.; Yi, J.K.; Pan, Y.; Wang, J.; Zhang, X.X.; Wang, J.X.; Yang, S.; Xi, J.H. Identification and tissue expression profiling of candidate UDP-glycosyltransferase genes expressed in Holotrichia parallela motschulsky antennae. Bull. Entomol. Res. 2018, 108, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-F.; Chai, C.-L.; Zhang, Z.; Liu, Z.-H.; Dai, F.-Y.; Lu, C.; Xiang, Z.-H. The UDP-glucosyltransferase multigene family in Bombyx mori. BMC Genom. 2008, 9, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.J.; Vogel, H.; Heckel, D.G. Comparative analysis of the UDP-glycosyltransferase multigene family in insects. Insect Biochem. Mol. Biol. 2012, 42, 133–147. [Google Scholar] [CrossRef]
- Daimon, T.; Hirayama, C.; Kanai, M.; Ruike, Y.; Meng, Y.; Kosegawa, E.; Nakamura, M.; Tsujimoto, G.; Katsuma, S.; Shimada, T. The silkworm Green b locus encodes a quercetin 5-O-glucosyltransferase that produces green cocoons with UV-shielding properties. Proc. Natl. Acad. Sci. USA 2010, 107, 11471–11476. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, M.; Wang, Y.; Sima, Y.; Zhang, D.; Li, J.; Yin, W.; Xu, S. Green cocoons in silkworm Bombyx mori resulting from the quercetin 5-O-glucosyltransferase of UGT86, is an evolved response to dietary toxins. Mol. Biol. Rep. 2013, 40, 3631–3639. [Google Scholar] [CrossRef]
- Luque, T.; Okano, K.; O’Reilly, D.R. Characterization of a novel silkworm (Bombyx mori) phenol UDP-glucosyltransferase. Eur. J. Biochem. 2002, 269, 819–825. [Google Scholar] [CrossRef]
- Ma, Z.; Li, C.; Pan, G.; Li, Z.; Han, B.; Xu, J.; Lan, X.; Chen, J.; Yang, D.; Chen, Q.; et al. Genome-wide transcriptional response of silkworm (Bombyx mori) to infection by the microsporidian Nosema bombycis. PLoS ONE 2013, 8, e84137. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.J.; Tang, X.D.; Xu, L.; Yan, W.; Li, Q.L.; Xiao, S.Y.; Fu, X.L.; Wang, W.; Li, N.; Shen, Z.Y. Early responses of silkworm midgut to microsporidium infection--A Digital Gene Expression analysis. J. Invertebr. Pathol. 2015, 124, 6–14. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wang, L.; Zhou, Z. Molecular and biochemical responses in the midgut of the silkworm, Bombyx mori, infected with Nosema bombycis. Parasites Vectors 2018, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- He, X.; He, X.; Liu, H.; Li, M.; Cai, S.; Fu, Z.; Lu, X. Proteomic analysis of BmN cells (Bombyx mori) in response to infection with Nosema bombycis. Acta Biochim. Biophys. Sin. 2014, 46, 982–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, M.H.; Cai, X.J.; Liu, M.; Lv, J.; Tang, H.; Tan, J.; Lu, C. Establishment and characterization of an ovarian cell line of the silkworm, Bombyx mori. Tissue Cell 2010, 42, 42–46. [Google Scholar] [CrossRef]
- Yu, B.; Sang, Q.; Pan, G.; Li, C.; Zhou, Z. A Toll-Spätzle Pathway in the Immune Response of Bombyx mori. Insects 2020, 11, 586. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Li, C.; Sun, B.; Zheng, R.; Li, Z.; Chen, J.; Long, M.; Li, T.; Pan, G.; Zhou, Z. Cloning and Expression Analysis of C-type Lectin 11 Gene in Bombyx mori. Sci. Seric. 2016, 042, 627–635. [Google Scholar] [CrossRef]
- Dong, Z.; Long, J.; Huang, L.; Hu, Z.; Chen, P.; Hu, N.; Zheng, N.; Huang, X.; Lu, C.; Pan, M. Construction and application of an HSP70 promoter-inducible genome editing system in transgenic silkworm to induce resistance to Nosema bombycis. Appl. Microbiol. Biotechnol. 2019, 103, 9583–9592. [Google Scholar] [CrossRef] [PubMed]
- Kittayapong, P.; Ninphanomchai, S.; Limohpasmanee, W.; Chansang, C.; Chansang, U.; Mongkalangoon, P. Combined sterile insect technique and incompatible insect technique: The first proof-of-concept to suppress Aedes aegypti vector populations in semi-rural settings in Thailand. PLoS Negl. Trop. Dis. 2019, 13, e0007771. [Google Scholar] [CrossRef] [Green Version]
- Bouyer, J.; Yamada, H.; Pereira, R.; Bourtzis, K.; Vreysen, M.J.B. Phased Conditional Approach for Mosquito Management Using Sterile Insect Technique. Trends Parasitol. 2020, 36, 325–336. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; The International Atomic Energy Agency. Guidance Framework for Testing the Sterile Insect Technique as a Vector Control Tool Against Aedes-Borne Diseases; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Kandul, N.P.; Liu, J.; Sanchez, C.H.; Wu, S.L.; Marshall, J.M.; Akbari, O.S. Transforming insect population control with precision guided sterile males with demonstration in flies. Nat. Commun. 2019, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef]
- Fu, G.; Lees, R.S.; Nimmo, D.; Aw, D.; Jin, L.; Gray, P.; Berendonk, T.U.; White-Cooper, H.; Scaife, S.; Kim Phuc, H.; et al. Female-specific flightless phenotype for mosquito control. Proc. Natl. Acad. Sci. USA 2010, 107, 4550–4554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Li, M.; He, X.; Cai, S.; He, X.; Lu, X. Transcriptome sequencing and characterization of ungerminated and germinated spores of Nosema bombycis. Acta Biochim. Biophys. Sin. 2016, 48, 246–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meech, R.; Mackenzie, P.I. Structure and function of uridine diphosphate glucuronosyltransferases. Clin. Exp. Pharmacol. Physiol. 1997, 24, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Israni, B.; Wouters, F.C.; Luck, K.; Seibel, E.; Ahn, S.J.; Paetz, C.; Reinert, M.; Vogel, H.; Erb, M.; Heckel, D.G.; et al. The Fall Armyworm Spodoptera frugiperda Utilizes Specific UDP-Glycosyltransferases to Inactivate Maize Defensive Benzoxazinoids. Front. Physiol. 2020, 11, 604754. [Google Scholar] [CrossRef]
- Ma, K.; Tang, Q.; Liang, P.; Li, J.; Gao, X. UDP-Glycosyltransferases from the UGT344 Family Are Involved in Sulfoxaflor Resistance in Aphis gossypii Glover. Insects 2021, 12, 356. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Cui, L.; Wang, Q.; Huang, W.; Yang, Q.; Ji, X.; Rui, C. Overexpression of Multiple UDP-Glycosyltransferase Genes Involved in Sulfoxaflor Resistance in Aphis gossypii Glover. J. Agric. Food Chem. 2021, 69, 5198–5205. [Google Scholar] [CrossRef]
- Chen, X.; Xia, J.; Shang, Q.; Song, D.; Gao, X. UDP-glucosyltransferases potentially contribute to imidacloprid resistance in Aphis gossypii glover based on transcriptomic and proteomic analyses. Pestic. Biochem. Physiol. 2019, 159, 98–106. [Google Scholar] [CrossRef]
- Chen, X.; Tang, C.; Ma, K.; Xia, J.; Song, D.; Gao, X.W. Overexpression of UDP-glycosyltransferase potentially involved in insecticide resistance in Aphis gossypii Glover collected from Bt cotton fields in China. Pest Manag. Sci. 2020, 76, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Liu, X.Y.; Shi, L.; Liu, J.L.; Shen, G.M.; Zhang, P.; Lu, W.C.; He, L. Functional analysis of UGT201D3 associated with abamectin resistance in Tetranychus cinnabarinus (Boisduval). Insect Sci. 2020, 27, 276–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.R.; Kong, C.; Lee, S.H.; Nathan, S. Detection of Burkholderia pseudomallei toxin-mediated inhibition of protein synthesis using a Caenorhabditis elegans ugt-29 biosensor. Sci. Rep. 2016, 6, 27475. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Yang, Q.; Wei, J.; Pan, G.; Li, C.; Zhou, Z. UDP-Glucosyltransferases Induced by Nosema bombycis Provide Resistance to Microsporidia in Silkworm (Bombyx mori). Insects 2021, 12, 799. https://doi.org/10.3390/insects12090799
Yu B, Yang Q, Wei J, Pan G, Li C, Zhou Z. UDP-Glucosyltransferases Induced by Nosema bombycis Provide Resistance to Microsporidia in Silkworm (Bombyx mori). Insects. 2021; 12(9):799. https://doi.org/10.3390/insects12090799
Chicago/Turabian StyleYu, Bin, Qiuhua Yang, Junhong Wei, Guoqing Pan, Chunfeng Li, and Zeyang Zhou. 2021. "UDP-Glucosyltransferases Induced by Nosema bombycis Provide Resistance to Microsporidia in Silkworm (Bombyx mori)" Insects 12, no. 9: 799. https://doi.org/10.3390/insects12090799
APA StyleYu, B., Yang, Q., Wei, J., Pan, G., Li, C., & Zhou, Z. (2021). UDP-Glucosyltransferases Induced by Nosema bombycis Provide Resistance to Microsporidia in Silkworm (Bombyx mori). Insects, 12(9), 799. https://doi.org/10.3390/insects12090799






